Lewy Body Dementias: A Coin with Two Sides?
Abstract
1. Introduction
2. Methods
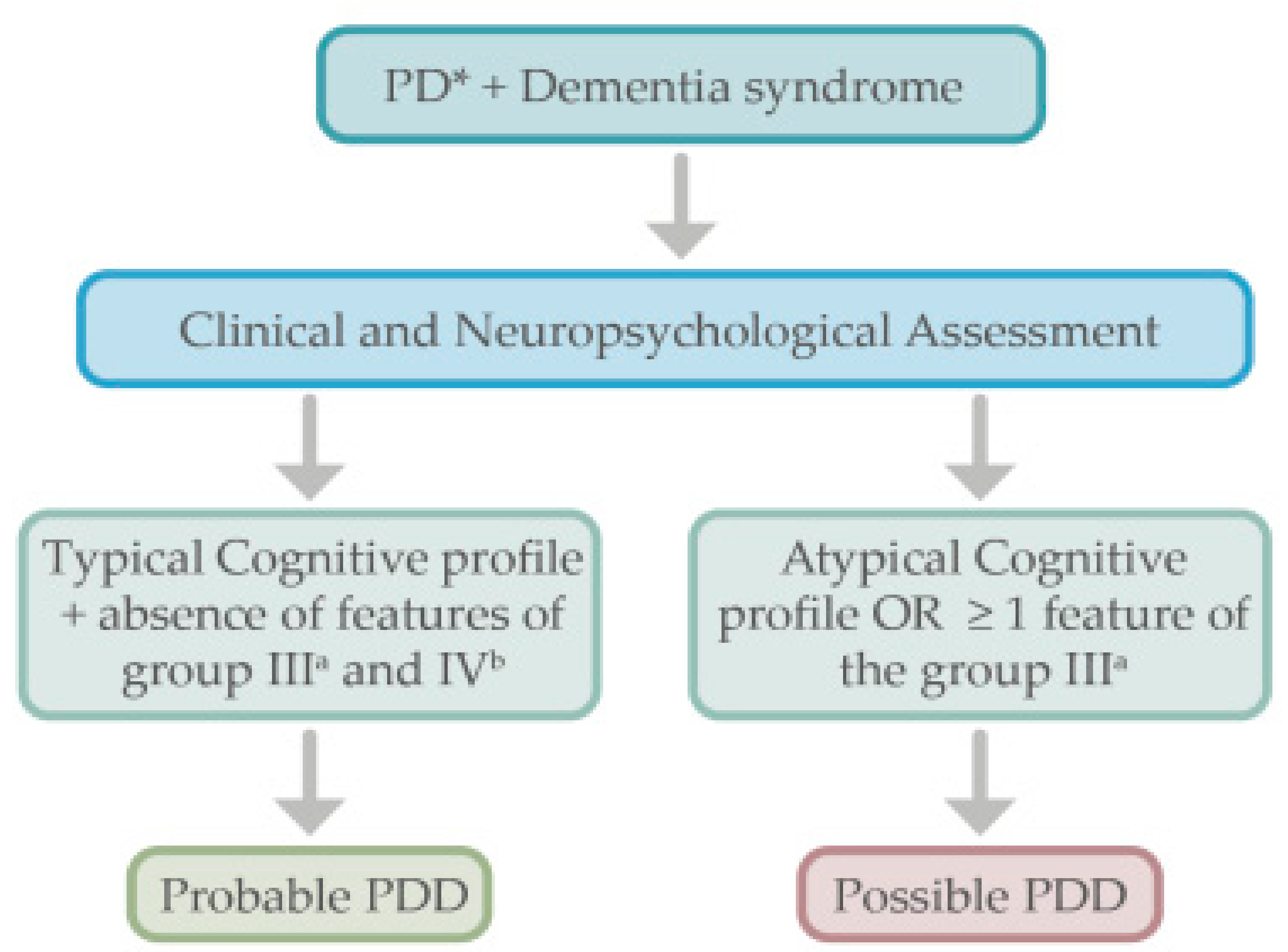
3. Current Diagnostic Criteria for Parkinson’s Disease Dementia and Lewy Body Dementia
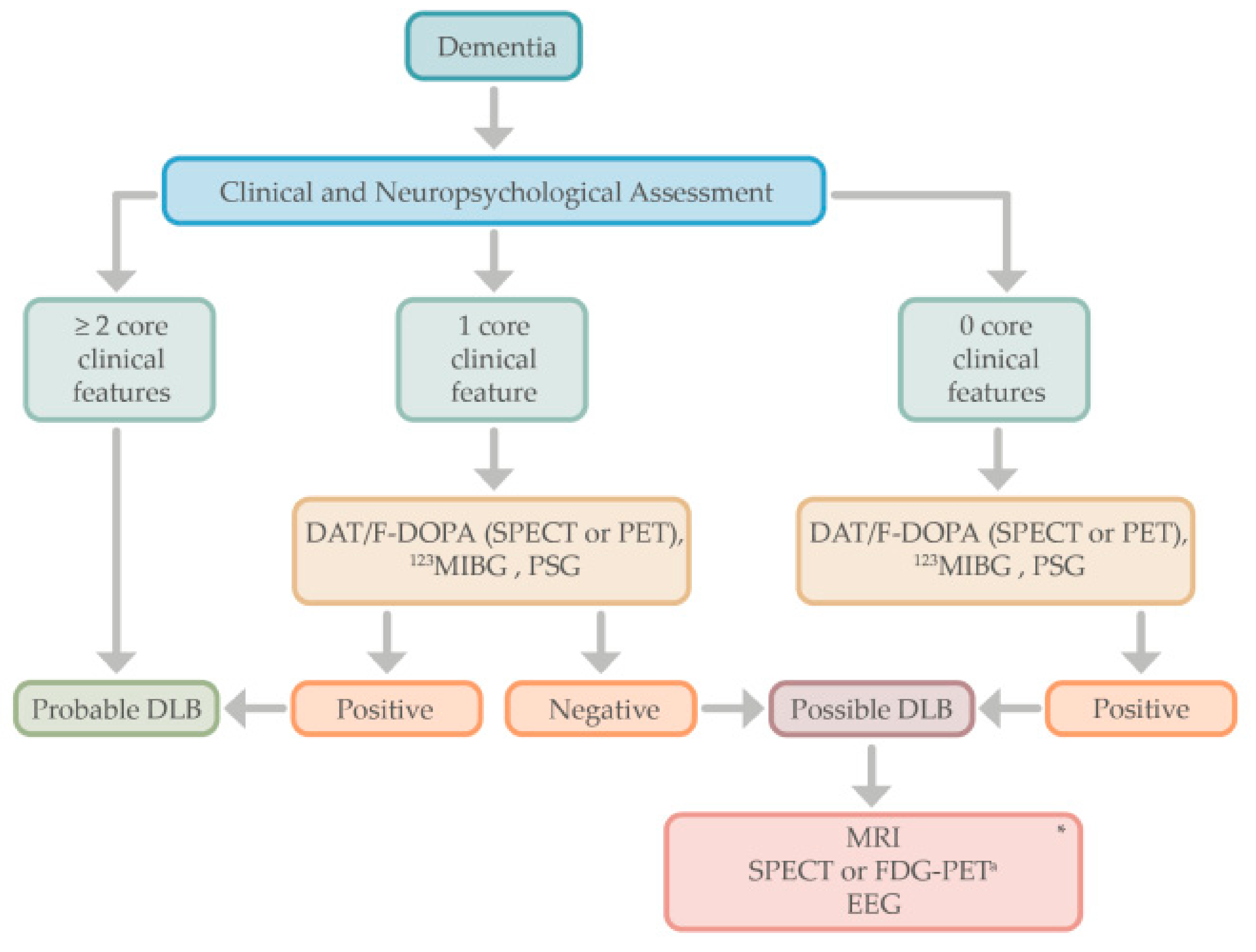
4. Diagnostic Approach
5. Cognitive Profile of Lewy Body Dementias
6. Behavioral and Neuropsychiatric Manifestations of Lewy Body Dementias
7. From Neuropathology to the Clinical Spectrum of Lewy Body Dementias
8. Biomarkers for Lewy Body Dementias
8.1. Magnetic Resonance Imaging in Lewy Body Dementias
8.1.1. Structural Magnetic Resonance Imaging
8.1.2. Functional Magnetic Resonance Imaging
8.2. Nuclear Medicine and Molecular Imaging in Lewy Body Dementias
8.3. Molecular Biomarkers in Fluids in Lewy Body Dementias
8.3.1. Cerebrospinal Fluid
8.3.2. Other Biological Fluid Biomarkers
8.4. Neurophysiological Biomarkers
9. Evolution and Prognosis of Lewy Body Dementias
10. Management of Lewy Body Dementias
11. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harding, A.J.; Halliday, G.M. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001, 102, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.C.; Eggers, C.; Kalbe, E.; Weisenbach, S.; Hohmann, C.; Vollmar, S.; Baudrexel, S.; Diederich, N.J.; Heiss, W.D.; Hilker, R. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 2010, 74, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Zaccai, J.; McCracken, C.; Brayne, C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing 2005, 34, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.B.; Fiest, K.M.; Roberts, J.I.; Maxwell, C.J.; Dykeman, J.; Pringsheim, T.; Steeves, T.; Smith, E.E.; Pearson, D.; Jetté, N. The prevalence and incidence of dementia with lewy bodies: A systematic review. Can. J. Neurol. Sci. 2016, 43, S83–S95. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Non-motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2016, 22, S119–S122. [Google Scholar] [CrossRef]
- Rodriguez-Oroz, M.C.; Jahanshahi, M.; Krack, P.; Litvan, I.; Macias, R.; Bezard, E.; Obeso, J.A. Initial clinical manifestations of Parkinson’s disease: Features and pathophysiological mechanisms. Lancet Neurol. 2009, 8, 1128–1139. [Google Scholar] [CrossRef]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef]
- Goldman, J.G.; Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin. Geriatr. Med. 2020, 36, 365–377. [Google Scholar] [CrossRef]
- Nicoletti, A.; Luca, A.; Baschi, R.; Cicero, C.E.; Mostile, G.; Davì, M.; Pilati, L.; Restivo, V.; Zappia, M.; Monastero, R. Incidence of mild cognitive impairment and dementia in Parkinson’s disease: The Parkinson’s disease cognitive impairment study. Front. Aging Neurosci. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Janvin, C.C.; Larsen, J.P.; Aarsland, D.; Hugdahl, K. Subtypes of mild cognitive impairment in Parkinson’s disease: Progression to dementia. Mov. Disord. 2006, 21, 1343–1349. [Google Scholar] [CrossRef]
- Gasca-Salas, C.; Estanga, A.; Clavero, P.; Aguilar-Palacio, I.; González-Redondo, R.; Obeso, J.A.; Rodriguez-Oroz, M.C. Longitudinal assessment of the pattern of cognitive decline in non-demented patients with advanced Parkinson’s disease. J. Parkinson’s Dis. 2014, 4, 677–686. [Google Scholar] [CrossRef]
- Litvan, I.; Aarsland, D.; Adler, C.H.; Goldman, J.G.; Kulisevsky, J.; Mollenhauer, B.; Rodriguez-Oroz, M.C.; Tröster, A.I.; Weintraub, D. MDS task force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Mov. Disord. 2011, 26, 1814–1824. [Google Scholar] [CrossRef]
- Hely, M.A.; Reid, W.G.J.; Adena, M.A.; Halliday, G.M.; Morris, J.G.L. The Sydney Multicenter Study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef]
- Mckeith, I.G.; Sci, M.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.-P.; Psych, M.; Weintraub, D.; Aarsland, D.; Galvin, J.; et al. Diagnosis and management of dementia with Lewy bodies Fourth consensus report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef]
- McKeith, I.G.; Dickson, D.W.; Lowe, J.; Emre, M.; O’Brien, J.T.; Feldman, H.; Cummings, J.; Duda, J.E.; Lippa, C.; Perry, E.K.; et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology 2005, 65, 1863–1872. [Google Scholar] [CrossRef]
- Gomperts, S.N. Lewy body dementias. Contin. Lifelong Learn. Neurol. 2016, 22, 435–463. [Google Scholar] [CrossRef]
- Mckeith, I.G.; Sci, M.; Ferman, T.J.; Thomas, A.J.; Blanc, F.; Boeve, B.F.; Fujishiro, H.; Kantarci, K.; Muscio, C.; O’brien, J.T.; et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 2020, 94, 743–755. [Google Scholar] [CrossRef]
- Galvin, J.E. Improving the clinical detection of Lewy body dementia with the Lewy body composite risk score. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2015, 1, 316–324. [Google Scholar] [CrossRef]
- Thomas, A.J.; Donaghy, P.; Roberts, G.; Colloby, S.J.; Barnett, N.A.; Petrides, G.; Lloyd, J.; Olsen, K.; Taylor, J.P.; McKeith, I.; et al. Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol. Med. 2019, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Yoshita, M.; Taki, J.; Yamada, M. A clinical role for [123I]MIBG myocardial scintigraphy in the distinction between dementia of the Alzheimer’s-type and dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry 2001, 71, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Mason, S.L.; Evans, J.R.; Foltynie, T.; Brayne, C.; Robbins, T.W.; Barker, R.A. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1258–1264. [Google Scholar] [CrossRef]
- Nobili, F.; Morbelli, S.; Arnaldi, D.; Ferrara, M.; Campus, C.; Brugnolo, A.; Mazzei, D.; Mehrdad, N.; Sambuceti, G.; Rodriguez, G. Radionuclide brain imaging correlates of cognitive impairment in Parkinson’s disease (PD). J. Neurol. Sci. 2011, 310, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Hirano, S.; Shinotoh, H.; Aotsuka, A.; Sato, K.; Tanaka, N.; Ota, T.; Asahina, M.; Fukushi, K.; Kuwabara, S.; et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 2009, 73, 273–278. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Kaufer, D.I.; Ivanco, L.S.; Lopresti, B.; Koeppe, R.A.; Davis, J.G.; Mathis, C.A.; Moore, R.Y.; DeKosky, S.T. Cortical cholinergic function is more severely affected in Parkinsonian dementia than in Alzheimer disease: An in vivo positron emission tomographic study. Arch. Neurol. 2003, 60, 1745–1748. [Google Scholar] [CrossRef]
- Litvan, I.; Mohr, E.; Williams, J.; Gomez, C.; Chase, T.N. Differential memory and executive functions in demented patients with Parkinson’s and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1991, 54, 25–29. [Google Scholar] [CrossRef]
- Ferman, T.J.; Smith, G.E.; Boeve, B.F.; Graff-Radford, N.R.; Lucas, J.A.; Knopman, D.S.; Petersen, R.C.; Ivnik, R.J.; Wszolek, Z.; Uitti, R.; et al. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer’s disease. Clin. Neuropsychol. 2006, 20, 623–636. [Google Scholar] [CrossRef]
- Mosimann, U.P.; Mather, G.; Wesnes, K.A.; O’Brien, J.T.; Burn, D.J.; McKeith, I.G. Visual perception in Parkinson disease dementia and dementia with Lewy bodies. Neurology 2004, 63, 2091–2096. [Google Scholar] [CrossRef]
- Robertson, A.D.; Messner, M.A.; Shirzadi, Z.; Kleiner-Fisman, G.; Lee, J.; Hopyan, J.; Lang, A.E.; Black, S.E.; MacIntosh, B.J.; Masellis, M. Orthostatic hypotension, cerebral hypoperfusion, and visuospatial deficits in Lewy body disorders. Park. Relat. Disord. 2016, 22, 80–86. [Google Scholar] [CrossRef]
- Smirnov, D.S.; Galasko, D.; Edland, S.D.; Filoteo, J.V.; Hansen, L.A.; Salmon, D.P. Cognitive decline profiles differ in Parkinson disease dementia and dementia with Lewy bodies. Neurology 2020, 94, e2076–e2087. [Google Scholar] [CrossRef]
- Whittington, C.J.; Podd, J.; Kan, M.M. Recognition memory impairment in Parkinson’s disease: Power and meta-analyses. Neuropsychology 2000, 14, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Filoteo, J.V.; Salmon, D.P.; Schiehser, D.M.; Kane, A.E.; Hamilton, J.M.; Rilling, L.M.; Lucas, J.A.; Zizak, V.; Galasko, D.R. Verbal learning and memory in patients with dementia with Lewy bodies or Parkinson’s disease with dementia. J. Clin. Exp. Neuropsychol. 2009, 31, 823–834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Dowd, S.; Schumacher, J.; Burn, D.J.; Bonanni, L.; Onofrj, M.; Thomas, A.; Taylor, J.P. Fluctuating cognition in the Lewy body dementias. Brain 2019, 142, 3338–3350. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.G.; Aarsland, D.; McKeith, I.; O’Brien, J.; Gray, A.; Cormaak, F.; Burn, D.; Cassidy, T.; Starfeldt, R.; Larsen, J.P.; et al. Fluctuations in attention: PD dementia vs DLB with parkinsonism. Neurology 2002, 59, 1714–1720. [Google Scholar] [CrossRef]
- Lee, D.R.; Taylor, J.P.; Thomas, A.J. Assessment of cognitive fluctuation in dementia: A systematic review of the literature. Int. J. Geriatr. Psychiatry 2012, 27, 989–998. [Google Scholar] [CrossRef]
- Van Dyk, K.; Towns, S.; Tatarina, O.; Yeung, P.; Dorrejo, J.; Zahodne, L.B.; Stern, Y. Assessing fluctuating cognition in dementia diagnosis. Am. J. Alzheimer’s Dis. Other Dement. 2016, 31, 137–143. [Google Scholar] [CrossRef]
- Dorrejo, J.; Zahodne, L.B.; Stern, Y. Interrater reliability of the clinician assessment of fluctuation. Am. J. Alzheimer’s Dis. Other Dement. 2016, 31, 137–143. [Google Scholar] [CrossRef]
- Fields, J.A. Cognitive and neuropsychiatric features in Parkinson’s and Lewy body dementias. Arch. Clin. Neuropsychol. 2017, 32, 786–801. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Korczyn, A.D. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 2018, 16, 34. [Google Scholar] [CrossRef]
