Associations of Patient Mood, Modulators of Quality of Life, and Pharmaceuticals with Amyotrophic Lateral Sclerosis Survival Duration
Abstract
1. Introduction
2. Materials and Methods
2.1. Temporal Assessments and Patient Characteristics
2.2. Assessment of Clincal Impression of Mood
2.3. Assessments of Physical Health and Modulators of Quality of Life
2.4. Other Pharmaceutical and Supplement Usage
2.5. Data Analysis and Statistical Methods
3. Results
3.1. Factors Assessed for Association with Clinical Impression of Mood (CIM)
3.2. Physical Health Factors Significantly Associated with Clinical Impression of Mood (CIM)
3.3. Quality of Life Factors (QoL) Significantly Associated with Clinical Impression of Mood (CIM)
3.4. Physical Health Factors Have Strong Association with Survival Duration
3.5. Quality of Life (QoL) Modulators Have Strong Association with Survival Duration
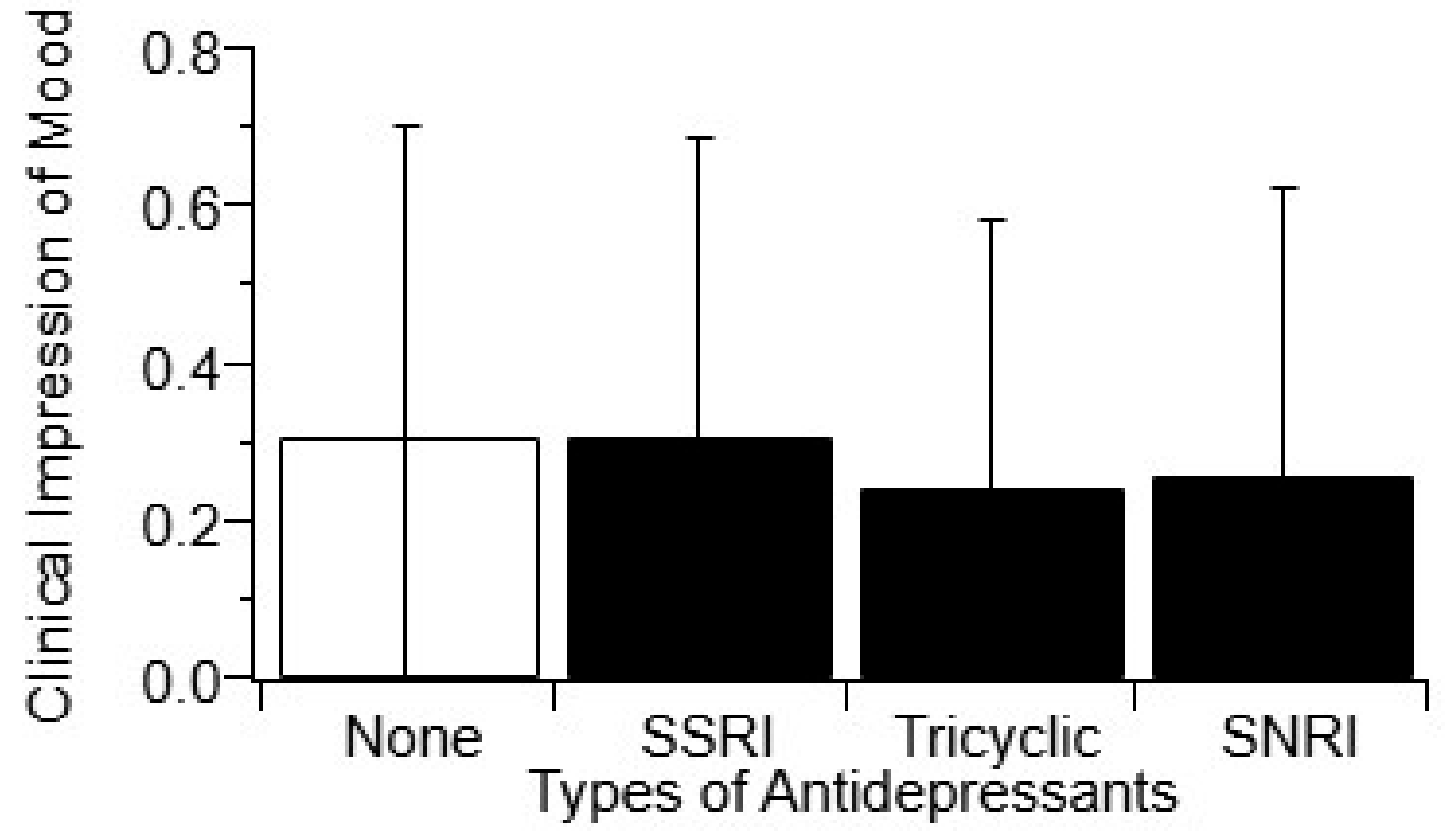
3.6. Antidepressant Usage Does not Impact Clincial Impression of Mood (CIM)
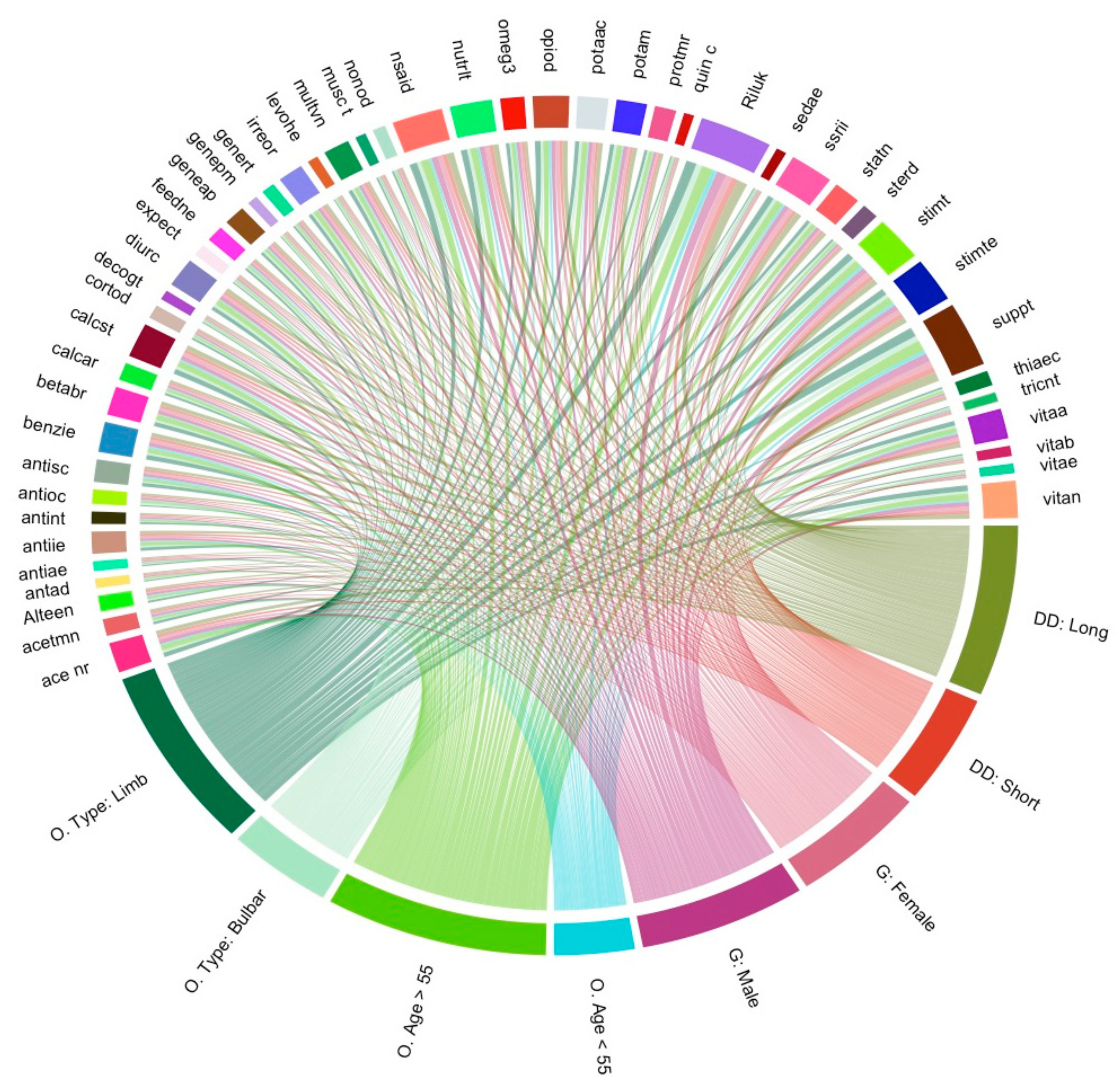
3.7. Visualisation of Medication and Supplement Usage Associations
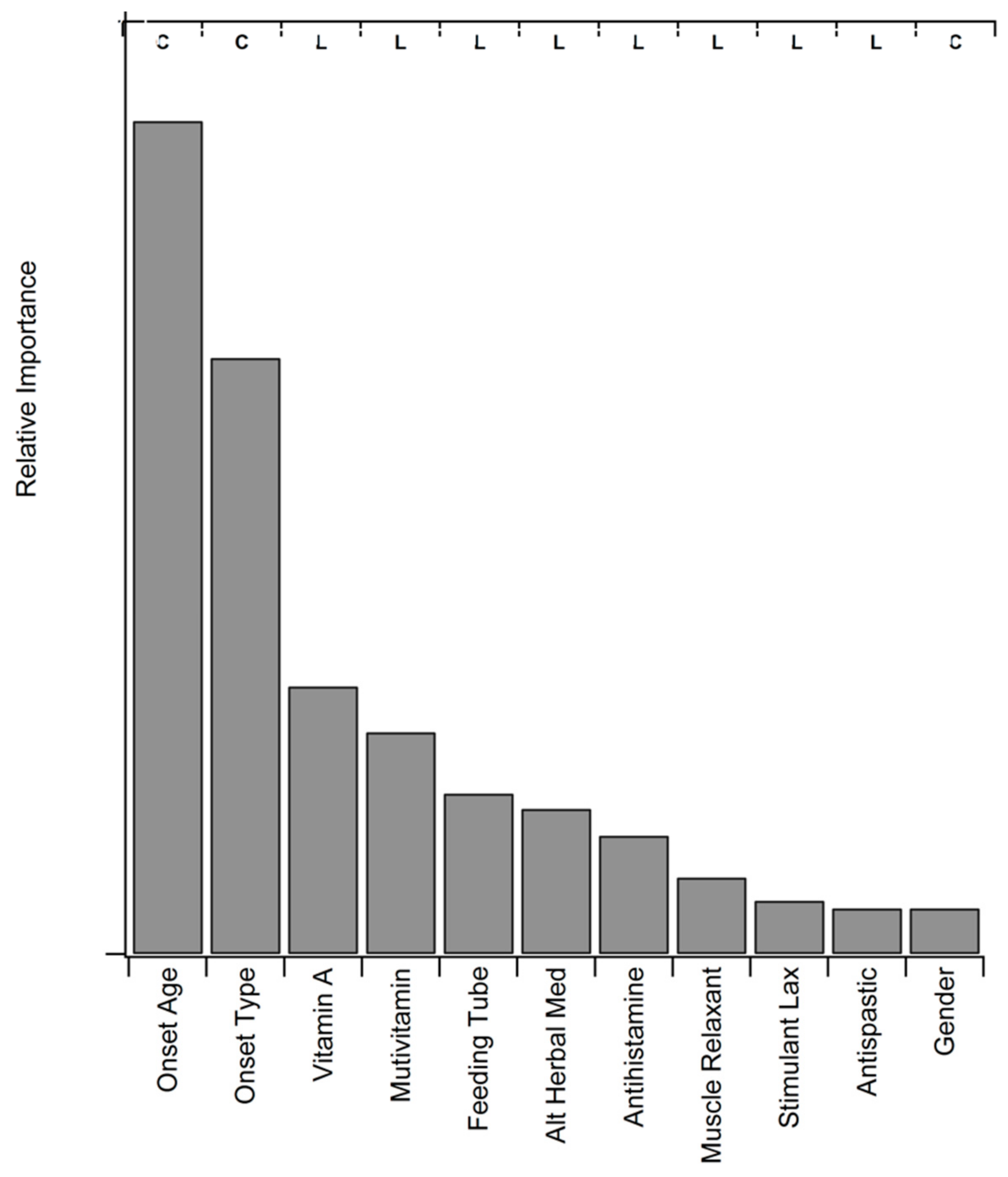
3.8. Statistical Assessment of Medication and Supplement Associations
4. Discussion
4.1. Mood and Social Support are Associated with Increased Survival Duration
4.2. Palliative ALS Interventions Associated with Improved Mood and Survival Duration
4.3. Antidepressant, Pain, Sleeping Medication Correlate with Survival Duration but not Mood
4.4. Pharmaceuticals for ALS-Related Muscle Spasm and Fasiculation Symptoms
4.5. Pharmaceuticals for ALS-Related Secretion Clearance Dysfunction
4.6. “Controversial” Interventions that May be Related to Decreased Survival Duration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zarei, S.; Carr, K.; Reiley, L.; Diaz, K.; Guerra, O.; Altamirano, P.F.; Pagani, W.; Lodin, D.; Orozco, G.; Chinea, A. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Petrov, D.; Mansfield, C.; Moussy, A.; Hermine, O. ALS Clinical Trials Review: 20 Years of Failure. Are We Any Closer to Registering a New Treatment? Front. Aging Neurosci. 2017, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute at the National Institutes of Health. NCI Dictionary of Cancer Terms. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/quality-of-life (accessed on 20 October 2019).
