Measuring Biases of Visual Attention: A Comparison of Four Tasks
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Participants
2.3. Clinician Administered Interviews
2.4. Self-Report Measures
2.5. Administration of Clinical Tests and Interviews
2.6. Equipment
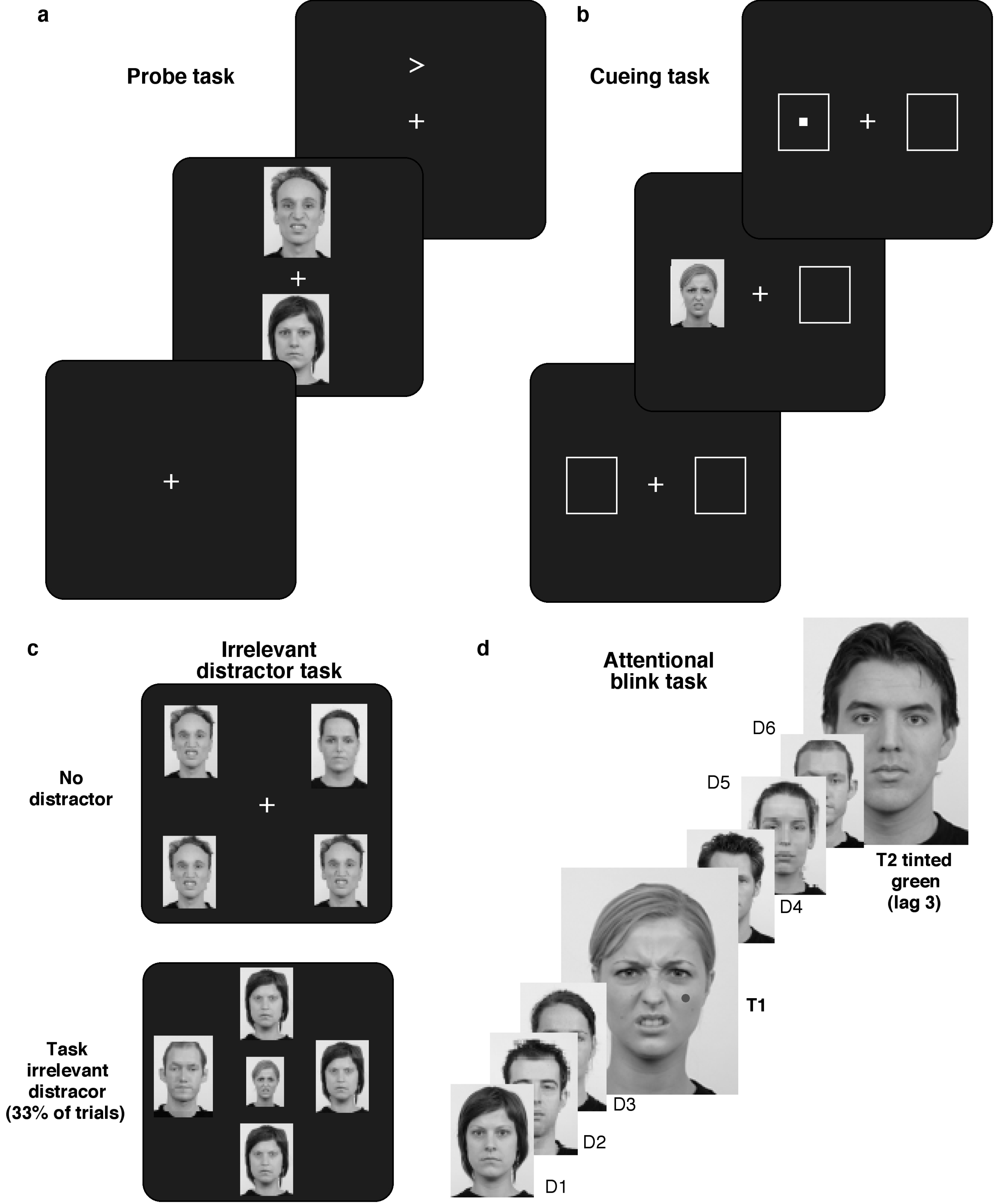
2.7. Visual Attention Tasks
2.8. Procedure
2.9. Data Analyses
- SAD model: Sensitivity to differences in attentional processing of threatening and neutral faces in the SAD group
- Control model: Sensitivity to differences in attentional processing of threatening and neutral faces in the control group
- Between-group model: Sensitivity to differences in anxious (SAD) and healthy (control) attentional processing of threatening and neutral faces.