- Lenka, A.; Pagonabarraga, J.; Pal, P.K.; Bejr-Kasem, H.; Kulisvesky, J. Minor hallucinations in Parkinson disease: A subtle symptom with major clinical implications. Neurology 2019, 93, 259–266. [Google Scholar] [CrossRef]
- Martínez-Horta, S.; Riba, J.; de Bobadilla, R.F.; Pagonabarraga, J.; Pascual-Sedano, B.; Antonijoan, R.M.; Romero, S.; Mañanas, M.À.; García-Sanchez, C.; Kulisevsky, J. Apathy in parkinson’s disease: Neurophysiological evidence of impaired incentive processing. J. Neurosci. 2014, 34, 5918–5926. [Google Scholar] [CrossRef][Green Version]
- Weintraub, D.; Mamikonyan, E. The Neuropsychiatry of Parkinson disease: A perfect storm. Am. J. Geriatr. Psychiatry 2019, 27, 998–1018. [Google Scholar] [CrossRef]
- Aarsland, D.; Ballard, C.; Larsen, J.P.; McKeith, I. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson’s disease with and without dementia. Int. J. Geriatr. Psychiatry 2001, 16, 528–536. [Google Scholar] [CrossRef]
- Marsh, L. Depression and Parkinson’s disease: Current knowledge. Curr. Neurol. Neurosci. Rep. 2013, 13, 409. [Google Scholar] [CrossRef]
- Patterson, L.; Rushton, S.P.; Attems, J.; Thomas, A.J.; Morris, C.M. Degeneration of dopaminergic circuitry influences depressive symptoms in Lewy body disorders. Brain Pathol. 2019, 29, 544–557. [Google Scholar] [CrossRef]
- Ishihara, L.; Brayne, C. A systematic review of depression and mental illness preceding Parkinson’s disease. Acta Neurol. Scand. 2006, 113, 211–220. [Google Scholar] [CrossRef]
- McKeith, I.; Cummings, J. Behavioural changes and psychological symptoms in dementia disorders. Lancet Neurol. 2005, 4, 735–742. [Google Scholar] [CrossRef]
- Kuring, J.K.; Mathias, J.L.; Ward, L. Prevalence of depression, anxiety and PTSD in people with dementia: A systematic review and meta-analysis. Neuropsychol. Rev. 2018, 28, 393–416. [Google Scholar] [CrossRef]
- Fritze, F.; Ehrt, U.; Hortobagyi, T.; Ballard, C.; Aarsland, D. Depressive symptoms in Alzheimer’s disease and Lewy body dementia: A one-year follow-up study. Dement. Geriatr. Cogn. Disord. 2011, 32, 143–149. [Google Scholar] [CrossRef]
- Aarsland, D.; Brønnick, K.; Ehrt, U.; De Deyn, P.P.; Tekin, S.; Emre, M.; Cummings, J.L. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: Frequency, profile and associated care giver stress. J. Neurol. Neurosurg. Psychiatry 2007, 78, 36–42. [Google Scholar] [CrossRef]
- Borroni, B.; Agosti, C.; Padovani, A. Behavioral and psychological symptoms in dementia with Lewy-bodies (DLB): Frequency and relationship with disease severity and motor impairment. Arch. Gerontol. Geriatr. 2008, 46, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Breitve, M.H.; Brønnick, K.; Chwiszczuk, L.J.; Hynninen, M.J.; Aarsland, D.; Rongve, A. Apathy is associated with faster global cognitive decline and early nursing home admission in dementia with Lewy bodies. Alzheimer’s Res. Ther. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Hynninen, M.J.; Breitve, M.H.; Rongve, A.; Aarsland, D.; Nordhus, I.H. The frequency and correlates of anxiety in patients with first-time diagnosed mild dementia. Int. Psychogeriatr. 2012, 24, 1771–1778. [Google Scholar] [CrossRef]
- Ffytche, D.H.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Weintraub, D.; Ballard, C.; Aarsland, D. The psychosis spectrum in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 81–95. [Google Scholar] [CrossRef]
- Fénelon, G.; Mahieux, F.; Huon, R.; Ziégler, M. Hallucinations in Parkinson’s disease. Prevalence, phenomenology and risk factors. Brain 2000, 123, 733–745. [Google Scholar] [CrossRef]
- Gomperts, S.N. Lewy body dementias: Dementia with lewy bodies and Parkinson disease dementia. Continuum 2016, 22, 435–463. [Google Scholar] [CrossRef]
- State, D.; Events, S.L. Clinical features of auditory hallucinations in patients with dementia with lewy bodies: A soundtrack of visual hallucinations. J. Clin. Psychiatry 2014, 2012, 11–12. [Google Scholar]
- Pagonabarraga, J.; Martinez-Horta, S.; Fernández de Bobadilla, R.; Pérez, J.; Ribosa-Nogué, R.; Marín, J.; Pascual-Sedano, B.; García, C.; Gironell, A.; Kulisevsky, J. Minor hallucinations occur in drug-naive Parkinson’s disease patients, even from the premotor phase. Mov. Disord. 2016, 31, 45–52. [Google Scholar] [CrossRef]
- Uchiyama, M.; Nishio, Y.; Yokoi, K.; Hirayama, K.; Imamura, T.; Shimomura, T.; Mori, E. Pareidolias: Complex visual illusions in dementia with Lewy bodies. Brain 2012, 135, 2458–2469. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Nishio, Y.; Yokoi, K.; Hosokai, Y.; Takeda, A.; Mori, E. Pareidolia in Parkinson’s disease without dementia: A positron emission tomography study. Park. Relat. Disord. 2015, 21, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Kövari, E.; Gold, G.; Herrmann, F.R.; Canuto, A.; Hof, P.R.; Bouras, C.; Giannakopoulos, P. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson’s disease. Acta Neuropathol. 2003, 106, 83–88. [Google Scholar] [CrossRef]
- Mattila, P.M.; Rinne, J.O.; Helenius, H.; Dickson, D.W.; Röyttä, M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol. 2000, 100, 285–290. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; De Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- McKeith, I.G. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the Consortium on DLB International Workshop. In Proceedings of the Journal of Alzheimer’s Disease; IOS Press: Amsterdam, The Netherlands, 2006; Volume 9, pp. 417–423. [Google Scholar]
- Irwin, D.J.; White, M.T.; Toledo, J.B.; Xie, S.X.; Robinson, J.L.; Van Deerlin, V.; Lee, V.M.Y.; Leverenz, J.B.; Montine, T.J.; Duda, J.E.; et al. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 2012, 72, 587–598. [Google Scholar] [CrossRef]
- Compta, Y.; Parkkinen, L.; O’Sullivan, S.S.; Vandrovcova, J.; Holton, J.L.; Collins, C.; Lashley, T.; Kallis, C.; Williams, D.R.; De Silva, R.; et al. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: Which is more important? Brain 2011, 134, 1493–1505. [Google Scholar] [CrossRef]
- Hepp, D.H.; Vergoossen, D.L.E.; Huisman, E.; Lemstra, A.W.; Bank, N.B.; Berendse, H.W.; Rozemuller, A.J.; Foncke, E.M.J.; Van De Berg, W.D.J. Distribution and load of amyloid-b pathology in Parkinson disease and dementia with lewy bodies. J. Neuropathol. Exp. Neurol. 2016, 75, 936–945. [Google Scholar] [CrossRef]
- Clinton, L.K.; Blurton-Jones, M.; Myczek, K.; Trojanowski, J.Q.; LaFerla, F.M. Synergistic interactions between Aβ, tau, and α-synuclein: Acceleration of neuropathology and cognitive decline. J. Neurosci. 2010, 30, 7281–7289. [Google Scholar] [CrossRef]
- Walker, L.; McAleese, K.E.; Thomas, A.J.; Johnson, M.; Martin-Ruiz, C.; Parker, C.; Colloby, S.J.; Jellinger, K.; Attems, J. Neuropathologically mixed Alzheimer’s and Lewy body disease: Burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol. 2015, 129, 729–748. [Google Scholar] [CrossRef]
- Masliah, E.; Rockenstein, E.; Veinbergs, I.; Sagara, Y.; Mallory, M.; Hashimoto, M.; Mucke, L. β-Amyloid peptides enhance α-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2001, 98, 12245–12250. [Google Scholar] [CrossRef]
- Giasson, B.I.; Forman, M.S.; Higuchi, M.; Golbe, L.I.; Graves, C.L.; Kotzbauer, P.T.; Trojanowski, J.Q.; Lee, V.M.Y. Initiation and synergistic fibrillization of tau and alpha-synuctein. Science 2003, 300, 636–640. [Google Scholar] [CrossRef]
- Pletnikova, O.; West, N.; Lee, M.K.; Rudow, G.L.; Skolasky, R.L.; Dawson, T.M.; Marsh, L.; Troncoso, J.C. Aβ deposition is associated with enhanced cortical α-synuclein lesions in Lewy body diseases. Neurobiol. Aging 2005, 26, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Lashley, T.; Holton, J.L.; Gray, E.; Kirkham, K.; O’Sullivan, S.S.; Hilbig, A.; Wood, N.W.; Lees, A.J.; Revesz, T. Cortical α-synuclein load is associated with amyloid-β plaque burden in a subset of Parkinson’s disease patients. Acta Neuropathol. 2008, 115, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.; Gelpi, E.; Martí, M.J.; Compta, Y. Lewy- and Alzheimer-type pathologies in midbrain and cerebellum across the Lewy body disorders spectrum. Neuropathol. Appl. Neurobiol. 2016, 42, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, M.E.; Graeber, M.B.; Gentleman, S.M.; Pearce, R.K.B. Striatal β-amyloid deposition in Parkinson disease with dementia. J. Neuropathol. Exp. Neurol. 2008, 67, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Reyes, S.; Landeck, N.; Bye, C.; Leanza, G.; Double, K.; Thompson, L.; Halliday, G.; Kirik, D. Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain 2014, 137, 2493–2508. [Google Scholar] [CrossRef] [PubMed]
- Ruffmann, C.; Calboli, F.C.F.; Bravi, I.; Gveric, D.; Curry, L.K.; de Smith, A.; Pavlou, S.; Buxton, J.L.; Blakemore, A.I.F.; Takousis, P.; et al. Cortical Lewy bodies and Aβ burden are associated with prevalence and timing of dementia in Lewy body diseases. Neuropathol. Appl. Neurobiol. 2016, 42, 436–450. [Google Scholar] [CrossRef]
- Schneider, J.A.; Arvanitakis, Z.; Yu, L.; Boyle, P.A.; Leurgans, S.E.; Bennett, D.A. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain 2012, 135, 3005–3014. [Google Scholar] [CrossRef]
- Deramecourt, V.; Bombois, S.; Maurage, C.A.; Ghestem, A.; Drobecq, H.; Vanmechelen, E.; Lebert, F.; Pasquier, F.; Delacourte, A. Biochemical staging of synucleinopathy and amyloid deposition in dementia with Lewy bodies. J. Neuropathol. Exp. Neurol. 2006, 65, 278–288. [Google Scholar] [CrossRef]
