Motor Coordination Disorders Evaluated through the Grid Test and Changes in the Nigral Nrf2 mRNA Expression in Rats with Pedunculopontine Lesion
Abstract
1. Introduction
2. Materials and Methods
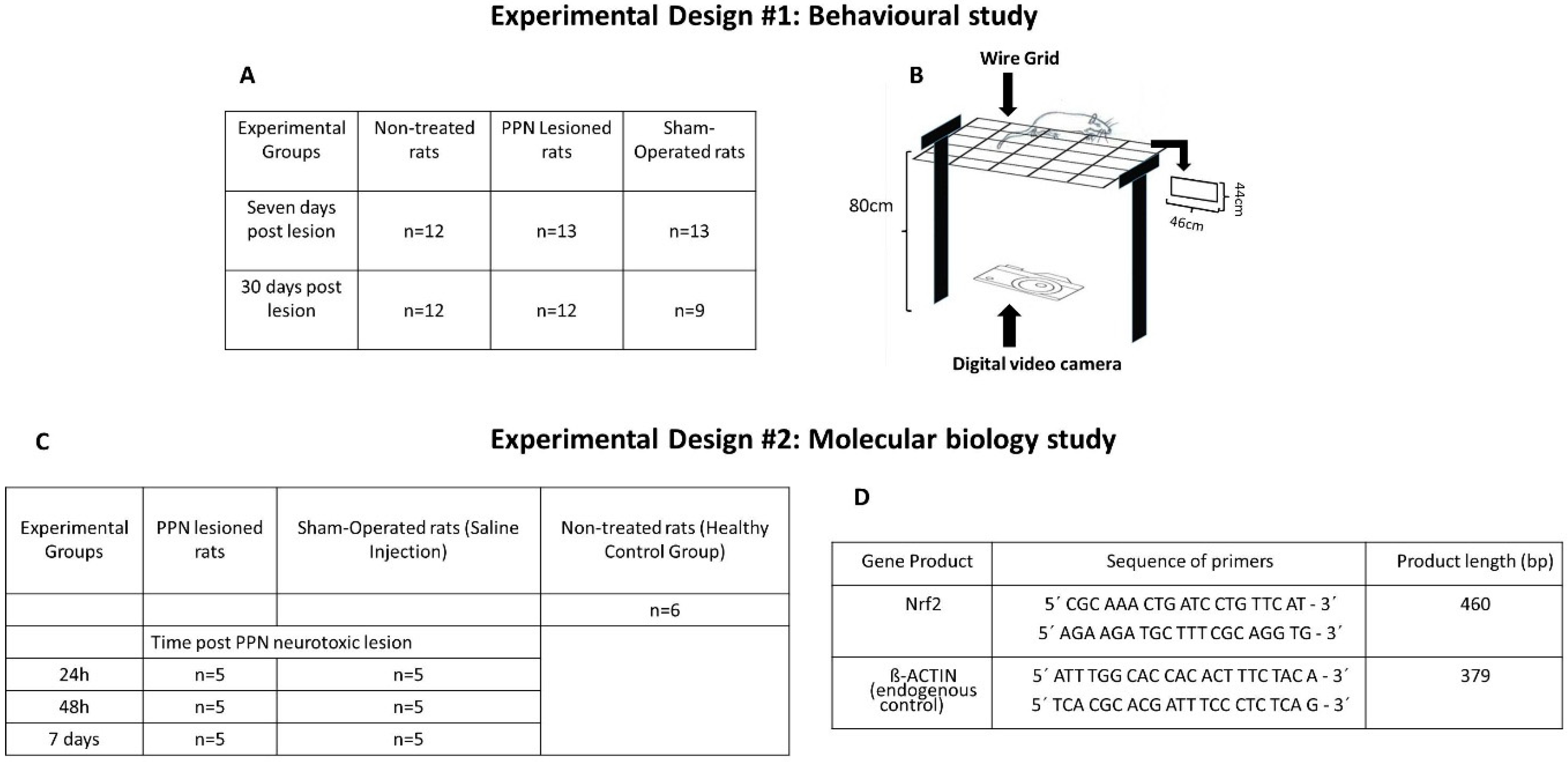
2.1. Experimental Subjects
2.2. Surgical Procedure
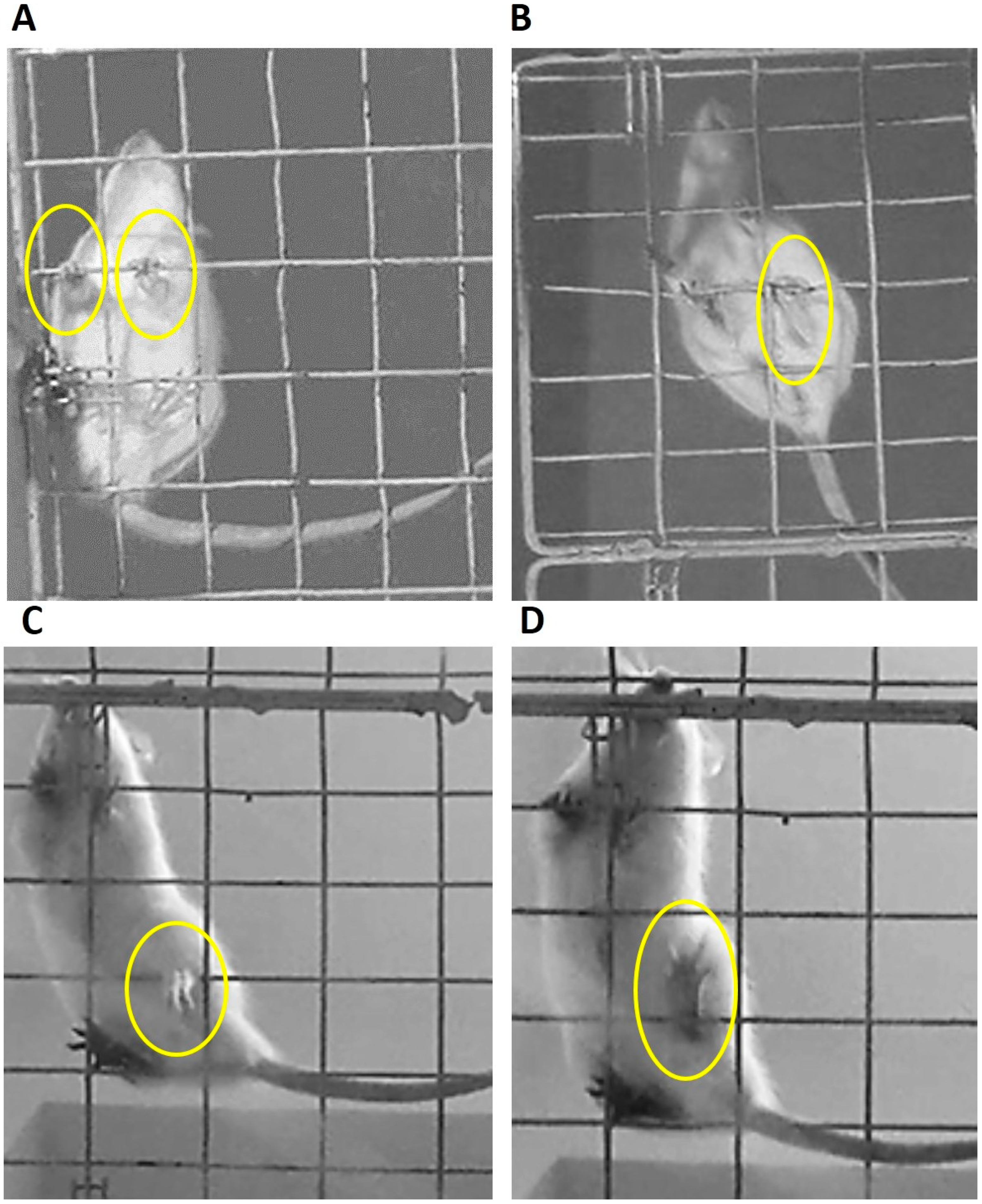
2.3. Evaluation of Motor Coordination
2.4. Experimental Set
- (a)
- Total number of failures committed by the limbs on each side,
- (b)
- Number of failures made with each limb individually,
- (c)
- Time delayed to the first failure.
2.5. Molecular Biology Studies
2.6. RT-PCR Analysis for Gene Expression of Nrf2
2.7. Morphological Studies
2.8. Immunohistochemistry
2.9. Statistical Analysis
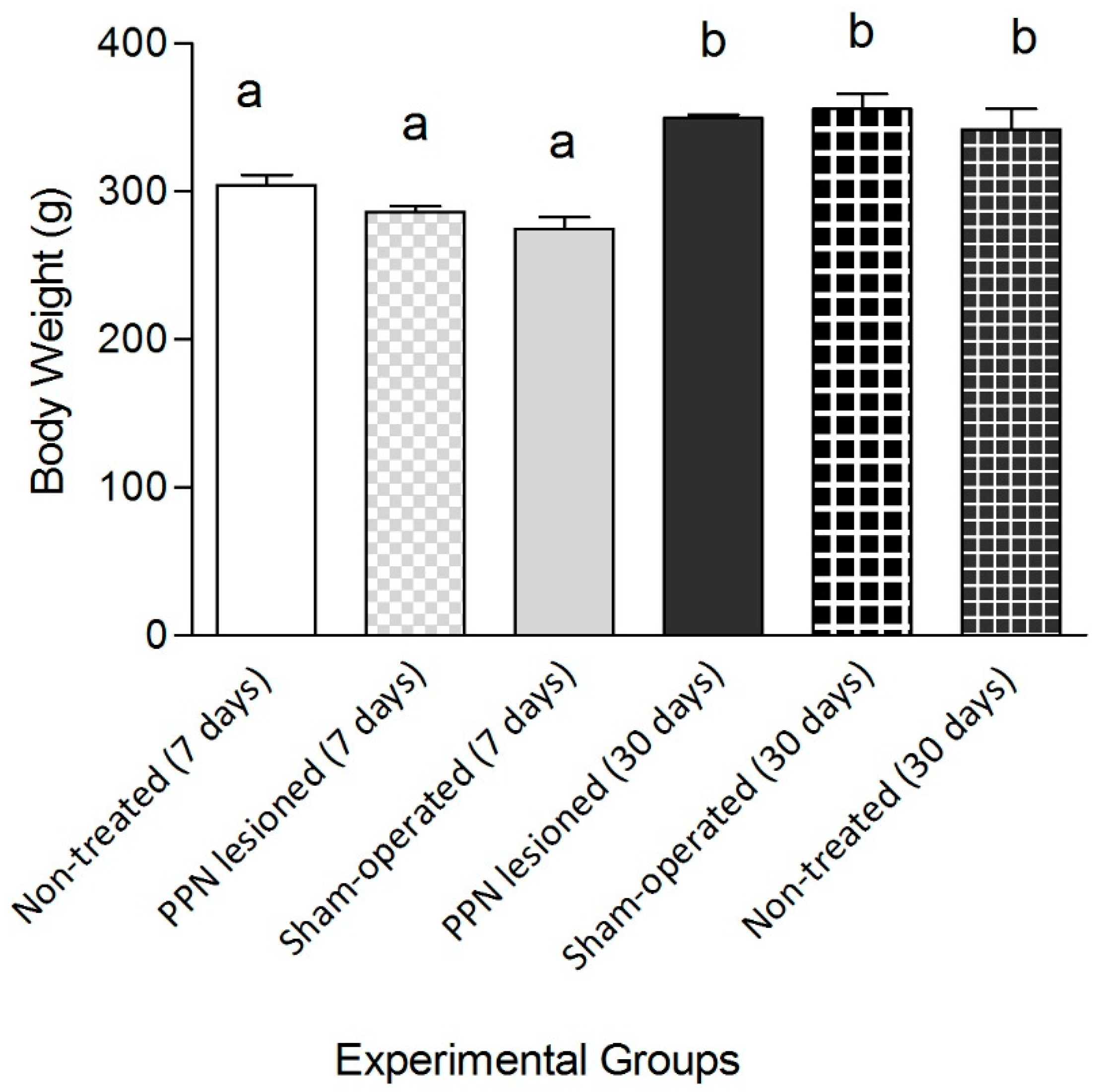
3. Results
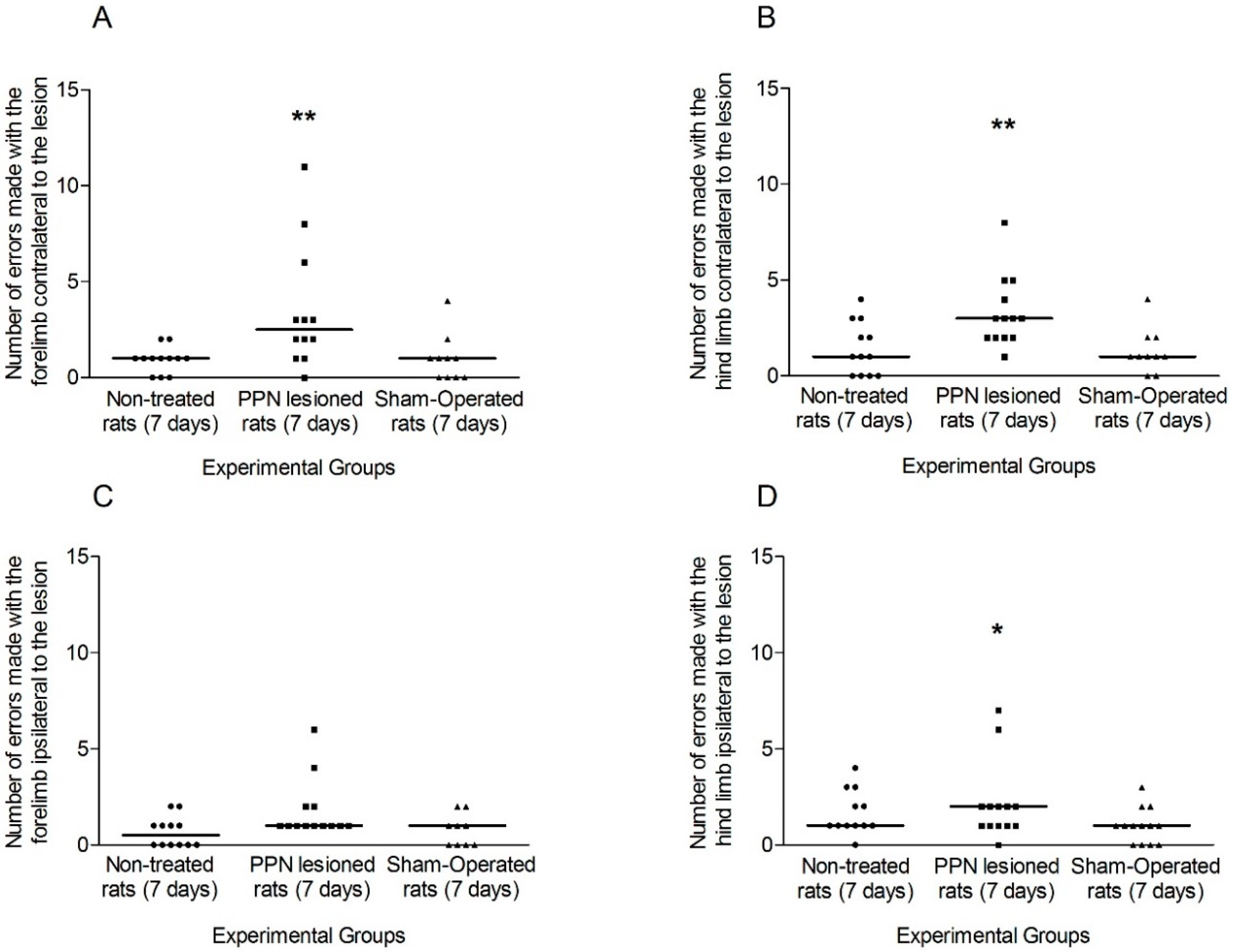
3.1. Evaluation of Motor Coordination through Grid Test
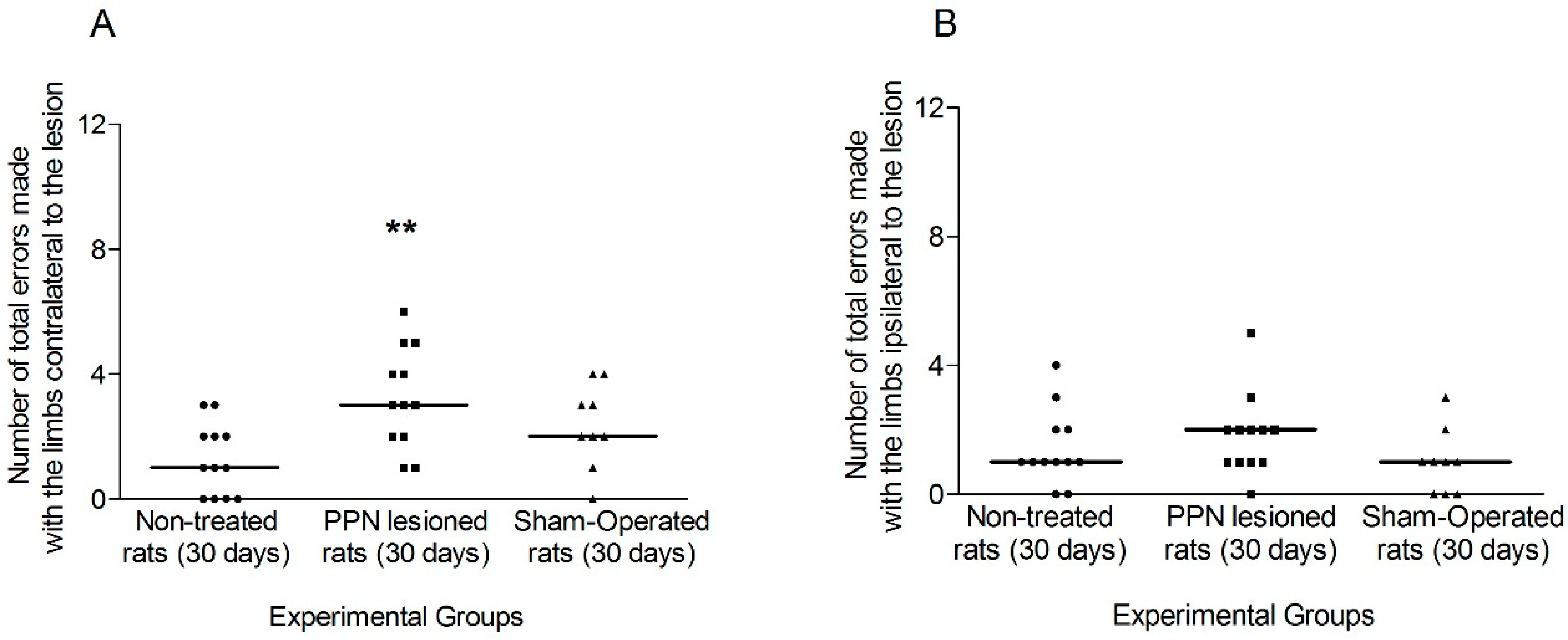
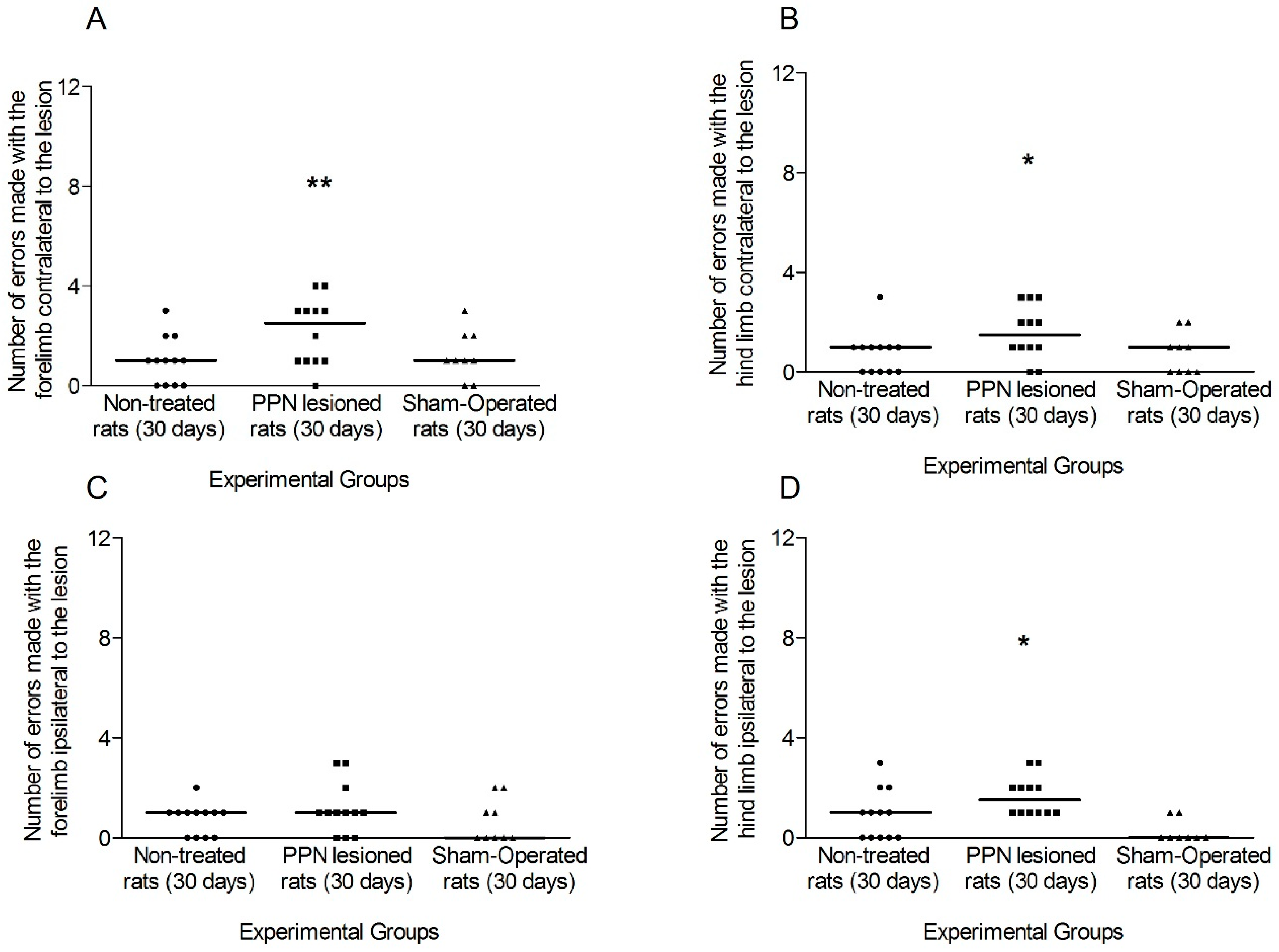
3.1.1. Motor Coordination Seven Days after PPN Lesion
3.1.2. Motor Coordination 30 Days after PPN Lesion
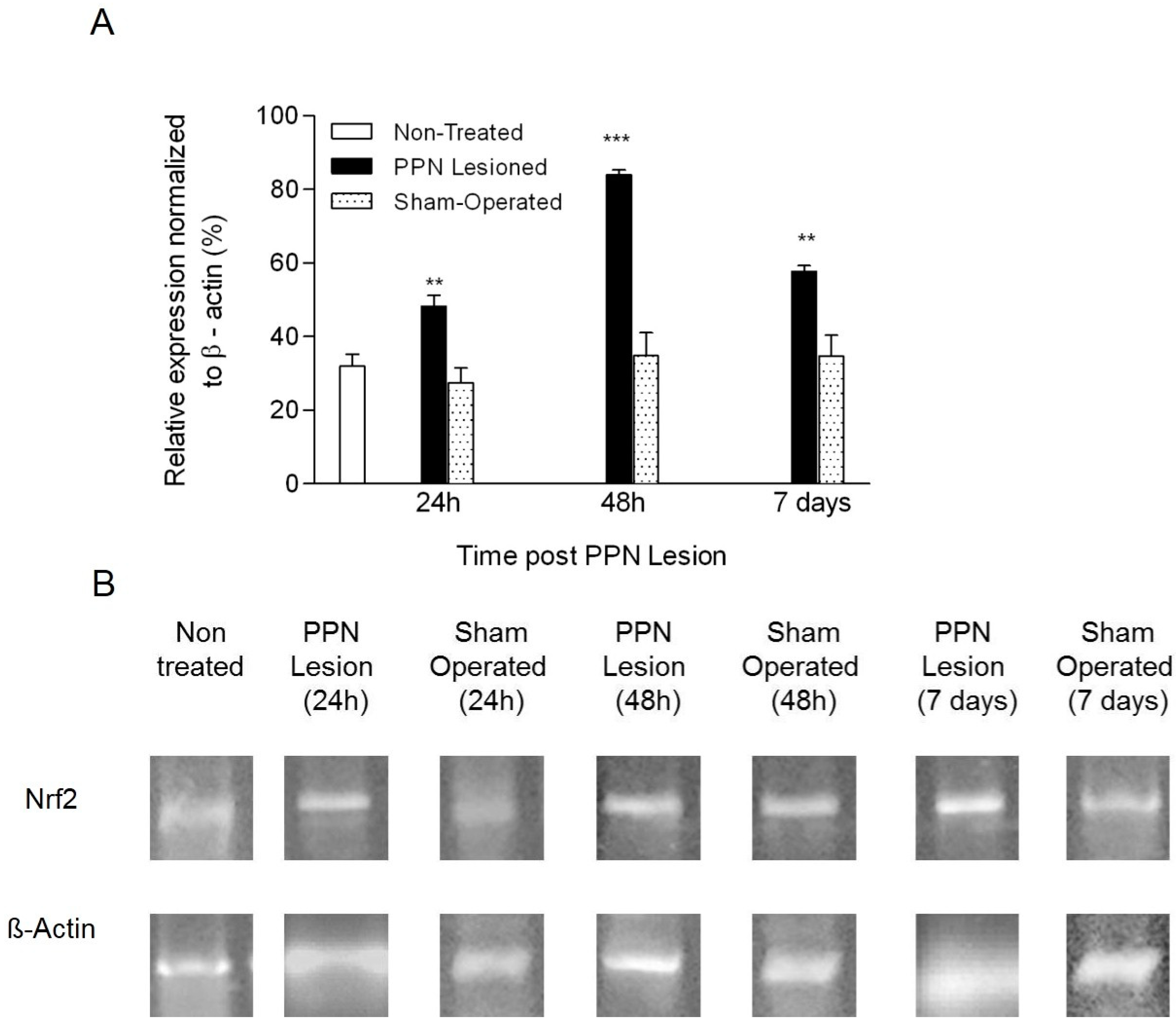
3.2. Molecular Biology Results
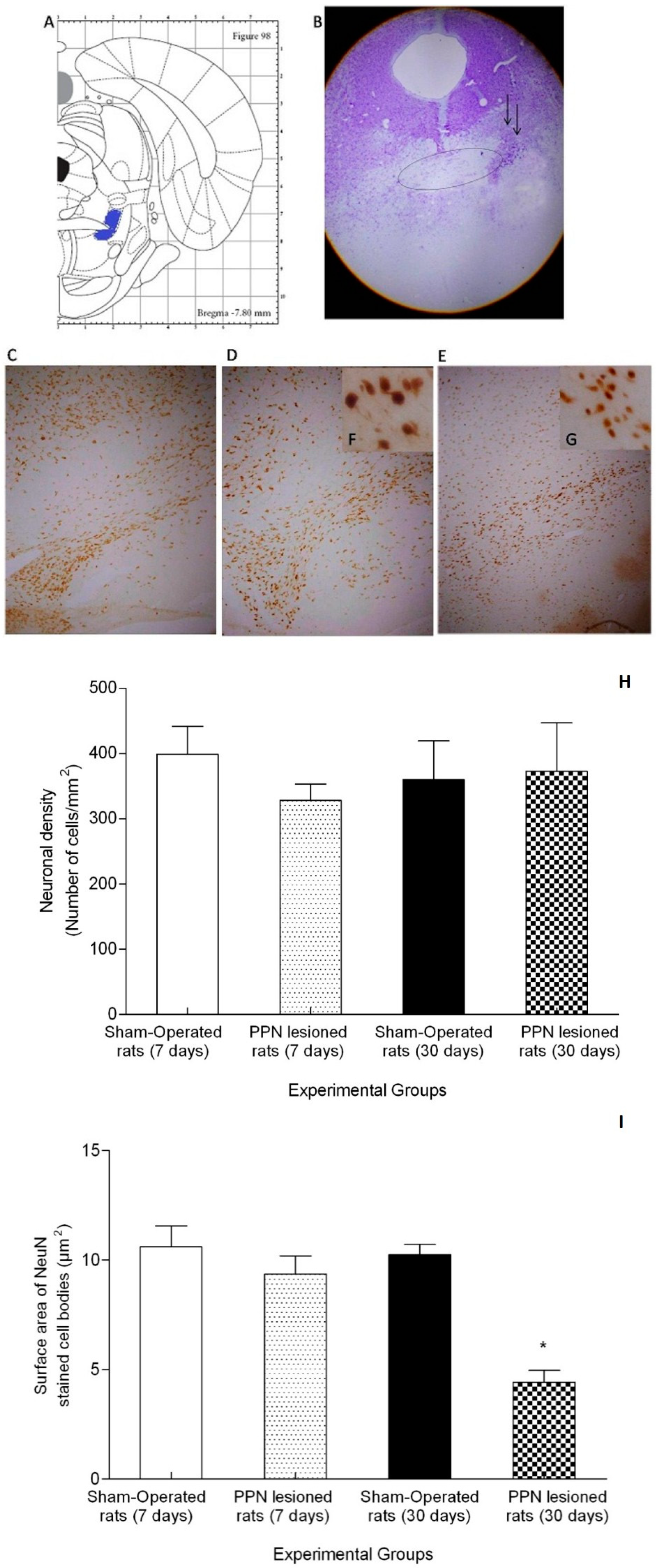
3.3. Morphological Results
4. Discussion
4.1. Neurotoxic PPN Injury Affects Motor Coordination during Ambulation over a Discontinuous and Elevated Horizontal Support Surface
4.2. Neurotoxic PPN Injury Modifies Nrf2 mRNA Expression in Nigral Tissue
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giguère, N.; Nanni, S.B.; Trudeau, L.-E. On Cell Loss and Selective Vulnerability of Neuronal Populations in Parkinson’s Disease. Front. Neurol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Schumacker, P.T.; Guzman, J.D.; Ilijic, E.; Yang, B.; Zampese, E. Calcium and Parkinson’s disease. Biochem. Biophys. Res. Commun. 