3. Results
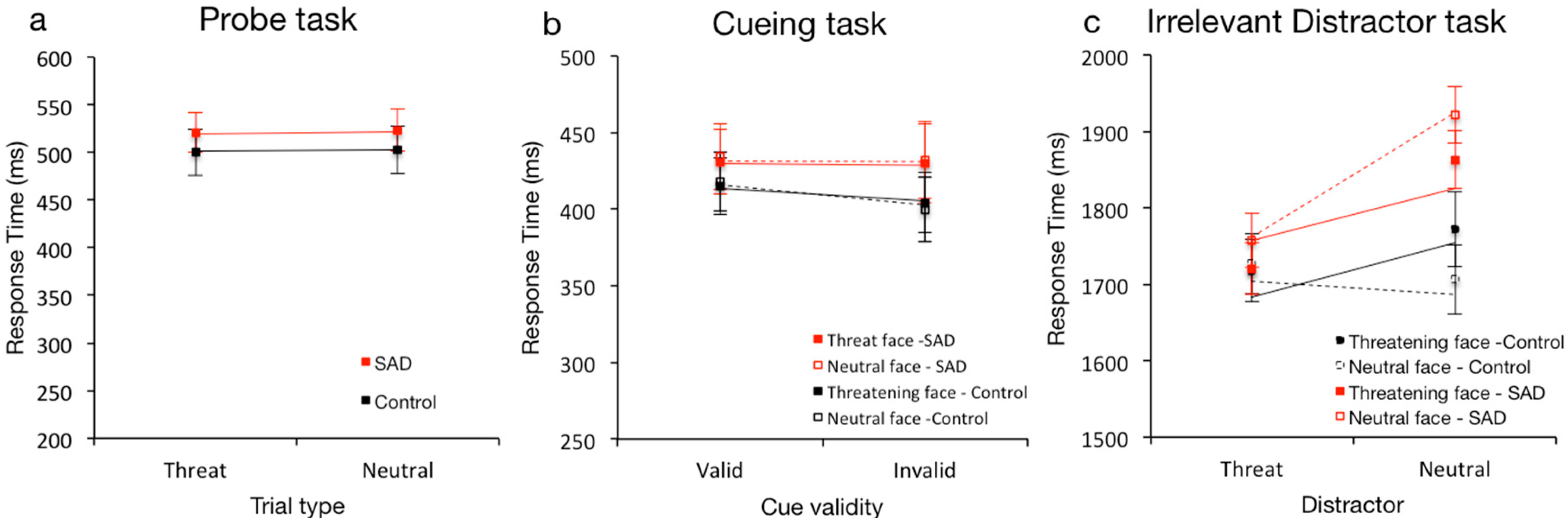
3.1. Differences in Attentional Processing of Threatening and Neutral Faces
3.2. Differences in Anxious (SAD) and Healthy (Control) Attentional Processing Patterns of Threatening and Neutral Faces
4. Discussion
5. Conclusions
6. Supplementary Information
6.1. Clinician Administered Interviews
6.2. Self-Report Measures
6.3. Administration of Clinical Tests and Interviews
Author Contributions
Funding
Conflicts of Interest
References
- Jonides, J.; Yantis, S. Uniqueness of abrupt visual onset in capturing attention. Percept. Psychophys. 1988, 43, 346–354. [Google Scholar] [CrossRef]
- Eastwood, J.D.; Smilek, D.; Merikle, P.M. Differential attentional guidance by unattended faces expressing positive and negative emotion. Percept. Psychophys. 2001, 63, 1004–1013. [Google Scholar] [CrossRef]
- Kristjánsson, Á.; Óladóttir, B.; Most, S.B. “Hot” facilitation of “cool” processing: Emotional distraction can enhance priming of visual search. J. Exp. Psychol. Hum. Percept. Perform. 2013, 39, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstaele, B.; Verschuere, B.; Tibboel, H.; De Houwer, J.; Crombez, G.; Koster, E.H. A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychol. Bull. 2014, 140, 682. [Google Scholar] [CrossRef] [PubMed]
- Vuilleumier, P.; Schwartz, S. Emotional facial expressions capture attention. Neurology 2001, 56, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Bar-Haim, Y.; Lamy, D.; Pergamin, L.; Bakermans-Kranenburg, M.J.; van Ijzendoorn, M.H. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol. Bull. 2007, 133, 1–24. [Google Scholar] [CrossRef]
- Clark, D.M.; Wells, A. A Cognitive model of social phobia. In Social Phobia: Diagnosis, Assessment, and Treatment; Heimberg, R.G., Liebowitz, M.R., Hope, D.A.O., Schneier, F.R., Eds.; Guilford Press: New York, NY, USA, 1995; pp. 69–79. [Google Scholar]
- Mathews, A.; MacLeod, C. Selective processing of threat cues in anxiety states. Behav. Res. Ther. 1985, 23, 563–569. [Google Scholar] [CrossRef]
- Sigurjónsdóttir, Ó.; Sigurðardóttir, S.; Björnsson, A.S.; Kristjánsson, Á. Barking up the wrong tree in attentional bias modification? Comparing the sensitivity of four tasks to attentional biases. J. Behav. Ther. Exp. Psychiatry 2015, 48, 9–16. [Google Scholar] [CrossRef]
- Öhman, A.; Flykt, A.; Esteves, F. Emotion drives attention: Detecting the snake in the grass. J. Exp. Psychol. Gen. 2001, 130, 466. [Google Scholar] [CrossRef]
- Amir, N.; Beard, C.; Taylor, C.T.; Klumpp, H.; Elias, J.; Burns, M.; Chen, X. Attention training in individuals with generalized social phobia: A randomized controlled trial. J. Consult. Clin. Psychol. 2009, 77, 961–973. [Google Scholar] [CrossRef]
- Bar-Haim, Y. Research review: Attention bias modification (ABM): A novel treatment for anxiety disorders. J. Child Psychol. Psychiatry 2010, 51, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.; Sawyer, A.T.; Hofmann, S.G. Efficacy of attentional bias modification using threat and appetitive stimuli: A meta-analytic review. Behav. Ther. 2012, 43, 724–740. [Google Scholar] [CrossRef] [PubMed]