- Petrou, M.; Dwamena, B.A.; Foerster, B.R.; Maceachern, M.P.; Bohnen, N.I.; Müller, M.L.; Albin, R.L.; Frey, K.A. Amyloid deposition in Parkinson’s disease and cognitive impairment: A systematic review. Mov. Disord. 2015, 30, 928–935. [Google Scholar] [CrossRef]
- Fiorenzato, E.; Biundo, R.; Cecchin, D.; Frigo, A.C.; Kim, J.; Weis, L.; Strafella, A.P.; Antonini, A. Brain amyloid contribution to cognitive dysfunction in early-stage Parkinson’s disease: The PPMI dataset. J. Alzheimer’s Dis. 2018, 66, 229–237. [Google Scholar] [CrossRef]
- Colom-Cadena, M.; Grau-Rivera, O.; Planellas, L.; Cerquera, C.; Morenas, E.; Helgueta, S.; Munoz, L.; Kulisevsky, J.; Marti, M.J.; Tolosa, E.; et al. Regional overlap of pathologies in lewy body disorders. J. Neuropathol. Exp. Neurol. 2017, 76, 216–224. [Google Scholar] [CrossRef]
- Kapasi, A.; DeCarli, C.; Schneider, J.A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017, 134, 171–186. [Google Scholar] [CrossRef]
- Smith, C.; Malek, N.; Grosset, K.; Cullen, B.; Gentleman, S.; Grosset, D.G. Neuropathology of dementia in patients with Parkinson’s disease: A systematic review of autopsy studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1234–1243. [Google Scholar] [CrossRef]
- Halliday, G.M.; Song, Y.J.C.; Harding, A.J. Striatal β-amyloid in dementia with Lewy bodies but not Parkinson’s disease. J. Neural Transm. 2011, 118, 713–719. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Attems, J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol. 2006, 112, 253–260. [Google Scholar] [CrossRef]
- Shah, N.; Frey, K.A.; Müller, M.L.T.M.; Petrou, M.; Kotagal, V.; Koeppe, R.A.; Scott, P.J.H.; Albin, R.L.; Bohnen, N.I. Striatal and cortical β-amyloidopathy and cognition in Parkinson’s disease. Mov. Disord. 2016, 31, 111–117. [Google Scholar] [CrossRef]
- Kalaitzakis, M.E.; Pearce, R.K.B.; Gentleman, S.M. Clinical correlates of pathology in the claustrum in Parkinson’s disease and dementia with Lewy bodies. Neurosci. Lett. 2009, 461, 12–15. [Google Scholar] [CrossRef]
- Walker, L.; Stefanis, L.; Attems, J. Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies—Current issues and future directions. J. Neurochem. 2019, 150, 467–474. [Google Scholar] [CrossRef]
- Horvath, J.; Herrmann, F.R.; Burkhard, P.R.; Bouras, C.; Kövari, E. Neuropathology of dementia in a large cohort of patients with Parkinson’s disease. Park. Relat. Disord. 2013, 19, 864–868. [Google Scholar] [CrossRef]
- Coughlin, D.G.; Hurtig, H.I.; Irwin, D.J. Pathological influences on clinical heterogeneity in Lewy body diseases. Mov. Disord. 2020, 35, 5–19. [Google Scholar] [CrossRef]
- Buchman, A.S.; Yu, L.; Wilson, R.S.; Leurgans, S.E.; Nag, S.; Shulman, J.M.; Barnes, L.L.; Schneider, J.A.; Bennett, D.A. Progressive parkinsonism in older adults is related to the burden of mixed brain pathologies. Neurology 2019, 92, E1821–E1830. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.K.; Larsen, J.P.; Aarsland, D. Gray matter atrophy in Parkinson disease with dementia and dementia with Lewy bodies. Neurology 2007, 69, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.J.; McKeith, I.G.; Burn, D.J.; Williams, E.D.; O’Brien, J.T. Cerebral atrophy in Parkinson’s disease with and without dementia: A comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 2004, 127. [Google Scholar] [CrossRef] [PubMed]
- Borroni, B.; Premi, E.; Formenti, A.; Turrone, R.; Alberici, A.; Cottini, E.; Rizzetti, C.; Gasparotti, R.; Padovani, A. Structural and functional imaging study in dementia with Lewy bodies and Parkinson’s disease dementia. Park. Relat. Disord. 2015, 21, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Stebbins, G.T.; Bernard, B.; Stoub, T.R.; Goetz, C.G.; deToledo-Morrell, L. Entorhinal cortex atrophy differentiates Parkinson’s disease patients with and without dementia. Mov. Disord. 2012, 27, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Weil, R.S.; Hsu, J.K.; Darby, R.R.; Soussand, L.; Fox, M.D. Neuroimaging in Parkinson’s disease dementia: Connecting the dots. Brain Commun. 2019, 1, fcz006. [Google Scholar] [CrossRef]
- Compta, Y.; Pereira, J.B.; Ríos, J.; Ibarretxe-Bilbao, N.; Junqué, C.; Bargalló, N.; Cámara, A.; Buongiorno, M.; Fernández, M.; Pont-Sunyer, C.; et al. Combined dementia-risk biomarkers in Parkinson’s disease: A prospective longitudinal study. Park. Relat. Disord. 2013, 19, 717–724. [Google Scholar] [CrossRef]
- Lee, J.E.; Cho, K.H.; Song, S.K.; Kim, H.J.; Lee, H.S.; Sohn, Y.H.; Lee, P.H. Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2014, 85, 7–16. [Google Scholar] [CrossRef]
- Zheng, D.; Chen, C.; Song, W.C.; Yi, Z.Q.; Zhao, P.W.; Zhong, J.G.; Dai, Z.Y.; Shi, H.C.; Pan, P.L. Regional gray matter reductions associated with mild cognitive impairment in Parkinson’s disease: A meta-analysis of voxel-based morphometry studies. Behav. Brain Res. 2019, 371, 111973. [Google Scholar] [CrossRef]
- Kandiah, N.; Zainal, N.H.; Narasimhalu, K.; Chander, R.J.; Ng, A.; Mak, E.; Au, W.L.; Sitoh, Y.Y.; Nadkarni, N.; Tan, L.C.S. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson’s disease. Park. Relat. Disord. 2014, 20, 1203–1208. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, B.; Song, S.K.; Sohn, Y.H.; Park, H.J.; Lee, P.H. A comparison of gray and white matter density in patients with Parkinson’s disease dementia and dementia with Lewy bodies using voxel-based morphometry. Mov. Disord. 2010, 25, 28–34. [Google Scholar] [CrossRef]
- Yousaf, T.; Dervenoulas, G.; Valkimadi, P.E.; Politis, M. Neuroimaging in Lewy body dementia. J. Neurol. 2019, 266, 1. [Google Scholar] [CrossRef]
- Burton, E.J.; McKeith, I.G.; Burn, D.J.; Firbank, M.J.; O’Brien, J.T. Progression of white matter hyperintensities in Alzheimer disease, dementia with Lewy bodies, and Parkinson disease dementia: A comparison with normal aging. Am. J. Geriatr. Psychiatry 2006, 14, 842–849. [Google Scholar] [CrossRef]
- Hayashi, H.; Kawakatsu, S.; Suzuki, A.; Shibuya, Y.; Kobayashi, R.; Sato, C.; Otani, K. Application of the VSRAD, a specific and sensitive voxel-based morphometry, to comparison of Entorhinal cortex atrophy between dementia with Lewy bodies and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2013, 34, 328–331. [Google Scholar] [CrossRef]
- Watson, R.; Colloby, S.J. Imaging in dementia with Lewy bodies: An overview. J. Geriatr. Psychiatry Neurol. 2016, 29, 254–260. [Google Scholar] [CrossRef]
- Blanc, F.; Colloby, S.J.; Cretin, B.; De Sousa, P.L.; Demuynck, C.; O’Brien, J.T.; Martin-Hunyadi, C.; McKeith, I.; Philippi, N.; Taylor, J.P. Grey matter atrophy in prodromal stage of dementia with Lewy bodies and Alzheimer’s disease. Alzheimer’s Res. Ther. 2016, 8, 31. [Google Scholar] [CrossRef]
- Kantarci, K.; Lesnick, T.; Ferman, T.J.; Przybelski, S.A.; Boeve, B.F.; Smith, G.E.; Kremers, W.K.; Knopman, D.S.; Jack, C.R.; Petersen, R.C. Hippocampal volumes predict risk of dementia with Lewy bodies in mild cognitive impairment. Neurology 2016, 87, 2317–2323. [Google Scholar] [CrossRef]
- Delgado-Alvarado, M.; Gago, B.; Navalpotro-Gomez, I.; Jiménez-Urbieta, H.; Rodriguez-Oroz, M.C. Biomarkers for dementia and mild cognitive impairment in Parkinson’s disease. Mov. Disord. 2016, 31, 861–881. [Google Scholar] [CrossRef]
- Melzer, T.R.; Watts, R.; Macaskill, M.R.; Pitcher, T.L.; Livingston, L.; Keenan, R.J.; Dalrymple-Alford, J.C.; Anderson, T.J. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology 2013, 80, 1841–1849. [Google Scholar] [CrossRef]
- Kamagata, K.; Motoi, Y.; Tomiyama, H.; Abe, O.; Ito, K.; Shimoji, K.; Suzuki, M.; Hori, M.; Nakanishi, A.; Sano, T.; et al. Relationship between cognitive impairment and white-matter alteration in Parkinson’s disease with dementia: Tract-based spatial statistics and tract-specific analysis. Eur. Radiol. 2013, 23, 1946–1955. [Google Scholar] [CrossRef]
- Chen, B.; Fan, G.G.; Liu, H.; Wang, S. Changes in anatomical and functional connectivity of Parkinson’s disease patients according to cognitive status. Eur. J. Radiol. 2015, 84, 1318–1324. [Google Scholar] [CrossRef]
- Hattori, T.; Orimo, S.; Aoki, S.; Ito, K.; Abe, O.; Amano, A.; Sato, R.; Sakai, K.; Mizusawa, H. Cognitive status correlates with white matter alteration in Parkinson’s disease. Hum. Brain Mapp. 2012, 33, 727–739. [Google Scholar] [CrossRef]
- Agosta, F.; Canu, E.; Stefanova, E.; Sarro, L.; Tomić, A.; Špica, V.; Comi, G.; Kostić, V.S.; Filippi, M. Mild cognitive impairment in Parkinson’s disease is associated with a distributed pattern of brain white matter damage. Hum. Brain Mapp. 2014, 35, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, I.O.; Stebbins, G.T.; Merkitch, D.; Goldman, J.G. White matter abnormalities in the corpus callosum with cognitive impairment in Parkinson disease. Neurology 2018, 91, E2244–E2255. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.; Blamire, A.M.; Colloby, S.J.; Wood, J.S.; Barber, R.; He, J.; O’Brien, J.T. Characterizing dementia with Lewy bodies by means of diffusion tensor imaging. Neurology 2012, 79, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Cheung, C.; Pang, S.; Shek-kwan Chang, R.; Lau, K.K.; Suckling, J.; Yu, K.; Ka-Fung Mak, H.; Chua, S.E.; Ho, S.L.; et al. Multimodal MRI of the hippocampus in Parkinson’s disease with visual hallucinations. Brain Struct. Funct. 2016, 221, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Delli Pizzi, S.; Maruotti, V.; Taylor, J.P.; Franciotti, R.; Caulo, M.; Tartaro, A.; Thomas, A.; Onofrj, M.; Bonanni, L. Relevance of subcortical visual pathways disruption to visual symptoms in dementia with Lewy bodies. Cortex 2014, 59, 12–21. [Google Scholar] [CrossRef]