2017, 483, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, M.; Michel, P.P.; Clark, S.D.; Hirsch, E.; François, C. Role of pedunculopontine cholinergic neurons in the vulnerability of nigral dopaminergic neurons in Parkinson’s disease. Exp. Neurol. 2016, 275, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K.; Kitai, S.T. Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neuroscience 1997, 78, 771–794. [Google Scholar] [CrossRef]
- Braak, H.; Bohl, J.R.; Müller, C.M.; Rüb, U.; De Vos, R.A.; Del Tredici, K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov. Disord. 2006, 21, 2042–2051. [Google Scholar] [CrossRef]
- Rizzi, G.; Tan, K.R. Dopamine and Acetylcholine, a Circuit Point of View in Parkinson’s Disease. Front. Neural Circuits 2017, 11, 110. [Google Scholar] [CrossRef]
- De Sarno, P.; Shestopal, S.A.; King, T.D.; Żmijewska, A.; Song, L.; Jope, R.S. Muscarinic Receptor Activation Protects Cells from Apoptotic Effects of DNA Damage, Oxidative Stress, and Mitochondrial Inhibition. J. Boil. Chem. 2003, 278, 11086–11093. [Google Scholar] [CrossRef]
- Jimenez-Martin, J.; Blanco-Lezcano, L.; González-Fraguela, M.; Díaz-Hung, M.-L.; Serrano-Sánchez, T.; Almenares, J.; Turner, L.F. Effect of neurotoxic lesion of pedunculopontine nucleus in nigral and striatal redox balance and motor performance in rats. Neuroscience 2015, 289, 300–314. [Google Scholar] [CrossRef]
- Blanco, L.; Jiménez, J.; Diaz, M.L.; Alberti, E.; Wong, M.; González, M.E.; Estupinán, B.; Serrano, T.; Francis, L.; Delgado, S. Motor Dysfunction and alterations in gluthathione concentration, cholinesterase activity and BDNF expression in substantia nigra pars compacta in rats with pedunculopontine lesion. Neuroscience 2017, 348, 83–97. [Google Scholar] [CrossRef]