- Lee, M.K.; Baek, S.K.; Kim, S.Y.; Heo, D.S.; Yun, Y.H.; Park, S.R.; Kim, J.S. Awareness of incurable cancer status and health-related quality of life among advanced cancer patients: A prospective cohort study. Palliat. Med. 2013, 27, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Khamankar, N.; Coan, G.; Weaver, B.; Mitchell, C.S. Associative Increases in Amyotrophic Lateral Sclerosis Survival Duration With Non-invasive Ventilation Initiation and Usage Protocols. Front. Neurol. 2018, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Majmudar, S.; Wu, J.; Paganoni, S. Rehabilitation in amyotrophic lateral sclerosis: Why it matters. Muscle Nerve 2014, 50, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Earll, L.; Giles, M.; McClenahan, R.; Stevens, D.; Morrison, V. Mood as a predictor of disability and survival in patients newly diagnosed with ALS MND. Br. J. Health Psychol. 1999, 4, 127–136. [Google Scholar] [CrossRef]
- Van Groenestijn, A.C.; Kruitwagen-van Reenen, E.T.; Visser-Meily, J.M.; van den Berg, L.H.; Schroder, C.D. Associations between psychological factors and health-related quality of life and global quality of life in patients with ALS: A systematic review. Health Qual. Life Outcomes 2016, 14, 107. [Google Scholar] [CrossRef]
- Chio, A.; Logroscino, G.; Hardiman, O.; Swingler, R.; Mitchell, D.; Beghi, E.; Traynor, B.G.; Eurals, C. Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 2009, 10, 310–323. [Google Scholar] [CrossRef]
- Lou, J.S.; Moore, D.; Gordon, P.H.; Miller, R. Correlates of quality of life in ALS: Lessons from the minocycline study. Amyotroph. Lateral Scler. 2010, 11, 116–121. [Google Scholar] [CrossRef]
- Simmons, Z. Patient-Perceived Outcomes and Quality of Life in ALS. Neurotherapeutics 2015, 12, 394–402. [Google Scholar] [CrossRef]
- Bourke, S.C.; McColl, E.; Shaw, P.J.; Gibson, G.J. Validation of quality of life instruments in ALS. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2004, 5, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Nowinski, C.; Peterman, A.; Victorson, D.; Miller, D.; Lai, J.S.; Moy, C. The Neurology Quality-of-Life Measurement Initiative. Arch. Phys. Med. Rehabil. 2011, 92, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.; Hickey, A.; O’Boyle, C.; Hardiman, O. Assessing individual quality of life in amyotrophic lateral sclerosis. Qual. Life Res. 2001, 10, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Epton, J.; Harris, R.; Jenkinson, C. Quality of life in amyotrophic lateral sclerosis/motor neuron disease: A structured review. Amyotroph. Lateral Scler. 2009, 10, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Felgoise, S.H.; Stewart, J.L.; Bremer, B.A.; Walsh, S.M.; Bromberg, M.B.; Simmons, Z. The SEIQoL-DW for assessing quality of life in ALS: Strengths and limitations. Amyotroph. Lateral Scler. 2009, 10, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- Jenkinson, C.; Fitzpatrick, R.; Brennan, C.; Swash, M. Evidence for the validity and reliability of the ALS assessment questionnaire: The ALSAQ-40. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 1999, 1, 33–40. [Google Scholar] [CrossRef]
- O’Boyle, C.A. Quality of Life assessment: A paradigm shift in healthcare? Ir. J. Psychol. 1997, 18, 51–66. [Google Scholar] [CrossRef]
- Bond, L.; Ganguly, P.; Khamankar, N.; Mallet, N.; Bowen, G.; Green, B.; Mitchell, C.S. A Comprehensive Examination of Percutaneous Endoscopic Gastrostomy and Its Association with Amyotrophic Lateral Sclerosis Patient Outcomes. Brain Sci. 2019, 9, 223. [Google Scholar] [CrossRef]
- Carr, A.J.; Higginson, I.J. Measuring quality of life—Are quality of life measures patient centred? Br. Med. J. 2001, 322, 1357–1360. [Google Scholar] [CrossRef]