- Kantarci, K.; Avula, R.; Senjem, M.L.; Samikoglu, A.R.; Zhang, B.; Weigand, S.D.; Przybelski, S.A.; Edmonson, H.A.; Vemuri, P.; Knopman, D.S.; et al. Dementia with Lewy bodies and Alzheimer disease: Neurodegenerative patterns characterized by DTI. Neurology 2010, 74, 1814–1821. [Google Scholar] [CrossRef]
- Balážová, Z.; Nováková, M.; Minsterová, A.; Rektorová, I. Structural and functional magnetic resonance imaging of dementia with Lewy bodies. Int. Rev. Neurobiol. 2019, 144, 95–141. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, H.J.; Park, B.S.; Song, S.K.; Sohn, Y.H.; Lee, J.D.; Lee, P.H. A comparative analysis of cognitive profiles and white-matter alterations using voxel-based diffusion tensor imaging between patients with Parkinson’s disease dementia and dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry 2010, 81, 320–326. [Google Scholar] [CrossRef]
- Pietracupa, S.; Martin-Bastida, A.; Piccini, P. Iron metabolism and its detection through MRI in parkinsonian disorders: A systematic review. Neurol. Sci. 2017, 38, 2095–2101. [Google Scholar] [CrossRef]
- Barber, T.R.; Griffanti, L.; Bradley, K.M.; McGowan, D.R.; Lo, C.; Mackay, C.E.; Hu, M.T.; Klein, J.C. Nigrosome 1 imaging in REM sleep behavior disorder and its association with dopaminergic decline. Ann. Clin. Transl. Neurol. 2020, 7, 26–35. [Google Scholar] [CrossRef]
- Schwarz, S.T.; Afzal, M.; Morgan, P.S.; Bajaj, N.; Gowland, P.A.; Auer, D.P. The “swallow tail” appearance of the healthy nigrosome—A new accurate test of Parkinson’s disease: A case-control and retrospective cross-sectional MRI study at 3T. PLoS ONE 2014, 9, e93814. [Google Scholar] [CrossRef]
- Rizzo, G.; De Blasi, R.; Capozzo, R.; Tortelli, R.; Barulli, M.R.; Liguori, R.; Grasso, D.; Logroscino, G. Loss of swallow tail sign on susceptibility-weighted imaging in dementia with Lewy bodies. J. Alzheimer’s Dis. 2019, 67, 61–65. [Google Scholar] [CrossRef]
- Oustwani, C.S.; Korutz, A.W.; Lester, M.S.; Kianirad, Y.; Simuni, T.; Hijaz, T.A. Can loss of the swallow tail sign help distinguish between Parkinson Disease and the Parkinson-Plus syndromes? Clin. Imaging 2017, 44, 66–69. [Google Scholar] [CrossRef]
- Murakami, Y.; Kakeda, S.; Watanabe, K.; Ueda, I.; Ogasawara, A.; Moriya, J.; Ide, S.; Futatsuya, K.; Sato, T.; Okada, K.; et al. Usefulness of quantitative susceptibility mapping for the diagnosis of Parkinson disease. Am. J. Neuroradiol. 2015, 36, 1102–1108. [Google Scholar] [CrossRef]
- Langkammer, C.; Pirpamer, L.; Seiler, S.; Deistung, A.; Schweser, F.; Franthal, S.; Homayoon, N.; Katschnig-Winter, P.; Koegl-Wallner, M.; Pendl, T.; et al. Quantitative susceptibility mapping in Parkinson’s disease. PLoS ONE 2016, 11, e162460. [Google Scholar] [CrossRef]
- Martin-Bastida, A.; Lao-Kaim, N.P.; Loane, C.; Politis, M.; Roussakis, A.A.; Valle-Guzman, N.; Kefalopoulou, Z.; Paul-Visse, G.; Widner, H.; Xing, Y.; et al. Motor associations of iron accumulation in deep grey matter nuclei in Parkinson’s disease: A cross-sectional study of iron-related magnetic resonance imaging susceptibility. Eur. J. Neurol. 2017, 24, 357–365. [Google Scholar] [CrossRef]
- He, N.; Ling, H.; Ding, B.; Huang, J.; Zhang, Y.; Zhang, Z.; Liu, C.; Chen, K.; Yan, F. Region-specific disturbed iron distribution in early idiopathic Parkinson’s disease measured by quantitative susceptibility mapping. Hum. Brain Mapp. 2015, 36, 4407–4420. [Google Scholar] [CrossRef]
- Guan, X.; Xuan, M.; Gu, Q.; Huang, P.; Liu, C.; Wang, N.; Xu, X.; Luo, W.; Zhang, M. Regionally progressive accumulation of iron in Parkinson’s disease as measured by quantitative susceptibility mapping. NMR Biomed. 2017, 30, 1–17. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Zhang, Y.; Wang, F.; Yu, H.; Zhang, C.; Jiang, Z.; Luo, W. Iron deposition in Parkinson’s disease by quantitative susceptibility mapping. BMC Neurosci. 2019, 20, 23. [Google Scholar] [CrossRef]
- Thomas, G.E.C.; Leyland, L.A.; Schrag, A.E.; Lees, A.J.; Acosta-Cabronero, J.; Weil, R.S. Brain iron deposition is linked with cognitive severity in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 418–425. [Google Scholar] [CrossRef]
- Cogswell, P.M.; Wiste, H.J.; Senjem, M.L.; Gunter, J.L.; Weigand, S.D.; Schwarz, C.G.; Arani, A.; Therneau, T.M.; Lowe, V.J.; Knopman, D.S.; et al. Associations of quantitative susceptibility mapping with Alzheimer’s disease clinical and imaging markers. Neuroimage 2021, 224, 117433. [Google Scholar] [CrossRef]
- Ravanfar, P.; Loi, S.M.; Syeda, W.T.; Van Rheenen, T.E.; Bush, A.I.; Desmond, P.; Cropley, V.L.; Lane, D.J.R.; Opazo, C.M.; Moffat, B.A.; et al. Systematic Review: Quantitative Susceptibility Mapping (QSM) of Brain Iron Profile in Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 618435. [Google Scholar] [CrossRef] [PubMed]
- Baggio, H.C.; Segura, B.; Sala-Llonch, R.; Marti, M.J.; Valldeoriola, F.; Compta, Y.; Tolosa, E.; Junqué, C. Cognitive impairment and resting-state network connectivity in Parkinson’s disease. Hum. Brain Mapp. 2015, 36, 199–212. [Google Scholar] [CrossRef]
- Bezdicek, O.; Ballarini, T.; Růžička, F.; Roth, J.; Mueller, K.; Jech, R.; Schroeter, M.L. Mild cognitive impairment disrupts attention network connectivity in Parkinson’s disease: A combined multimodal MRI and meta-analytical study. Neuropsychologia 2018, 112, 105–115. [Google Scholar] [CrossRef]
- Schumacher, J.; Peraza, L.R.; Firbank, M.; Thomas, A.J.; Kaiser, M.; Gallagher, P.; O’Brien, J.T.; Blamire, A.M.; Taylor, J.P. Functional connectivity in dementia with Lewy bodies: A within- and between-network analysis. Hum. Brain Mapp. 2018, 39, 1118–1129. [Google Scholar] [CrossRef]
- Peraza, L.R.; Kaiser, M.; Firbank, M.; Graziadio, S.; Bonanni, L.; Onofrj, M.; Colloby, S.J.; Blamire, A.; O’Brien, J.; Taylor, J.P. FMRI resting state networks and their association with cognitive fluctuations in dementia with Lewy bodies. NeuroImage Clin. 2014, 4, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, A.; Gomperts, S.N.; Johnson, K.A.; Growdon, J.H.; Van Dijk, K.R.A. Putamen-midbrain functional connectivity is related to striatal dopamine transporter availability in patients with Lewy body diseases. NeuroImage Clin. 2015, 8, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Peraza, L.R.; Colloby, S.J.; Deboys, L.; O’Brien, J.T.; Kaiser, M.; Taylor, J.P. Regional functional synchronizations in dementia with Lewy bodies and Alzheimer’s disease. Int. Psychogeriatr. 2016, 28, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Franciotti, R.; Falasca, N.W.; Bonanni, L.; Anzellotti, F.; Maruotti, V.; Comani, S.; Thomas, A.; Tartaro, A.; Taylor, J.P.; Onofrj, M. Default network is not hypoactive in dementia with fluctuating cognition: An Alzheimer disease/dementia with Lewy bodies comparison. Neurobiol. Aging 2013, 34, 1148–1158. [Google Scholar] [CrossRef]
- Peraza, L.R.; Colloby, S.J.; Firbank, M.J.; Greasy, G.S.; McKeith, I.G.; Kaiser, M.; O’Brien, J.; Taylor, J.P. Resting state in Parkinson’s disease dementia and dementia with Lewy bodies: Commonalities and differences. Int. J. Geriatr. Psychiatry 2015, 30, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.S.; Okamura, N.; Arai, H.; Higuchi, M.; Matsui, T.; Tashiro, M.; Shinkawa, M.; Itoh, M.; Ido, T.; Sasaki, H. 18F-fluorodopa PET study of striatal dopamine uptake in the diagnosis of dementia with Lewy bodies. Neurology 2000, 55, 1575–1576. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.; O’Brien, J.; Walker, Z.; Tatsch, K.; Booij, J.; Darcourt, J.; Padovani, A.; Giubbini, R.; Bonuccelli, U.; Volterrani, D.; et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: A phase III, multicentre study. Lancet Neurol. 2007, 6, 305–313. [Google Scholar] [CrossRef]
- Thomas, A.J.; Attems, J.; Colloby, S.J.; O’Brien, J.T.; Mckeith, I.; Walker, R.; Lee, L.; Burn, D.; Lett, D.J.; Walker, Z. Autopsy validation of 123 I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology 2017, 88, 276–283. [Google Scholar] [CrossRef]
- Rossi, C.; Volterrani, D.; Nicoletti, V.; Manca, G.; Frosini, D.; Kiferle, L.; Unti, E.; De Feo, P.; Bonuccelli, U.; Ceravolo, R. “Parkinson-dementia” diseases: A comparison by double tracer SPECT studies. Park. Relat. Disord. 2009, 15, 762–766. [Google Scholar] [CrossRef]
- Yonga, S.W.; Yoonb, J.K.; Leea, Y.S.A.; Lee, P.H. A comparison of cerebral glucose metabolism in Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies. Eur. J. Neurol. 2007, 14, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Firbank, M.J.; Davison, C.; Barnett, N.; Bamford, C.; Donaldson, C.; Olsen, K.; Herholz, K.; Williams, D.; Lloyd, J. 18F-FDG PET and perfusion SPECT in the diagnosis of Alzheimer and Lewy body dementias. J. Nucl. Med. 2014, 55, 1959–1965. [Google Scholar] [CrossRef]
- González-Redondo, R.; García-García, D.; Clavero, P.; Gasca-Salas, C.; García-Eulate, R.; Zubieta, J.L.; Arbizu, J.; Obeso, J.A.; Rodríguez-Oroz, M.C. Grey matter hypometabolism and atrophy in Parkinson’s disease with cognitive impairment: A two-step process. Brain 2014, 137, 2356–2367. [Google Scholar] [CrossRef]