- Blanco-Lezcano, L.; Amador, E.A.; Díaz-Hung, M.-L.; González-Fraguela, M.E.; Díaz, B.E.; Sánchez, T.S.; Francis-Turner, L.; Jiménez-Martin, J.; Vega-Hurtado, Y.; Fernández-Jiménez, I. Tyrosine Hydroxylase, Vesicular Monoamine Transporter and Dopamine Transporter mRNA Expression in Nigrostriatal Tissue of Rats with Pedunculopontine Neurotoxic Lesion. Behav. Sci. 2018, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Blanco Lezcano, L.; Alberti Amador, E.; González Fraguela, M.E.; De Larrea, G.Z.-L.; Serrano Pérez, R.M.; Jiménez-Luna, N.A.; Sánchez, T.S.; Francis-Turner, L.; Camejo-Rodriguez, D.; Vega-Hurtado, Y.; et al. Nurr1, Pitx3, and α7 nAChRs mRNA Expression in Nigral Tissue of Rats with Pedunculopontine Neurotoxic Lesion. Medicine 2019, 55, 616. [Google Scholar] [CrossRef] [PubMed]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free. Radic. Boil. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019, 2019, 1–20. [Google Scholar] [CrossRef]
- Łoboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Víctor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1–44. [Google Scholar] [CrossRef]
- Dunbar, J.; Hitchcock, K.; Latimer, M.; Rugg, E.; Ward, N.; Winn, P. Excitotoxic lesions of the pedunculopontine tegmental nucleus of the rat. II. Examination of eating and drinking, rotation, and reaching and grasping following unilateral ibotenate or quinolinate lesions. Brain Res. 1992, 589, 194–206. [Google Scholar] [CrossRef]
- MacLaren, D.A.A.; Santini, J.A.; Russell, A.L.; Marković, T.; Clark, S.D. Deficits in motor performance after pedunculopontine lesions in rats - impairment depends on demands of task. Eur. J. Neurosci. 2014, 40, 3224–3236. [Google Scholar] [CrossRef]
- Gut, N.K.; Winn, P. Deep brain stimulation of different pedunculopontine targets in a novel rodent model of parkinsonism. J. Neurosci. 2015, 35, 4792–4803. [Google Scholar] [CrossRef] [PubMed]