- Chio, A.; Gauthier, A.; Montuschi, A.; Calvo, A.; Di Vito, N.; Ghiglione, P.; Mutani, R. A cross sectional study on determinants of quality of life in ALS. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Leigh, P.N.; Swash, M.; Iwasaki, Y.; Ludolph, A.; Meininger, V.; Miller, R.G.; Mitsumoto, H.; Shaw, P.; Tashiro, K.; Van Den Berg, L. Amyotrophic lateral sclerosis: A consensus viewpoint on designing and implementing a clinical trial. Amyotroph. Lateral Scler. 2004, 5, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, H.; Del Bene, M. Improving the quality of life for people with ALS: The challenge ahead. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.A.; Simmons, Z.; Bremer, B.A.; Walsh, S.M.; Fischer, S. Quality of life in ALS is maintained as physical function declines. Neurology 2001, 56, 442–444. [Google Scholar] [CrossRef]
- Mundt, J.C.; Vogel, A.P.; Feltner, D.E.; Lenderking, W.R. Vocal acoustic biomarkers of depression severity and treatment response. Biol. Psychiatry 2012, 72, 580–587. [Google Scholar] [CrossRef]
- Williamson, J.R.; Quatieri, T.F.; Helfer, B.S.; Horwitz, R.; Yu, B.; Mehta, D.D. Vocal biomarkers of depression based on motor incoordination. In Proceedings of the 3rd ACM International Workshop on Audio/Visual Emotion Challenge, Barcelona, Spain, 21–25 October 2013; Association for Computing Machinery: New York, NY, USA, 2013; pp. 41–48. [Google Scholar]
- Kim, S.Y.; Kim, J.M.; Kim, S.W.; Shin, I.S.; Bae, K.Y.; Shim, H.J.; Hwang, J.E.; Bae, W.K.; Cho, S.H.; Chung, I.J.; et al. Does awareness of terminal status influence survival and quality of life in terminally ill cancer patients? Psychooncology 2013, 22, 2206–2213. [Google Scholar] [CrossRef]
- Averill, A.J.; Kasarskis, E.J.; Segerstrom, S.C. Psychological health in patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2007, 8, 243–254. [Google Scholar] [CrossRef]
- Cupp, J.; Simmons, Z.; Berg, A.; Felgoise, S.H.; Walsh, S.M.; Stephens, H.E. Psychological health in patients with ALS is maintained as physical function declines. Amyotroph. Lateral Scler. 2011, 12, 290–296. [Google Scholar] [CrossRef]
- Koschnitzky, J.E.; Quinlan, K.A.; Lukas, T.J.; Kajtaz, E.; Kocevar, E.J.; Mayers, W.F.; Siddique, T.; Heckman, C.J. Effect of fluoxetine on disease progression in a mouse model of ALS. J. Neurophysiol. 2014, 111, 2164–2176. [Google Scholar] [CrossRef]
- Peng, Q.; Masuda, N.; Jiang, M.; Li, Q.; Zhao, M.; Ross, C.A.; Duan, W. The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington’s disease mouse model. Exp. Neurol. 2008, 210, 154–163. [Google Scholar] [CrossRef]
- Wang, H.; Guan, Y.; Wang, X.; Smith, K.; Cormier, K.; Zhu, S.; Stavrovskaya, I.G.; Huo, C.; Ferrante, R.J.; Kristal, B.S.; et al. Nortriptyline delays disease onset in models of chronic neurodegeneration. Eur. J. Neurosci. 2007, 26, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Taler, M.; Miron, O.; Gil-Ad, I.; Weizman, A. Neuroprotective and procognitive effects of sertraline: In vitro and in vivo studies. Neurosci. Lett. 2013, 550, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Then, C.K.; Liu, K.H.; Liao, M.H.; Chung, K.H.; Wang, J.Y.; Shen, S.C. Antidepressants, sertraline and paroxetine, increase calcium influx and induce mitochondrial damage-mediated apoptosis of astrocytes. Oncotarget 2017, 8, 115490–115502. [Google Scholar] [CrossRef] [PubMed]
- Then, C.K.; Chi, N.F.; Chung, K.H.; Kuo, L.; Liu, K.H.; Hu, C.J.; Shen, S.C.; Lin, Y.K. Risk analysis of use of different classes of antidepressants on subsequent dementia: A nationwide cohort study in Taiwan. PLoS ONE 2017, 12, e0175187. [Google Scholar] [CrossRef]
- Hollinger, S.K.; Okosun, I.S.; Mitchell, C.S. Antecedent Disease and Amyotrophic Lateral Sclerosis: What Is Protecting Whom? Front. Neurol. 2016, 7, 47. [Google Scholar] [CrossRef]
- Mitchell, C.S.; Hollinger, S.K.; Goswami, S.D.; Polak, M.A.; Lee, R.H.; Glass, J.D. Antecedent Disease is Less Prevalent in Amyotrophic Lateral Sclerosis. Neurodegener. Dis. 2015, 15, 109–113. [Google Scholar] [CrossRef]
- Freedman, D.M.; Kuncl, R.W.; Cahoon, E.K.; Rivera, D.R.; Pfeiffer, R.M. Relationship of statins and other cholesterol-lowering medications and risk of amyotrophic lateral sclerosis in the US elderly. Amyotroph. Lateral. Scler Front. Degener. 2018, 19, 538–546. [Google Scholar] [CrossRef]