- Higuchi, M.; Tashiro, M.; Arai, H.; Okamura, N.; Hara, S.; Higuchi, S.; Itoh, M.; Shin, R.W.; Trojanowski, J.Q.; Sasaki, H. Glucose hypometabolism and neuropathological correlates in brains of dementia with Lewy bodies. Exp. Neurol. 2000, 162, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Tsui, W.H.; Herholz, K.; Pupi, A.; Drzezga, A.; Lucignani, G.; Reiman, E.M.; Holthoff, V.; Kalbe, E.; Sorbi, S.; et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J. Nucl. Med. 2008, 49, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, H.; Baba, T.; Nishio, Y.; Iizuka, O.; Matsuda, M.; Inoue, K.; Kikuchi, A.; Hasegawa, T.; Aoki, M.; Takeda, A.; et al. On the utility of MIBG SPECT/CT in evaluating cardiac sympathetic dysfunction in lewy body diseases. PLoS ONE 2016, 11, e152746. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kurita, A.; Hashimoto, M.; Fukumitsu, N.; Abo, M.; Ito, Y.; Urashima, M.; Inoue, K. Impaired myocardial 123I-metaiodobenzylguanidine uptake in Lewy body disease: Comparison between dementia with Lewy bodies and Parkinson’s disease. J. Neurol. Sci. 2006, 240, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, G.; Morello, R.; Lebasnier, A.; Agostini, D.; Defer, G.L. Accuracy and cutoff values of delayed heart to mediastinum ratio with 123I-metaiodobenzylguanidine cardiac scintigraphy for Lewy body disease diagnoses. BMC Neurol. 2015, 15, 83. [Google Scholar] [CrossRef]
- Watanabe, H.; Ieda, T.; Katayama, T.; Takeda, A.; Aiba, I.; Doyu, M.; Hirayama, M.; Sobue, G. Cardiac 123I-meta-iodobenzylguanidine (MIBG) uptake in dementia with lewy bodies: Comparison with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2001, 70, 781–783. [Google Scholar] [CrossRef]
- Orimo, S.; Yogo, M.; Nakamura, T.; Suzuki, M.; Watanabe, H. 123I-meta-iodobenzylguanidine (MIBG) cardiac scintigraphy in α-synucleinopathies. Ageing Res. Rev. 2016, 30, 122–133. [Google Scholar] [CrossRef]
- Chung, E.J.; Kim, S.J. (123)I-Metaiodobenzylguanidine Myocardial Scintigraphy in Lewy Body-Related Disorders: A Literature Review. J. Mov. Disord. 2015, 8, 55–66. [Google Scholar] [CrossRef]
- Armstrong, M.J. Lewy body dementias. Contin. Lifelong Learn. Neurol. 2019, 25, 128–146. [Google Scholar] [CrossRef]
- Donaghy, P.; Thomas, A.J.; O’Brien, J.T. Amyloid PET imaging in lewy body disorders. Am. J. Geriatr. Psychiatry 2015, 23, 23–37. [Google Scholar] [CrossRef]
- Gomperts, S.N. Imaging the role of amyloid in PD dementia and dementia with Lewy bodies. Curr. Neurol. Neurosci. Rep. 2014, 14, 472. [Google Scholar] [CrossRef]
- Gomperts, S.N.; Marquie, M.; Locascio, J.J.; Bayer, S.; Johnson, K.A.; Growdon, J.H. PET radioligands reveal the basis of dementia in Parkinson’s disease and dementia with lewy bodies. Neurodegener. Dis. 2016, 16, 118–124. [Google Scholar] [CrossRef]
- Gomperts, S.N.; Locascio, J.J.; Marquie, M.; Santarlasci, A.L.; Rentz, D.M.; Maye, J.; Johnson, K.A.; Growdon, J.H. Brain amyloid and cognition in Lewy body diseases. Mov. Disord. 2012, 27, 965–973. [Google Scholar] [CrossRef]
- Foster, E.R.; Campbell, M.C.; Burack, M.A.; Hartlein, J.; Flores, H.P.; Cairns, N.J.; Hershey, T.; Perlmutter, J.S. Amyloid imaging of Lewy body-associated disorders. Mov. Disord. 2010, 25, 2516–2523. [Google Scholar] [CrossRef]
- Shirvan, J.; Clement, N.; Ye, R.; Katz, S.; Schultz, A.; Johnson, K.A.; Gomez-Isla, T.; Frosch, M.; Growdon, J.H.; Gomperts, S.N. Neuropathologic correlates of amyloid and dopamine transporter imaging in Lewy body disease. Neurology 2019, 93, E476–E484. [Google Scholar] [CrossRef]
- Chen, Q.; Lowe, V.J.; Boeve, B.F.; Przybelski, S.A.; Miyagawa, T.; Senjem, M.L.; Jack, C.R.; Lesnick, T.G.; Kremers, W.K.; Fields, J.A.; et al. β-amyloid PET and 123 I-FP-CIT SPECT in mild cognitive impairment at risk for Lewy body dementia. Neurology 2021, 96, e1180–e1189. [Google Scholar] [CrossRef]
- Nedelska, Z.; Schwarz, C.G.; Lesnick, T.G.; Boeve, B.F.; Przybelski, S.A.; Lowe, V.J.; Kremers, W.K.; Gunter, J.L.; Senjem, M.L.; Graff-Radford, J.; et al. Association of longitudinal β-amyloid accumulation determined by positron emission tomography with clinical and cognitive decline in adults with probable Lewy body dementia. JAMA Netw. Open 2019, 2, e1916439. [Google Scholar] [CrossRef]
- Irwin, D.J.; Grossman, M.; Weintraub, D.; Hurtig, H.I.; Duda, J.E.; Xie, S.X.; Lee, E.B.; Van Deerlin, V.M.; Lopez, O.L.; Kofler, J.K.; et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: A retrospective analysis. Lancet Neurol. 2017, 16, 55. [Google Scholar] [CrossRef]
- Gomperts, S.N.; Locascio, J.J.; Makaretz, S.J.; Schultz, A.; Caso, C.; Vasdev, N.; Sperling, R.; Growdon, J.H.; Dickerson, B.C.; Johnson, K. Tau positron emission tomographic imaging in the lewy body diseases. JAMA Neurol. 2016, 73, 1334–1341. [Google Scholar] [CrossRef]
- Hall, B.; Mak, E.; Cervenka, S.; Aigbirhio, F.I.; Rowe, J.B.; O’Brien, J.T. In vivo tau PET imaging in dementia: Pathophysiology, radiotracer quantification, and a systematic review of clinical findings. Ageing Res. Rev. 2017, 36, 50–63. [Google Scholar] [CrossRef]
- Whitwell, J.L. Tau imaging in Parkinsonism: What have we learned so far? Mov. Disord. Clin. Pract. 2018, 5, 118–130. [Google Scholar] [CrossRef]
- Kantarci, K.; Lowe, V.J.; Boeve, B.F.; Senjem, M.L.; Tosakulwong, N.; Lesnick, T.G.; Spychalla, A.J.; Gunter, J.L.; Fields, J.A.; Graff-Radford, J.; et al. AV-1451 tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann. Neurol. 2017, 81, 58–67. [Google Scholar] [CrossRef]
- Kotzbauer, P.T.; Tu, Z.; Mach, R.H. Current status of the development of PET radiotracers for imaging alpha synuclein aggregates in Lewy bodies and Lewy neurites. Clin. Transl. Imaging 2017, 5, 3–14. [Google Scholar] [CrossRef]
- Verdurand, M.; Levigoureux, E.; Zeinyeh, W.; Berthier, L.; Mendjel-Herda, M.; Cadarossanesaib, F.; Bouillot, C.; Iecker, T.; Terreux, R.; Lancelot, S.; et al. In silico, in vitro, and in vivo evaluation of new candidates for α-synuclein PET imaging. Mol. Pharm. 2018, 15, 3153–3166. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, H.; Padakanti, P.; Li, J.; Yang, H.; Fan, J.; Mach, R.; Kotzbauer, P.; Tu, Z. Radiosynthesis and in vivo evaluation of two PET radioligands for imaging α-synuclein. Appl. Sci. 2014, 4, 66–78. [Google Scholar] [CrossRef]
- Maurer, A.; Leonov, A.; Ryazanov, S.; Herfert, K.; Kuebler, L.; Buss, S.; Schmidt, F.; Weckbecker, D.; Linder, R.; Bender, D.; et al. 11C Radiolabeling of anle253b: A putative PET tracer for Parkinson’s disease that binds to α-synuclein fibrils in vitro and crosses the blood-brain barrier. ChemMedChem 2020, 15, 411–415. [Google Scholar] [CrossRef]
- Surendranathan, A.; Su, L.; Mak, E.; Passamonti, L.; Hong, Y.T.; Arnold, R.; Rodríguez, P.V.; Bevan-Jones, W.R.; Brain, S.A.E.; Fryer, T.D.; et al. Early microglial activation and peripheral inflammation in dementia with Lewy bodies. Brain 2018, 141, 3415–3427. [Google Scholar] [CrossRef]
- Nicastro, N.; Surendranathan, A.; Mak, E.; Rowe, J.B.; O’Brien, J.T. 11C-PK11195 PET imaging and white matter changes in Parkinson’s disease dementia. Ann. Clin. Transl. Neurol. 2019, 6, 2133–2136. [Google Scholar] [CrossRef]
- Iannaccone, S.; Cerami, C.; Alessio, M.; Garibotto, V.; Panzacchi, A.; Olivieri, S.; Gelsomino, G.; Moresco, R.M.; Perani, D. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Park. Relat. Disord. 2013, 19, 47–52. [Google Scholar] [CrossRef]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Es, M.; Hammers, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.L.; Willemsen, A.T.M.; Doorduin, J.; de Vries, E.F.J.; Dierckx, R.A.; Leenders, K.L. [11C]-PK11195 PET: Quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson’s disease? Park. Relat. Disord. 2010, 16, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Aman, Y.; Ahmed, I.; Chetelat, G.; Landeau, B.; Ray Chaudhuri, K.; Brooks, D.J.; Edison, P. Influence of microglial activation on neuronal function in Alzheimer’s and Parkinson’s disease dementia. Alzheimer’s Dement. 2015, 11, 608–621.e7. [Google Scholar] [CrossRef]
- Edison, P.; Ahmed, I.; Fan, Z.; Hinz, R.; Gelosa, G.; Ray Chaudhuri, K.; Walker, Z.; Turkheimer, F.E.; Brooks, D.J. Microglia, amyloid, and glucose metabolism in parkinson’s disease with and without dementia. Neuropsychopharmacology 2013, 38, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chinchilla, T.; Quiroga-Varela, A.; Molinet-Dronda, F.; Belloso-Iguerategui, A.; Merino-Galan, L.; Jimenez-Urbieta, H.; Gago, B.; Rodriguez-Oroz, M.C. [18F]-DPA-714 PET as a specific in vivo marker of early microglial activation in a rat model of progressive dopaminergic degeneration. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, J.L.; Ayton, S.; Batrla, R.; Bednar, M.M.; Bittner, T.; Cummings, J.; Fagan, A.M.; Hampel, H.; Mielke, M.M.; Mikulskis, A.; et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 2018, 136, 821. [Google Scholar] [CrossRef] [PubMed]
- Parnetti, L.; Paciotti, S.; Farotti, L.; Bellomo, G.; Sepe, F.N.; Eusebi, P. Parkinson’s and Lewy body dementia CSF biomarkers. Clin. Chim. Acta 2019, 495, 318–325. [Google Scholar] [CrossRef]
- Siderowf, A.; Xie, S.X.; Hurtig, H.; Weintraub, D.; Duda, J.; Chen-Plotkin, A.; Shaw, L.M.; Van Deerlin, V.; Trojanowski, J.Q.; Clark, C. CSF amyloid β 1-42 predicts cognitive decline in Parkinson disease. Neurology 2010, 75, 1055–1061. [Google Scholar] [CrossRef]
- Liu, C.; Cholerton, B.; Shi, M.; Ginghina, C.; Cain, K.C.; Auinger, P.; Zhang, J. CSF tau and tau/Aβ42 predict cognitive decline in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 271–276. [Google Scholar] [CrossRef]