- Kunkel-Bagden, E.; Dai, H.-N.; Bregman, B.S. Methods to Assess the Development and Recovery of Locomotor Function after Spinal Cord Injury in Rats. Exp. Neurol. 1993, 119, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Tillerson, J.L.; Caudle, W.M.; Reverón, M.E.; Miller, G.W. Detection of Behavioral Impairments Correlated to Neurochemical Deficits in Mice Treated with Moderate Doses of 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Exp. Neurol. 2002, 178, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Tillerson, J.L.; Miller, G.W. Grid performance test to measure behavioral impairment in the MPTP-treated-mouse model of parkinsonism. J. Neurosci. Methods 2003, 123, 189–200. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Onyszchuk, G.; He, Y.-Y.; Berman, N.E.; Brooks, W.M. Detrimental Effects of Aging on Outcome from Traumatic Brain Injury: A Behavioral, Magnetic Resonance Imaging, and Histological Study in Mice. J. Neurotrauma 2008, 25, 153–171. [Google Scholar] [CrossRef]
- Kim, S.T.; Son, H.J.; Choi, J.H.; Ji, I.J.; Hwang, O. Vertical grid test and modified horizontal grid test are sensitive methods for evaluating motor dysfunctions in the MPTP mouse model of Parkinson’s disease. Brain Res. 2010, 1306, 176–183. [Google Scholar] [CrossRef]
- Nissbrandt, H.; Carlsson, A. Turnover of Dopamine and Dopamine Metabolites in Rat Brain: Comparison Between Striatum and Substantia Nigra. J. Neurochem. 1987, 49, 959–967. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Almaguer-Melian, W.; Mercerón-Martínez, D.; Pavón-Fuentes, N.; Alberti-Amador, E.; Leon-Martinez, R.; Ledón, N.; Ocaña, S.D.; Bergado, J.A. Erythropoietin Promotes Neural Plasticity and Spatial Memory Recovery in Fimbria-Fornix-Lesioned Rats. Neurorehabilit. Neural Repair 2015, 29, 979–988. [Google Scholar] [CrossRef]