- Golomb, B.A.; Verden, A.; Messner, A.K.; Koslik, H.J.; Hoffman, K.B. Amyotrophic Lateral Sclerosis Associated with Statin Use: A Disproportionality Analysis of the FDA’s Adverse Event Reporting System. Drug Saf. 2018, 41, 403–413. [Google Scholar] [CrossRef]
- Clemens, K.E.; Klaschik, E. Morphine in the management of dyspnoea in ALS. A pilot study. Eur. J. Neurol. 2008, 15, 445–450. [Google Scholar] [CrossRef]
- Umegaki, H.; Tagami, N. Anesthetic management of an ALS patient with remifentanil. Masui 2008, 57, 1139–1142. [Google Scholar]
- Stephens, H.E.; Lehman, E.; Raheja, D.; Yang, C.; Walsh, S.; McArthur, D.B.; Simmons, Z. Pain in amyotrophic lateral sclerosis: Patient and physician perspectives and practices. Amyotroph. Lateral Scler. Front. Degener. 2015, 17, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.S.; Cates, A.; Kim, R.B.; Hollinger, S.K. Undergraduate Biocuration: Developing Tomorrow’s Researchers While Mining Today’s Data. J. Undergrad. Neurosci. Educ. 2015, 14, A56–A65. [Google Scholar] [PubMed]
- Pfohl, S.R.; Kim, R.B.; Coan, G.S.; Mitchell, C.S. Unraveling the Complexity of Amyotrophic Lateral Sclerosis Survival Prediction. Front. Neuroinform. 2018, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Strobl, C.; Boulesteix, A.L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinform. 2007, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Korner, S.; Kollewe, K.; Abdulla, S.; Zapf, A.; Dengler, R.; Petri, S. Interaction of physical function, quality of life and depression in Amyotrophic lateral sclerosis: Characterization of a large patient cohort. BMC Neurol. 2015, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Murrell, R. Quality of life and neurological illness: A review of the literature. Neuropsychol. Rev. 1999, 9, 209–229. [Google Scholar] [CrossRef] [PubMed]
- Lo Coco, G.; Lo Coco, D.; Cicero, V.; Oliveri, A.; Lo Verso, G.; Piccoli, F.; La Bella, V. Individual and health-related quality of life assessment in amyotrophic lateral sclerosis patients and their caregivers. J. Neurol. Sci. 2005, 238, 11–17. [Google Scholar] [CrossRef]
- Gauthier, A.; Vignola, A.; Calvo, A.; Cavallo, E.; Moglia, C.; Sellitti, L.; Mutani, R.; Chio, A. A longitudinal study on quality of life and depression in ALS patient-caregiver couples. Neurology 2007, 68, 923–926. [Google Scholar] [CrossRef]
- Bourke, S.C.; Shaw, P.J.; Gibson, G.J. Respiratory function vs sleep-disordered breathing as predictors of QOL in ALS. Neurology 2001, 57, 2040–2044. [Google Scholar] [CrossRef]
- Simmons, Z. Management strategies for patients with amyotrophic lateral sclerosis from diagnosis through death. Neurologist 2005, 11, 257–270. [Google Scholar] [CrossRef]
- Burkhardt, C.; Neuwirth, C.; Sommacal, A.; Andersen, P.M.; Weber, M. Is survival improved by the use of NIV and PEG in amyotrophic lateral sclerosis (ALS)? A post-mortem study of 80 ALS patients. PLoS ONE 2017, 12, e0177555. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.L.; Cunningham, J.I.; Eyerman, D.J.; Dean, R.L., 3rd; Deaver, D.R.; Sanchez, C. Opioid system modulators buprenorphine and samidorphan alter behavior and extracellular neurotransmitter concentrations in the Wistar Kyoto rat. Neuropharmacology 2019, 146, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Coan, G.; Mitchell, C.S. An Assessment of Possible Neuropathology and Clinical Relationships in 46 Sporadic Amyotrophic Lateral Sclerosis Patient Autopsies. Neurodegener. Dis. 2015, 15, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Thakore, N.J.; Pioro, E.P. Depression in ALS in a large self-reporting cohort Author Response. Neurology 2016, 87, 1631–1632. [Google Scholar] [CrossRef] [PubMed]
- Thakore, N.J.; Pioro, E.P. Depression in ALS in a large self-reporting cohort. Neurology 2016, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Handy, C.R.; Krudy, C.; Boulis, N.; Federici, T. Pain in amyotrophic lateral sclerosis: A neglected aspect of disease. Neurol. Res. Int. 2011, 2011, 403808. [Google Scholar] [CrossRef]