- Ferreira, D.; Przybelski, S.A.; Lesnick, T.G.; Lemstra, A.W.; Londos, E.; Blanc, F.; Nedelska, Z.; Schwarz, C.G.; Graff-Radford, J.; Senjem, M.L.; et al. β-Amyloid and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology 2020, 95, e3257–e3268. [Google Scholar] [CrossRef]
- Van Steenoven, I.; Aarsland, D.; Weintraub, D.; Londos, E.; Blanc, F.; Van Der Flier, W.M.; Teunissen, C.E.; Mollenhauer, B.; Fladby, T.; Kramberger, M.G.; et al. Cerebrospinal fluid Alzheimer’s disease biomarkers across the spectrum of lewy body diseases: Results from a large multicenter cohort. J. Alzheimer’s Dis. 2016, 54, 287–295. [Google Scholar] [CrossRef]
- Van Steenoven, I.; Van Der Flier, W.M.; Scheltens, P.; Teunissen, C.E.; Lemstra, A.W. Amyloid-β peptides in cerebrospinal fluid of patients with dementia with Lewy bodies. Alzheimer’s Res. Ther. 2019, 11, 8–10. [Google Scholar] [CrossRef]
- Bibl, M.; Mollenhauer, B.; Esselmann, H.; Lewczuk, P.; Klafki, H.W.; Sparbier, K.; Smirnov, A.; Cepek, L.; Trenkwalder, C.; Rüther, E.; et al. CSF amyloid-β-peptides in Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease dementia. Brain 2006, 129, 1177–1187. [Google Scholar] [CrossRef]
- Hall, S.; Surova, Y.; Öhrfelt, A.; Blennow, K.; Zetterberg, H.; Hansson, O. Longitudinal measurements of cerebrospinal fluid biomarkers in Parkinson’s disease. Mov. Disord. 2016, 31, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Wennström, M.; Hall, S.; Nägga, K.; Londos, E.; Minthon, L.; Hansson, O. Cerebrospinal fluid levels of IL-6 are decreased and correlate with cognitive status in DLB patients. Alzheimer’s Res. Ther. 2015, 7, 63. [Google Scholar] [CrossRef]
- Hansson, O.; Hall, S.; Öhrfelt, A.; Zetterberg, H.; Blennow, K.; Minthon, L.; Nägga, K.; Londos, E.; Varghese, S.; Majbour, N.K.; et al. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimer’s Res. Ther. 2014, 6, 25. [Google Scholar] [CrossRef]
- Stewart, T.; Liu, C.; Ginghina, C.; Cain, K.C.; Auinger, P.; Cholerton, B.; Shi, M.; Zhang, J. Cerebrospinal fluid α-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am. J. Pathol. 2014, 184, 966–975. [Google Scholar] [CrossRef]
- Bougea, A.; Stefanis, L.; Paraskevas, G.P.; Emmanouilidou, E.; Efthymiopoulou, E.; Vekrelis, K.; Kapaki, E. Neuropsychiatric symptoms and α-Synuclein profile of patients with Parkinson’s disease dementia, dementia with Lewy bodies and Alzheimer’s disease. J. Neurol. 2018, 265, 2295–2301. [Google Scholar] [CrossRef]
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orrù, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020, 140, 49–62. [Google Scholar] [CrossRef]
- Bongianni, M.; Ladogana, A.; Capaldi, S.; Klotz, S.; Baiardi, S.; Cagnin, A.; Perra, D.; Fiorini, M.; Poleggi, A.; Legname, G.; et al. α-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann. Clin. Transl. Neurol. 2019, 6, 2120–2126. [Google Scholar] [CrossRef]
- Majbour, N.K.; Vaikath, N.N.; Eusebi, P.; Chiasserini, D.; Ardah, M.; Varghese, S.; Haque, M.E.; Tokuda, T.; Auinger, P.; Calabresi, P.; et al. Longitudinal changes in CSF alpha-synuclein species reflect Parkinson’s disease progression. Mov. Disord. 2016, 31, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Factor, S.A.; Scullin, M.K.; Sollinger, A.B.; Land, J.O.; Wood-Siverio, C.; Zanders, L.; Freeman, A.; Bliwise, D.L.; McDonald, W.M.; Goldstein, F.C. Cognitive correlates of hallucinations and delusions in Parkinson’s disease. J. Neurol. Sci. 2014, 347, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Førland, M.G.; Öhrfelt, A.; Dalen, I.; Tysnes, O.B.; Blennow, K.; Zetterberg, H.; Pedersen, K.F.; Alves, G.; Lange, J. Evolution of cerebrospinal fluid total α-synuclein in Parkinson’s disease. Park. Relat. Disord. 2018, 49, 4–8. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Caspell-Garcia, C.J.; Coffey, C.S.; Taylor, P.; Singleton, A.; Shaw, L.M.; Trojanowski, J.Q.; Frasier, M.; Simuni, T.; Iranzo, A.; et al. Longitudinal analyses of cerebrospinal fluid α-Synuclein in prodromal and early Parkinson’s disease. Mov. Disord. 2019, 34, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Alvarado, M.; Gago, B.; Gorostidi, A.; Jiménez-Urbieta, H.; Dacosta-Aguayo, R.; Navalpotro-Gómez, I.; Ruiz-Martínez, J.; Bergareche, A.; Martí-Massó, J.F.; Martínez-Lage, P.; et al. Tau/α-synuclein ratio and inflammatory proteins in Parkinson’s disease: An exploratory study. Mov. Disord. 2017, 32, 1066–1073. [Google Scholar] [CrossRef]
- Van Steenoven, I.; Majbour, N.K.; Vaikath, N.N.; Berendse, H.W.; van der Flier, W.M.; van de Berg, W.D.J.; Teunissen, C.E.; Lemstra, A.W.; El-Agnaf, O.M.A. α-Synuclein species as potential cerebrospinal fluid biomarkers for dementia with lewy bodies. Mov. Disord. 2018, 33, 1724–1733. [Google Scholar] [CrossRef]
- Eusebi, P.; Giannandrea, D.; Biscetti, L.; Abraha, I.; Chiasserini, D.; Orso, M.; Calabresi, P.; Parnetti, L. Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2017, 32, 1389–1400. [Google Scholar] [CrossRef]
- García-Ayllón, M.S.; Monge-Argilés, J.A.; Monge-García, V.; Navarrete, F.; Cortés-Gómez, M.A.; Sánchez-Payá, J.; Manzanares, J.; Gasparini-Berenguer, R.; Leiva-Santana, C.; Sáez-Valero, J. Measurement of CSF α-synuclein improves early differential diagnosis of mild cognitive impairment due to Alzheimer’s disease. J. Neurochem. 2019, 150, 218–230. [Google Scholar] [CrossRef]
- Mavroudis, I.; Petridis, F.; Kazis, D. Cerebrospinal fluid, imaging, and physiological biomarkers in dementia with Lewy bodies. Am. J. Alzheimer’s Dis. Other Dement. 2019, 34, 421–432. [Google Scholar] [CrossRef]
- Atik, A.; Stewart, T.; Zhang, J. Alpha-synuclein as a biomarker for Parkinson’s disease. In Proceedings of the Brain Pathology; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2016; Volume 26, pp. 410–418. [Google Scholar]
- Williams, S.M.; Schulz, P.; Sierks, M.R. Oligomeric α-synuclein and β-amyloid variants as potential biomarkers for Parkinson’s and Alzheimer’s diseases. Eur. J. Neurosci. 2016, 43, 3–16. [Google Scholar] [CrossRef]
- Perra, D.; Bongianni, M.; Novi, G.; Janes, F.; Bessi, V.; Capaldi, S.; Sacchetto, L.; Tagliapietra, M.; Schenone, G.; Morbelli, S.; et al. Alpha-synuclein seeds in olfactory mucosa and cerebrospinal fluid of patients with dementia with Lewy bodies. Brain Commun. 2021, 3, 1–11. [Google Scholar] [CrossRef]
- Stefani, A.; Iranzo, A.; Holzknecht, E.; Perra, D.; Bongianni, M.; Gaig, C.; Heim, B.; Serradell, M.; Sacchetto, L.; Garrido, A.; et al. Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain 2021, 144, 1118–1126. [Google Scholar] [CrossRef]
- Zhao, Y.; Xin, Y.; Meng, S.; He, Z.; Hu, W. Neurofilament light chain protein in neurodegenerative dementia: A systematic review and network meta-analysis. Neurosci. Biobehav. Rev. 2019, 102, 123–138. [Google Scholar] [CrossRef]
- Chaudhry, A.; Houlden, H.; Rizig, M. Novel fluid biomarkers to differentiate frontotemporal dementia and dementia with Lewy bodies from Alzheimer’s disease: A systematic review. J. Neurol. Sci. 2020, 415, 116886. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Lee, W.J.; Wang, S.J.; Fuh, J.L. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci. Rep. 2018, 8, 17368. [Google Scholar] [CrossRef] [PubMed]
- Schade, S.; Mollenhauer, B. Biomarkers in biological fluids for dementia with Lewy bodies. Alzheimer’s Res. Ther. 2014, 6, 72. [Google Scholar] [CrossRef]
- King, E.; O’Brien, J.; Donaghy, P.; Williams-Gray, C.H.; Lawson, R.A.; Morris, C.M.; Barnett, N.; Olsen, K.; Martin-Ruiz, C.; Burn, D.; et al. Inflammation in mild cognitive impairment due to Parkinson’s disease, Lewy body disease, and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2019, 34, 1244–1250. [Google Scholar] [CrossRef]
- King, E.; O’brien, J.T.; Donaghy, P.; Morris, C.; Barnett, N.; Olsen, K.; Martin-Ruiz, C.; Taylor, J.P.; Thomas, A.J. Peripheral inflammation in mild cognitive impairment with possible and probable Lewy body disease and Alzheimer’s disease. Int. Psychogeriatr. 2019, 31, 551–560. [Google Scholar] [CrossRef]
- King, E.; Thomas, A. Systemic inflammation in Lewy body diseases a systematic review. Alzheimer Dis. Assoc. Disord. 2017, 31, 346–356. [Google Scholar] [CrossRef]
- Maetzler, W.; Stapf, A.K.; Schulte, C.; Hauser, A.K.; Lerche, S.; Wurster, I.; Schleicher, E.; Melms, A.; Berg, D. Serum and cerebrospinal fluid uric acid levels in lewy body disorders: Associations with disease occurrence and amyloid-β pathway. J. Alzheimer’s Dis. 2011, 27, 119–126. [Google Scholar] [CrossRef]
- Sommer, I.; Griebler, U.; Kien, C.; Auer, S.; Klerings, I.; Hammer, R.; Holzer, P.; Gartlehner, G. Vitamin D deficiency as a risk factor for dementia: A systematic review and meta-analysis. BMC Geriatr. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhong, S.; Liang, Y.; Zhang, X.; Zhang, R.; Kang, K.; Qu, H.; Xu, Y.; Zhao, C.; Zhao, M. Serum uric acid and the risk of dementia: A systematic review and meta-analysis. Front. Aging Neurosci. 2021, 13, 625690. [Google Scholar] [CrossRef]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef]
- Oh, Y.S.; Kim, J.S.; Park, H.E.; Song, I.U.; Park, J.W.; Yang, D.W.; Son, B.C.; Lee, S.H.; Lee, K.S. Association between urine protein/creatinine ratio and cognitive dysfunction in Lewy body disorders. J. Neurol. Sci. 2016, 362, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.X.; Sedor, S.; McGeary, I.; Cornblath, E.J.; Peng, C.; Riddle, D.M.; Li, H.L.; Zhang, B.; Brown, H.J.; Olufemi, M.F.; et al. Glucocerebrosidase activity modulates neuronal susceptibility to pathological α-synuclein insult. Neuron 2020, 105, 822–836.e7. [Google Scholar] [CrossRef] [PubMed]