- Šedý, J.; Urdziková, L.; Jendelová, P.; Sykova, E. Methods for behavioral testing of spinal cord injured rats. Neurosci. Biobehav. Rev. 2008, 32, 550–580. [Google Scholar] [CrossRef]
- Pajoohesh-Ganji, A.; Byrnes, K.R.; Fatemi, G.; Faden, A.I. A combined scoring method to assess behavioral recovery after mouse spinal cord injury. Neurosci. Res. 2010, 67, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Z’Graggen, W.J.; Metz, G.A.S.; Kartje, G.L.; Schwab, M.E. Functional recovery and enhanced cortico-fugal plasticity in the adult rat after unilateral pyramidal tract section and blockade of myelin associated neurite growth inhibitors. J. Neurosci. 1998, 18, 4744–4757. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.L.; Kutchko, K.M.; Fowler, S.C.; Berman, N.E.; Levant, B. Sensorimotor behavioral tests for use in a juvenile rat model of traumatic brain injury: Assessment of sex differences. J. Neurosci. Methods 2011, 199, 214–222. [Google Scholar] [CrossRef] [PubMed]
- French, I.T.; Muthusamy, K.A. A Review of the Pedunculopontine Nucleus in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10. [Google Scholar] [CrossRef]
- Pahapill, P.A.; Lozano, A.M. The pedunculopontine nucleus and Parkinson’s disease. Brain 2000, 123, 1767–1783. [Google Scholar] [CrossRef]
- Metz, G.A.S.; Merkler, D.; Dietz, V.; Schwab, M.E.; Fouad, K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000, 883, 165–177. [Google Scholar] [CrossRef]
- Takakusaki, K.; Chiba, R.; Nozu, T.; Okumura, T. Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J. Neural Transm. 2015, 123, 695–729. [Google Scholar] [CrossRef]
- Nielsen, J.B. How we walk: Central control of muscle activity during human walking. Neuroscience 2003, 9, 195–204. [Google Scholar] [CrossRef]
- Welniarz, Q.; Dusart, I.; Gallea, C.; Roze, E.; Gallea, C. One hand clapping: Lateralization of motor control. Front. Neuroanat. 2015, 9, 75. [Google Scholar] [CrossRef]