- Weishaupt, J.H.; Bartels, C.; Polking, E.; Dietrich, J.; Rohde, G.; Poeggeler, B.; Mertens, N.; Sperling, S.; Bohn, M.; Huther, G.; et al. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J. Pineal Res. 2006, 41, 313–323. [Google Scholar] [CrossRef]
- Bond, L.; Bernhardt, K.; Madria, P.; Sorrentino, K.; Scelsi, H.; Mitchell, C.S. A Metadata Analysis of Oxidative Stress Etiology in Preclinical Amyotrophic Lateral Sclerosis: Benefits of Antioxidant Therapy. Front. Neurosci. 2018, 12, 10. [Google Scholar] [CrossRef]
- De Carvalho, M.; Swash, M. Fasciculation potentials: A study of amyotrophic lateral sclerosis and other neurogenic disorders. Muscle Nerve 1998, 21, 336–344. [Google Scholar] [CrossRef]
- Shimizu, T.; Fujimaki, Y.; Nakatani-Enomoto, S.; Matsubara, S.; Watabe, K.; Rossini, P.M.; Ugawa, Y. Complex fasciculation potentials and survival in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2014, 125, 1059–1064. [Google Scholar] [CrossRef]
- Gonzalez, F. Diphenhydramine may be useful as a palliative treatment for patients dying with Parkinson’s disease and tremors: A case report and discussion. Am. J. Hosp. Palliat Care 2009, 26, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Kaneb, H.M.; Sharp, P.S.; Rahmani-Kondori, N.; Wells, D.J. Metformin treatment has no beneficial effect in a dose-response survival study in the SOD1(G93A) mouse model of ALS and is harmful in female mice. PLoS ONE 2011, 6, e24189. [Google Scholar] [CrossRef] [PubMed]
- Scelsa, S.N.; Khan, I. Blood pressure elevations in riluzole-treated patients with amyotrophic lateral sclerosis. Eur. Neurol. 2000, 43, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kato, S.; Hayashi, M.; Hayashi, H.; Tanabe, H. Amyotrophic lateral sclerosis with hypertensive attacks: Blood pressure changes in response to drug administration. Clin. Auton Res. 1996, 6, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Irvin, C.W.; Kim, R.B.; Mitchell, C.S. Seeking homeostasis: Temporal trends in respiration, oxidation, and calcium in SOD1 G93A Amyotrophic Lateral Sclerosis mice. Front. Cell Neurosci. 2015, 9, 248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herskovits, A.Z.; Hunter, T.A.; Maxwell, N.; Pereira, K.; Whittaker, C.A.; Valdez, G.; Guarente, L.P. SIRT1 deacetylase in aging-induced neuromuscular degeneration and amyotrophic lateral sclerosis. Aging Cell 2018, 17, e12839. [Google Scholar] [CrossRef]
- Tang, B.L. Could Sirtuin Activities Modify ALS Onset and Progression? Cell Mol. Neurobiol. 2017, 37, 1147–1160. [Google Scholar] [CrossRef]

| N = 1585 Patients | |
|---|---|
| Gender | N (%) |
| Male | 945 (59.62) |
| Female | 640 (40.38) |
| Race | |
| Caucasian | 923 (58.23) |
| African American | 196 (12.37) |
| Hispanic/Latino | 19 (1.20) |
| Asian | 17 (1.07) |
| Native American | 1 (0.06) |
| Mixed/Other | 12 (0.76) |
| Unspecified | 417 (26.31) |
| ALS Onset Type | |
| Limb | 1098 (69.27) |
| Bulbar | 428 (27.00) |
| Other/unclassifiable | 59 (3.72) |
| ALS Onset Age | |
| <55 years | 509 (32.11) |
| ≥55 years | 602 (67.89) |
| Category | Assessed Factors |
|---|---|
| Respiratory | forced vital capacity (FVC), percent predicted FVC (% predict), negative inspiratory force (NIF), oxygen saturation, ALSRFRS-R respiratory sub-score |
| Pain | general pain |
| Disability | disability present, ALRFRS-R total score, paraplegia, quadriplegia, hemiparesis |
| Muscle Control | head drop, jaw jerk, toe walk, atrophy, fasciculation |
| Oral Muscle Control | drooling, tongue atrophy, tongue fasciculation, dysphagia |
| Vocal Control | dysarthria, dysphasia |
| PEG tube | regular use of surgically inserted PEG tube for nutrition and/or hydration |
| Therapy | assistive device usage, cough assist usage, suction usage, Bi-PAP usage |