- Chiasserini, D.; Biscetti, L.; Eusebi, P.; Salvadori, N.; Frattini, G.; Simoni, S.; De Roeck, N.; Tambasco, N.; Stoops, E.; Vanderstichele, H.; et al. Differential role of CSF fatty acid binding protein 3, α-synuclein, and Alzheimer’s disease core biomarkers in Lewy body disorders and Alzheimer’s dementia. Alzheimer’s Res. Ther. 2017, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.K.; Bender, A.; Laub, C.; Högen, T.; Schlaudraff, F.; Liss, B.; Klopstock, T.; Elstner, M. Lewy body pathology is associated with mitochondrial DNA damage in Parkinson’s disease. Neurobiol. Aging 2013, 34, 2231–2233. [Google Scholar] [CrossRef] [PubMed]
- Moccia, M.; Picillo, M.; Erro, R.; Vitale, C.; Longo, K.; Amboni, M.; Santangelo, G.; Palladino, R.; Capo, G.; Orefice, G.; et al. Presence and progression of non-motor symptoms in relation to uric acid in de novo Parkinson’s disease. Eur. J. Neurol. 2015, 22, 93–98. [Google Scholar] [CrossRef]
- Annanmaki, T.; Pessala-Driver, A.; Hokkanen, L.; Murros, K. Uric acid associates with cognition in Parkinson’s disease. Park. Relat. Disord. 2008, 14, 576–578. [Google Scholar] [CrossRef]
- Chatzikonstantinou, S.; McKenna, J.; Karantali, E.; Petridis, F.; Kazis, D.; Mavroudis, I. Electroencephalogram in dementia with Lewy bodies: A systematic review. Aging Clin. Exp. Res. 2020, 33, 1197–1208. [Google Scholar] [CrossRef]
- Bonanni, L.; Thomas, A.; Tiraboschi, P.; Perfetti, B.; Varanese, S.; Onofrj, M. EEG comparisons in early Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease with dementia patients with a 2-year follow-up. Brain 2008, 131, 690–705. [Google Scholar] [CrossRef]
- Stylianou, M.; Murphy, N.; Peraza, L.R.; Graziadio, S.; Cromarty, R.; Killen, A.; O’ Brien, J.T.; Thomas, A.J.; LeBeau, F.E.N.; Taylor, J.P. Quantitative electroencephalography as a marker of cognitive fluctuations in dementia with Lewy bodies and an aid to differential diagnosis. Clin. Neurophysiol. 2018, 129, 1209–1220. [Google Scholar] [CrossRef]
- Law, Z.K.; Todd, C.; Mehraram, R.; Schumacher, J.; Baker, M.R.; LeBeau, F.E.N.; Yarnall, A.; Onofrj, M.; Bonanni, L.; Thomas, A.; et al. The role of EEG in the diagnosis, prognosis and clinical correlations of dementia with Lewy bodies—A systematic review. Diagnostics 2020, 10, 616. [Google Scholar] [CrossRef]
- Bonanni, L.; Perfetti, B.; Bifolchetti, S.; Taylor, J.P.; Franciotti, R.; Parnetti, L.; Thomas, A.; Onofrj, M. Quantitative electroencephalogram utility in predicting conversion of mild cognitive impairment to dementia with Lewy bodies. Neurobiol. Aging 2015, 36, 434–445. [Google Scholar] [CrossRef]
- Pase, M.P.; Himali, J.J.; Grima, N.A.; Beiser, A.S.; Satizabal, C.L.; Aparicio, H.J.; Thomas, R.J.; Gottlieb, D.J.; Auerbach, S.H.; Seshadri, S. Sleep architecture and the risk of incident dementia in the community. Neurology 2017, 89, 1244–1250. [Google Scholar] [CrossRef]
- Ferman, T.J.; Boeve, B.F.; Smith, G.E.; Lin, S.C.; Silber, M.H.; Pedraza, O.; Wszolek, Z.; Graff-Radford, N.R.; Uitti, R.; Van Gerpen, J.; et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology 2011, 77, 875–882. [Google Scholar] [CrossRef]
- Garcia-Ptacek, S.; Kramberger, M.G. Parkinson disease and dementia. J. Geriatr. Psychiatry Neurol. 2016, 29, 261–270. [Google Scholar] [CrossRef]
- Garcia-Ptacek, S.; Farahmand, B.; Kareholt, I.; Religa, D.; Cuadrado, M.L.; Eriksdotter, M. Mortality risk after dementia diagnosis by dementia type and underlying factors: A cohort of 15,209 patients based on the swedish dementia registry. J. Alzheimer’s Dis. 2014, 41, 467–477. [Google Scholar] [CrossRef]
- Akbar, U.; McQueen, R.B.; Bemski, J.; Carter, J.; Goy, E.R.; Kutner, J.; Johnson, M.J.; Miyasaki, J.M.; Kluger, B. Prognostic predictors relevant to end-of-life palliative care in Parkinson’s disease and related disorders: A systematic review. J. Neurol. Neurosurg. Psychiatry 2021, 92, 629–636. [Google Scholar] [CrossRef]
- Kramberger, M.G.; Auestad, B.; Garcia-Ptacek, S.; Abdelnour, C.; Olmo, J.G.; Walker, Z.; Lemstra, A.W.; Londos, E.; Blanc, F.; Bonanni, L.; et al. Long-term cognitive decline in dementia with lewy bodies in a large multicenter, international cohort. J. Alzheimer’s Dis. 2017, 57, 787–795. [Google Scholar] [CrossRef]
- Mueller, C.; Soysal, P.; Rongve, A.; Isik, A.T.; Thompson, T.; Maggi, S.; Smith, L.; Basso, C.; Stewart, R.; Ballard, C.; et al. Survival time and differences between dementia with Lewy bodies and Alzheimer’s disease following diagnosis: A meta-analysis of longitudinal studies. Ageing Res. Rev. 2019, 50, 72–80. [Google Scholar] [CrossRef]
- Moylett, S.; Price, A.; Cardinal, R.N.; Aarsland, D.; Mueller, C.; Stewart, R.; O’Brien, J.T. Clinical presentation, diagnostic features, and mortality in dementia with lewy bodies. J. Alzheimer’s Dis. 2019, 67, 995–1005. [Google Scholar] [CrossRef]
- Alvarez, M.V.G.; Evidente, V.G.H. Understanding drug-induced parkinsonism: Separating pearls from oy-sters. Neurology 2008, 70, 32–34. [Google Scholar] [CrossRef]
- Wang, H.F.; Yu, J.T.; Tang, S.W.; Jiang, T.; Tan, C.C.; Meng, X.F.; Wang, C.; Tan, M.S.; Tan, L. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: Systematic review with meta-analysis and trial sequential analysis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; McKeith, I.G.; Burn, D.J.; Boeve, B.F.; Weintraub, D.; Bamford, C.; Allan, L.M.; Thomas, A.J.; O’Brien, J.T. New evidence on the management of Lewy body dementia. Lancet Neurol. 2020, 19, 157–169. [Google Scholar] [CrossRef]
- Dementia: Assessment, Management and Support for People Living with Dementia and Their Carers. NICE guideline [NG97]. Available online: https://www.nice.org.uk/guidance/ng97 (accessed on 17 June 2021).
- Stinton, C.; McKeith, I.; Taylor, J.P.; Lafortune, L.; Mioshi, E.; Mak, E.; Cambridge, V.; Mason, J.; Thomas, A.; O’Brien, J.T. Pharmacological management of lewy body dementia: A systematic review and meta-analysis. Am. J. Psychiatry 2015, 172, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Khondoker, M.; Magill, N.; Stewart, R.; Landau, S. A systematic review and meta-analysis of the effectiveness of acetylcholinesterase inhibitors and memantine in treating the cognitive symptoms of dementia. Dement. Geriatr. Cogn. Disord. 2018, 45, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Goetz, C.G.; Brandabur, M.; Sanfilippo, M.; Stebbins, G.T. Effects of dopaminergic medications on psychosis and motor function in dementia with Lewy bodies. Mov. Disord. 2008, 23, 2248–2250. [Google Scholar] [CrossRef]
- Matsunaga, S.; Kishi, T.; Iwata, N. Combination therapy with zonisamide and antiparkinson drugs for Parkinson’s disease: A meta-analysis. J. Alzheimer’s Dis. 2017, 56, 1229–1239. [Google Scholar] [CrossRef]
- Murata, M.; Odawara, T.; Hasegawa, K.; Iiyama, S.; Nakamura, M.; Tagawa, M.; Kosaka, K. Adjunct zonisamide to levodopa for DLB parkinsonism: A randomized double-blind phase 2 study. Neurology 2018, 90, e664–e672. [Google Scholar] [CrossRef]
- Samudra, N.; Patel, N.; Womack, K.B.; Khemani, P.; Chitnis, S. Psychosis in Parkinson disease: A review of etiology, phenomenology, and management. Drugs Aging 2016, 33, 855–863. [Google Scholar] [CrossRef]
- Seppi, K.; Ray Chaudhuri, K.; Coelho, M.; Fox, S.H.; Katzenschlager, R.; Perez Lloret, S.; Weintraub, D.; Sampaio, C.; Chahine, L.; Hametner, E.M.; et al. Update on treatments for nonmotor symptoms of Parkinson’s disease—An evidence-based medicine review. Mov. Disord. 2019, 34, 180–198. [Google Scholar] [CrossRef]
- Hershey, L.A.; Coleman-Jackson, R. Pharmacological management of dementia with Lewy bodies. Drugs Aging 2019, 36, 309–319. [Google Scholar] [CrossRef]
- Chan, P.C.; Lee, H.H.; Hong, C.T.; Hu, C.J.; Wu, D. REM sleep behavior disorder (RBD) in dementia with lewy bodies (DLB). Behav. Neurol. 2018, 2018, 9421098. [Google Scholar] [CrossRef]
- Goldman, J.G.; Forsberg, L.K.; Boeve, B.F.; Armstrong, M.J.; Irwin, D.J.; Ferman, T.J.; Galasko, D.; Galvin, J.E.; Kaufer, D.; Leverenz, J.; et al. Challenges and opportunities for improving the landscape for Lewy body dementia clinical trials. Alzheimer’s Res. Ther. 2020, 12, 137. [Google Scholar] [CrossRef]
- Siderowf, A.; Aarsland, D.; Mollenhauer, B.; Goldman, J.G.; Ravina, B. Biomarkers for cognitive impairment in Lewy body disorders: Status and relevance for clinical trials. Mov. Disord. 2018, 33, 528–536. [Google Scholar] [CrossRef]

| DLB 1 | PDD 2 | ||
|---|---|---|---|
| Central features | Essential for a diagnosis: Dementia, in early stages with memory impairment, may not necessarily occur but is usually evident with progression. Deficits in tests of attention, executive function, and visuoperceptual ability may be especially prominent and occur early. | Core Features (I) | Essential for a diagnosis (both must be present): Diagnosis of Parkinson disease according to Queen Square Brain Bank criteria and Dementia syndrome with impairment in more than one cognitive domain |
| Core clinical features | The first three typically occur early and may persist throughout the course:
| Associated clinical features (II) |
|
| PDD; lack of behavioral symptoms, however, does not exclude the diagnosis | ||
| Supportive clinical features | Severe sensitivity to antipsychotic agents; postural instability; repeated falls; syncope or other transient episodes of unresponsiveness; severe autonomic dysfunction, e.g., constipation, orthostatic hypotension, urinary incontinence; hypersomnia; hyposmia; hallucinations in other modalities; systematized delusions; apathy, anxiety, and depression. | None of the group (III) features present | Features which do not exclude PDD, but make the diagnosis uncertain:
|
| Indicative biomarkers |
| None of the group (IV) features present | Features suggesting other conditions or diseases as cause of mental impairment, which, when present, make it impossible to reliably diagnose PDD: 1. Cognitive and behavioral symptoms appearing solely in the context of other conditions such as acute confusion due to (a.) systemic diseases or abnormalities (b.) drug intoxication 2. Major Depression according to DSM IV |
| Supportive biomarkers |
| Supportive or indicative biomarkers | No supportive or indicative biomarkers are needed for the diagnosis of PDD as per Emre et al. (2007) diagnostic criteria. |
| Diagnosis of probable or possible DLB | Probable: (a) ≥2 core clinical features of DLB are present, with or without the presence of indicative biomarkers, OR (b). Only one core clinical feature is present, but with ≥1 indicative biomarkers. Probable DLB should not be diagnosed on the basis of biomarkers alone. Possible: (a). Only one core clinical feature of DLB is present, with no indicative biomarker evidence, OR (b). ≥1 indicative biomarkers is present but there are no core clinical features. | Diagnosis of probable or possible PDD | Probable: (a) Core features: Both must be present; (b). Associated clinical features: Typical profile of cognitive deficits and the presence of at least one behavioral symptom (lack of behavioral symptoms, however, does not exclude the diagnosis); (c) None of the group III features present; (d) None of the group IV features present. Possible: (a) Core features: Both must be present (b). Associated clinical features: Atypical profile of cognitive impairment in one or more domains (e.g., fluent aphasia, or pure storage-failure type amnesia) and behavioral symptoms may or may not be present; OR (c) One or more of the group III features present, (d) None of the group IV features present |
| Essential for a diagnosis of MCI-LB is MCI defined by the presence of each of the following: 1. Concern by the patient, informant, or clinician regarding cognitive decline. 2. Objective evidence of impairment in one or more cognitive domains. The cognitive impairment may include any domain, but is more likely to be associated with attention-executive and/or visual processing deficits. 3. Preserved or minimally affected performance of previously attained independence in functional abilities, which do not meet the criteria for dementia. |
| Core clinical features: 1. Fluctuating cognition with variations in attention and alertness. 2. Recurrent visual hallucinations. 3. REM Behavior disorder. 4. One or more spontaneous cardinal features of parkinsonism: bradykinesia, rest tremor, or rigidity. |
| Supportive clinical features: Severe sensitivity to antipsychotic agents; postural instability; repeated falls; syncope or other transient episodes of unresponsiveness; prolonged or recurrent delirium; autonomic dysfunction, e.g., constipation, orthostatic hypotension, urinary incontinence; hypersomnia; hyposmia; hallucinations in other modalities including passage, and sense of presence phenomena; systematized delusions; apathy, anxiety, and depression. |
| Proposed biomarkers a. Reduced dopamine transporter uptake in basal ganglia demonstrated by SPECT or PET. b. Polysomnographic confirmation of REM sleep without atonia. c. Reduced meta-iodobenzylguanidine (MIBG) uptake on myocardial scintigraphy |
| Potential biomarkers of MCI-LB: a. Quantitative EEG showing slowing and dominant frequency variability. b. Relative preservation of medial temporal lobe structures on structural imaging. c. Insular thinning and gray matter volume loss on MRI. d. Low occipital uptake on perfusion/metabolism scan. |
| Note: MCI plus supportive clinical features or potential biomarkers are insufficient to diagnose MCI-LB but may raise suspicion of it and prompt biomarker investigation, which may add weight to an existing MCI-LB diagnosis. MCI-LB is less likely in the presence of any other physical illness or brain disease including cerebrovascular disease, and sufficient to account in part or in total for the clinical picture, although these do not exclude an MCI-LB diagnosis and may serve to indicate mixed or multiple pathologies contributing to the clinical presentation. |
| Probable or possible mild cognitive impairment due to Lewy body dementia (MCI-LB): • Probable MCI-LB can be diagnosed if: Two or more core clinical features of DLB are present, or only one core clinical feature is present, but with one or more proposed biomarkers. Probable MCI should not be diagnosed based on biomarkers alone. • Possible MCI-LB can be diagnosed if: Only one core clinical feature of DLB is present, with no proposed biomarkers, or one or more of the proposed biomarkers is present, but there are no core clinical features. |
| Biomarkers | PDD versus DLB | PDD/DLB versus AD |
|---|---|---|
| MRI | VBM: Diffuse cortical atrophy in both PDD and DLB; gray matter reductions in the temporal, parietal and occipital lobes in DLB [93,102]. DTI: controversial, corticostriatal disruption in PDD is more frontal and in DLB more posterior (parietal and occipital) [39,102,120]. SWI: Nigrosome 1 “swallow tail sign” differentiates PD from controls [124]. No studies comparing DLB from PDD. QSM: Higher iron load in hippocampus, thalamus, parietal, frontal, and occipital cortices correlate with cognition in PD [127,128,129,130,131,132,133]. No studies in DLB. fMRI: No conclusive differences between PDD and DLB [95,139,140]. | VBM: Insular cortical thinning may differentiate DBL from AD [99,100]. Preserved hippocampal volumes in DBL differentiate from AD [14]. DTI: Posterior regions affected in DLB more than AD [116]. SWI: Nigrosome 1 “swallow tail sign” differentiates DLB from AD with sensitivity (80%) [125]. QSM: Positive associations between susceptibility and amyloid PET in the pallidum and putamen of AD [134]. No studies in DLB. fMRI: No major differences between DLB and AD groups [138]. |
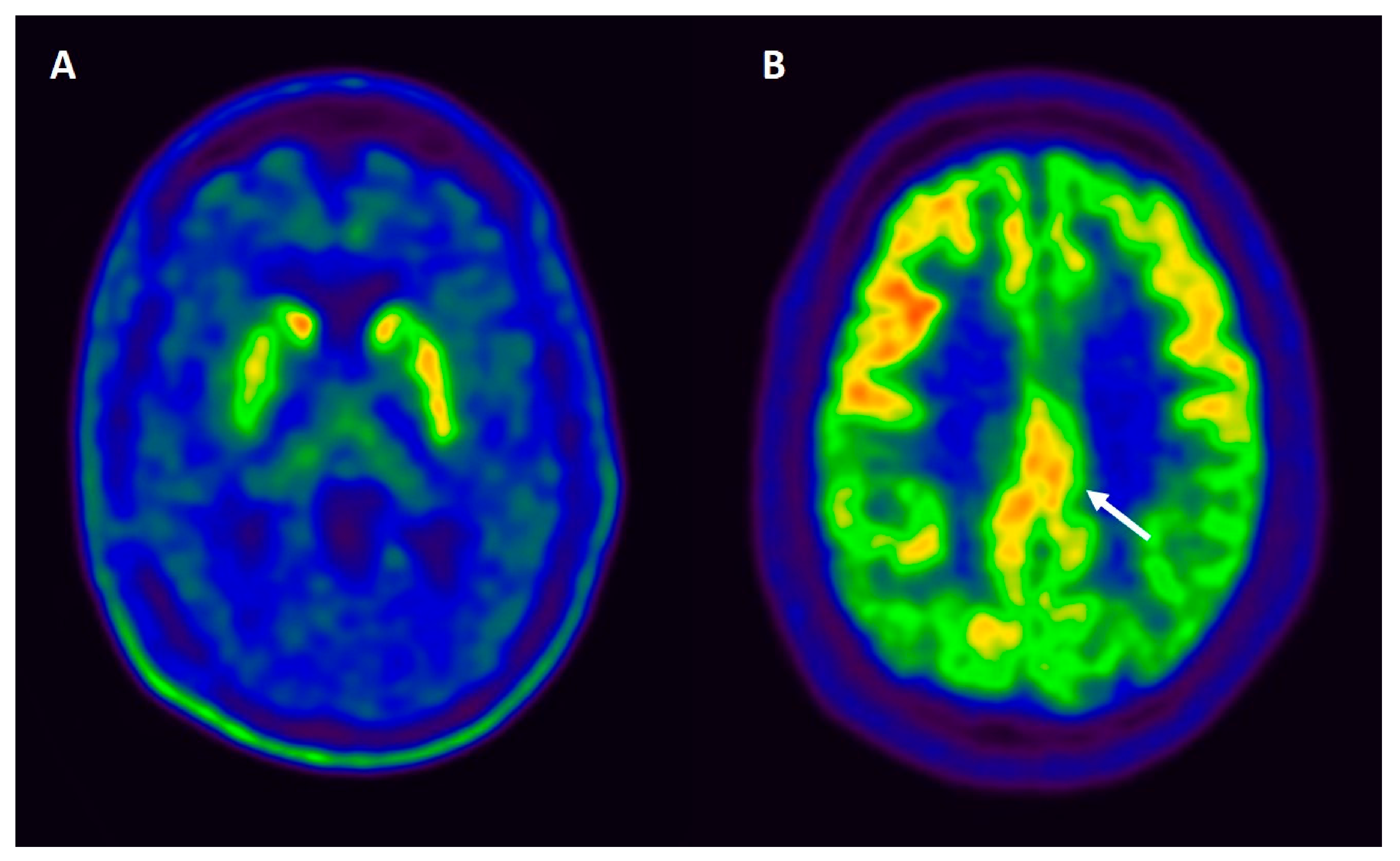
| PET and SPECT | DAT: Reduced dopamine transporter uptake in PD and DLB. SPECT perfusion and FDG-PET: Similar perfusion profiles in PDD and DLB, with posterior hypoperfusion and hypometabolism (inferior parietal and occipital cortices) [149,152]. More prominent hypometabolism in the anterior cingulate in DLB than PDD [148]. | DAT: Reduced striatal DAT uptake in DLB, useful in the differential diagnosis from AD, sensitivity (78%), and specificity (90%) [145]. SPECT perfusion and FDG-PET: Greater hypoperfusion in the parietooccipital cortex in DLB and PDD compared to AD [147]. Occipital hypometabolism combined with a less prominent metabolic decline in the medial temporal lobe in DBL vs. AD [148]. The “cingulate island” sign has high sensitivity (100%) in differentiating DLB from AD [14]. |
| PET amyloid and tau | amyloid: Increasing amyloid pathology PD < PD-MCI < PDD < DLB. Positive amyloid PET is associated with worse global cognition in LBD [161,162,163,172]. tau: Under investigation, no conclusive data. | amyloid: Positive amyloid PET indicative of amyloid pathology (AD or co-pathology with LBD) tau: Under investigation, no conclusive data. |
| [123I]MIBG Scintigraphy | Lower uptake in DLB than those with PDD [154], but the latest can also be positive. | Would help to differentiate AD from LBD [21,156,157]. |
| Molecular fluid biomarkers | CSF: (1) Low CSF Aβ42 levels predict cognitive impairment in PD and DLB [98,109,186,187,188,189]. Oxidized α-helical form of Aβ1-40 peptide significantly increased in patients with DLB in comparison to PDD [191]. (2) Lower α-syn in DLB and PD than in controls [193,196,208]. It may not be useful to differentiate between PDD and DLB [208]. (3) Other biomarkers: Inflammatory factors may increase in PDD and DLB but may not differentiate them [218]. NfL was also elevated in DLB, but no significant differences compared to PDD [213,216]. | CSF: (1) AD pattern (low Aβ42, high t-tau and p-tau): AD > DLB > PDD > PD [191]. (2) α- oligomeric α-syn higher in DLB and PDD compared with AD and controls [195,197,205], also at prodromal stages [207] (3) Other biomarkers: unspecific elevation of NfL levels in DLB, which do not distinguish from other dementias [213]. |
| Other potential biomarkers | Microglia activation: Increased 11C-PK11195 binding in several regions in PDD and DLB [178,179,180,181], but still under investigation (no conclusive data). | Microglia activation: Under investigation, no conclusive data. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milán-Tomás, Á.; Fernández-Matarrubia, M.; Rodríguez-Oroz, M.C. Lewy Body Dementias: A Coin with Two Sides? Behav. Sci. 2021, 11, 94. https://doi.org/10.3390/bs11070094
Milán-Tomás Á, Fernández-Matarrubia M, Rodríguez-Oroz MC. Lewy Body Dementias: A Coin with Two Sides? Behavioral Sciences. 2021; 11(7):94. https://doi.org/10.3390/bs11070094
Chicago/Turabian StyleMilán-Tomás, Ángela, Marta Fernández-Matarrubia, and María Cruz Rodríguez-Oroz. 2021. "Lewy Body Dementias: A Coin with Two Sides?" Behavioral Sciences 11, no. 7: 94. https://doi.org/10.3390/bs11070094
APA StyleMilán-Tomás, Á., Fernández-Matarrubia, M., & Rodríguez-Oroz, M. C. (2021). Lewy Body Dementias: A Coin with Two Sides? Behavioral Sciences, 11(7), 94. https://doi.org/10.3390/bs11070094