- Meredith, G.E.; Kang, U.J. Behavioral models of Parkinson’s disease in rodents: A new look at an old problem. Mov. Disord. 2006, 21, 1595–1606. [Google Scholar] [CrossRef]
- Mazzone, P.; Scarnati, E.; Garcia-Rill, E. Commentary: The pedunculopontine nucleus: Clinical experience, basic questions and future directions. J. Neural Transm. 2010, 118, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Allbutt, H.N.; Henderson, J.M. Use of the narrow beam test in the rat, 6-hydroxydopamine model of Parkinson’s disease. J. Neurosci. Methods 2007, 159, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Martin, A.; Lessmann, L.; Cerkez, D.; Gasser, T.; Schulz, J.B. Bilateral changes in neuronal activity of the basal ganglia in the unilateral 6-hydroxydopamine rat model. J. Neurosci. Res. 2008, 86, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.P.; Bolam, J.P.; Bevan, M.D. Glutamate-enriched Inputs from the Mesopontine Tegmentum to the Entopeduncular Nucleus in the Rat. Eur. J. Neurosci. 1996, 8, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; McBean, G.; Cindrić, M.; Egea, J.; López, M.G.; Rada, P.; Zarkovic, N.; Cuadrado, A. Redox control of microglial function: Molecular mechanisms and functional significance. Antioxid. Redox Signal. 2014, 21, 1766–1801. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular Stress Responses, The Hormesis Paradigm, and Vitagenes: Novel Targets for Therapeutic Intervention in Neurodegenerative Disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Bayin, E.C.; Genc, S.; Genc, K. The Nrf2/ARE Pathway: A Promising Target to Counteract Mitochondrial Dysfunction in Parkinson’s Disease. Park. Dis. 2011, 2011, 314082. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell Survival Responses to Environmental Stresses Via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Narasimhan, M.; Riar, A.K.; Rathinam, M.L.; Vedpathak, D.; Henderson, G.; Mahimainathan, L. Hydrogen peroxide responsive miR153 targets Nrf2/ARE cytoprotection in paraquat induced dopaminergic neurotoxicity. Toxicol. Lett. 2014, 228, 179–191. [Google Scholar] [CrossRef]
- Johnson, D.A.; Johnson, J.A. Nrf2—A therapeutic target for the treatment of neurodegenerative diseases. Free. Radic. Boil. Med. 2015, 88, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Caudle, W.M.; Colebrooke, R.E.; Emson, P.C.; Miller, G.W. Altered vesicular dopamine storage in Parkinson’s disease: A premature demise. Trends Neurosci. 2008, 31, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Aguilar, A.; Luna-López, A.; Ventura-Gallegos, J.L.; Lazzarini, R.; Galván-Arzate, S.; González-Puertos, V.Y.; Morán, J.; Santamaría, A.; Königsberg, M. Primary cultured astrocytes from old rats are capable to activate the Nrf2 response against MPP+ toxicity after tBHQ pretreatment. Neurobiol. Aging 2014, 35, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Iglesias, O.; Cacabelos, R. Pharmacoepigenomic Interventions as Novel Potential Treatments for Alzheimer’s and Parkinson’s Diseases. Int. J. Mol. Sci. 2018, 19, 3199. [Google Scholar] [CrossRef]


| Experimental Groups | ||||
|---|---|---|---|---|
| Variable | Non-Treated Rats | PPN Lesioned Rats | Sham-Operated Rats | Statistic |
| Time delayed before the first fault | 11.90 ± 3.26 | 5.01 ± 1.10 | 11.37 ± 3.13 | (F(2, 35) = 2.10 p > 0.05) |
| Experimental Groups | ||||
|---|---|---|---|---|
| Variable | Non-Treated Rats | PPN Lesioned Rats | Sham-Operated Rats | Statistic |