| QoL Medication | antidepressant usage, drooling medication usage, non-opioid pain usage, opioid pain usage, NSAID usage, sleeping medication usage, muscle-related medication usage |
| Depression | depression reported |
| Social | accompaniment to appointment, family or friend support, hospice care, issues in home reported, reported changes in behavior |
| Sleep | reported sleeping problems |
| Factor | N | p-Value | Relationship to CIM |
|---|---|---|---|
| Cough assist | 1484 | ** | Users (+) = (↓CIM) |
| Bi-pap usage | 1979 | ** | Users (+) = (↓CIM) |
| Jaw jerk | 2112 | ** | ↑jaw jerk = (↑CIM) |
| Toe walk | 142 | * | ↑toe walk = (↓CIM) |
| Dysphagia | 840 | * | dysphagia (+) = (↑CIM) |
| Drooling | 2702 | * | drooling (+) = (↑CIM) |
| General pain | 671 | ** | pain (+) = (↑CIM) |
| No family reported | 4175 | * | No family reported (+) = (↑CIM) |
| ALSFRS-R total | 848 | * | ↑ALSFRS-R = (↓CIM) |
| FVC percent predict | 1300 | ** | ↑FVC %predict = (↓CIM) |
| Forced vital capacity (FVC) | 1272 | ** | ↑FVC = (↓CIM) |
| Category | F User Ratio | M User Ratio | Gender p-Value | Bulbar User Ratio | Limb User Ratio | User Onset p-Value | User Age (yrs) | Age p-Value | Short Dur Ratio | Long Dur Ratio | Surv Dur p-Value | △ Surv (mo.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| alternative herb med | 0.14 | 0.17 | 0.17 | 0.15 | 59.4 | 0.11 | 0.21 | ** | 9.7 | |||
| vitamin a | 0.31 | 0.29 | 0.25 | 0.32 | * | 60.1 | 0.22 | 0.33 | ** | 8.9 | ||
| multivitamin | 0.23 | 0.22 | 0.20 | 0.24 | 60.3 | 0.18 | 0.28 | ** | 8.2 | |||
| muscle relaxant | 0.13 | 0.10 | * | 0.06 | 0.13 | ** | 56.0 | ** | 0.07 | 0.13 | * | 13.4 |
| antispastic | 0.23 | 0.21 | 0.16 | 0.24 | ** | 54.5 | ** | 0.17 | 0.25 | * | 16.5 | |
| vitamin | 0.38 | 0.34 | * | 0.31 | 0.38 | * | 60.4 | 0.29 | 0.39 | * | 6.2 | |
| stimulant | 0.45 | 0.38 | ** | 0.40 | 0.42 | 59.6 | 0.37 | 0.46 | * | 7.7 | ||
| sedative | 0.09 | 0.08 | 0.08 | 0.09 | 56.4 | ** | 0.07 | 0.12 | * | 4.6 | ||
| stimulant laxative | 0.45 | 0.37 | ** | 0.40 | 0.42 | 59.7 | 0.36 | 0.45 | * | 7.1 | ||
| antihistamine | 0.23 | 0.18 | * | 0.20 | 0.20 | 59.7 | 0.17 | 0.24 | * | 8.4 | ||
| vitamin e | 0.07 | 0.08 | 0.07 | 0.09 | 57.2 | * | 0.07 | 0.11 | * | 12.4 | ||
| nutritional supplement | 0.41 | 0.37 | * | 0.39 | 0.40 | 59.4 | * | 0.32 | 0.40 | * | 9.7 | |
| tricyclic anti-depressant | 0.09 | 0.07 | 0.10 | 0.07 | 56.8 | * | 0.07 | 0.11 | * | 16.4 | ||
| non-opioid | 0.14 | 0.10 | * | 0.10 | 0.13 | 59.3 | 0.09 | 0.14 | * | 15.3 | ||
| anticonvulsant | 0.15 | 0.12 | 0.11 | 0.14 | 58.8 | 0.10 | 0.14 | * | 8.7 | |||
| general sleep | 0.22 | 0.19 | 0.21 | 0.20 | 60.7 | 0.16 | 0.21 | * | 4.9 | |||
| anticholinergic | 0.15 | 0.11 | * | 0.27 | 0.07 | ** | 61.3 | 0.12 | 0.16 | * | 6.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bond, L.; Bowen, G.; Mertens, B.; Denson, K.; Jordan, K.; Vidakovic, B.; Mitchell, C.S. Associations of Patient Mood, Modulators of Quality of Life, and Pharmaceuticals with Amyotrophic Lateral Sclerosis Survival Duration. Behav. Sci. 2020, 10, 33. https://doi.org/10.3390/bs10010033
Bond L, Bowen G, Mertens B, Denson K, Jordan K, Vidakovic B, Mitchell CS. Associations of Patient Mood, Modulators of Quality of Life, and Pharmaceuticals with Amyotrophic Lateral Sclerosis Survival Duration. Behavioral Sciences. 2020; 10(1):33. https://doi.org/10.3390/bs10010033
Chicago/Turabian StyleBond, Leila, Gloria Bowen, Benjamin Mertens, Keelie Denson, Kathleen Jordan, Branislav Vidakovic, and Cassie S. Mitchell. 2020. "Associations of Patient Mood, Modulators of Quality of Life, and Pharmaceuticals with Amyotrophic Lateral Sclerosis Survival Duration" Behavioral Sciences 10, no. 1: 33. https://doi.org/10.3390/bs10010033
APA StyleBond, L., Bowen, G., Mertens, B., Denson, K., Jordan, K., Vidakovic, B., & Mitchell, C. S. (2020). Associations of Patient Mood, Modulators of Quality of Life, and Pharmaceuticals with Amyotrophic Lateral Sclerosis Survival Duration. Behavioral Sciences, 10(1), 33. https://doi.org/10.3390/bs10010033