| Time delayed before the first fault | 17.45 ± 5.26 | 7.34 ± 2.05 | 14.45 ± 4.44 | (F(2,30) = 1.70 p > 0.05) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Lezcano, L.; Alberti Amador, E.; González Fraguela, M.E.; Larrea, G.Z.L.d.; Pérez Serrano, R.M.; Jiménez Luna, N.A.; Camejo Rodríguez, D.; Serrano Sánchez, T.; Francis Turner, L.; Estupiñán Díaz, B.; et al. Motor Coordination Disorders Evaluated through the Grid Test and Changes in the Nigral Nrf2 mRNA Expression in Rats with Pedunculopontine Lesion. Behav. Sci. 2020, 10, 156. https://doi.org/10.3390/bs10100156
Blanco-Lezcano L, Alberti Amador E, González Fraguela ME, Larrea GZLd, Pérez Serrano RM, Jiménez Luna NA, Camejo Rodríguez D, Serrano Sánchez T, Francis Turner L, Estupiñán Díaz B, et al. Motor Coordination Disorders Evaluated through the Grid Test and Changes in the Nigral Nrf2 mRNA Expression in Rats with Pedunculopontine Lesion. Behavioral Sciences. 2020; 10(10):156. https://doi.org/10.3390/bs10100156
Chicago/Turabian StyleBlanco-Lezcano, Lisette, Esteban Alberti Amador, María Elena González Fraguela, Guadalupe Zaldívar Lelo de Larrea, Rosa Martha Pérez Serrano, Nadia Angélica Jiménez Luna, Dianet Camejo Rodríguez, Teresa Serrano Sánchez, Liliana Francis Turner, Bárbara Estupiñán Díaz, and et al. 2020. "Motor Coordination Disorders Evaluated through the Grid Test and Changes in the Nigral Nrf2 mRNA Expression in Rats with Pedunculopontine Lesion" Behavioral Sciences 10, no. 10: 156. https://doi.org/10.3390/bs10100156
APA StyleBlanco-Lezcano, L., Alberti Amador, E., González Fraguela, M. E., Larrea, G. Z. L. d., Pérez Serrano, R. M., Jiménez Luna, N. A., Camejo Rodríguez, D., Serrano Sánchez, T., Francis Turner, L., Estupiñán Díaz, B., Vega Hurtado, Y., & Fernández Jiménez, I. (2020). Motor Coordination Disorders Evaluated through the Grid Test and Changes in the Nigral Nrf2 mRNA Expression in Rats with Pedunculopontine Lesion. Behavioral Sciences, 10(10), 156. https://doi.org/10.3390/bs10100156