- Sigurjónsdóttir, Ó.; Björnsson, A.S.; Ludvigsdottir, S.J.; Kristjánsson, Á. Money talks in attention bias modification: Reward in a dot-probe task affects attentional biases. Vis. Cogn. 2015, 23, 118–132. [Google Scholar]
- MacLeod, C.; Mathews, A.; Tata, P. Attentional bias in emotional disorders. J. Abnorm. Psychol. 1986, 95, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kristjánsson, Á.; Mackeben, M.; Nakayama, K. Rapid, object based learning in the deployment of transient attention. Perception 2001, 20, 1375–1387. [Google Scholar]
- Posner, M.I. Orienting of attention. Q. J. Exp. Psychol. 1980, 32, 3–25. [Google Scholar] [CrossRef]
- Schmuckle, S.C. Unreliability of the dot probe task. Eur. J. Personal. 2005, 19, 595–605. [Google Scholar] [CrossRef]
- Kristjánsson, Á.; Nakayama, K. The attentional blink in space and time. Vis. Res. 2002, 42, 2039–2050. [Google Scholar] [CrossRef][Green Version]
- Raymond, J.E.; Shapiro, K.L.; Arnell, K.M. Temporary suppression of visual processing in an RSVP task: An attentional blink? J. Exp. Psychol. Hum. Percept. Perform. 1992, 18, 849–860. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Janavs, J.; Weiller, E.; Keskiner, A.; Dunbar, G.C. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur. Psychiatry 1997, 12, 232–241. [Google Scholar] [CrossRef]
- Lecrubier, Y.; Sheehan, D.V.; Weiller, E.; Amorim, P.; Bonora, I.; Sheehan, K.H.; Dunbar, G.C. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. Eur. Psychiatry 1997, 12, 224–231. [Google Scholar] [CrossRef]
- Sigurðsson, B.H. Samanburður á Tveimur Stöðluðum Greiningarviðtölum og Tveimur Sjálfsmatskvörðum: MINI, CIDI, PHQ og DASS. Bachelor’s Thesis, University of Iceland, Reykjavík, Iceland, 2008. Unpublished work. [Google Scholar]
- Phillips, K.A. The Broken Mirror: Understanding and Treating Body Dysmorphic Disorder (Revised and Expanded Edition); Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Liebowitz, M.R. Social phobia. Mod. Probl. Pharm. 1987, 22, 141–173. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.C. Measurement of quality of life: Current state of the art. Arch. Phys. Med. Rehabil. 1982, 63, 56–59. [Google Scholar] [PubMed]
- Hrafnsson, Ó.; Guðmundsson, M. Psychometric Properties of the Icelandic Version of the Quality of Life Scale (QOLS). Bachelor’s Thesis, University of Iceland, Reykjavík, Iceland, 2007. Unpublished work. [Google Scholar]
- Langner, O.; Dotsch, R.; Bijlstra, G.; Wigboldus, D.H.J.; Hawk, S.T.; van Knippenberg, A. Presentation and validation of the Radboud Faces Database. Cogn. Emot. 2010, 24, 1377–1388. [Google Scholar] [CrossRef]
- Magezi, D.A. Linear mixed-effects models for within-participant psychology experiments: An introductory tutorial and free, graphical user interface (LMMgui). Front. Psychol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Koster, E.H.; Crombez, G.; Verschuere, B.; De Houwer, J. Selective attention to threat in the dot probe paradigm: Differentiating vigilance and difficulty to disengage. Behav. Res. Ther. 2004, 42, 1183–1192. [Google Scholar] [CrossRef]
- Salemink, E.; van den Hout, M.A.; Kindt, M. Selective attention and threat: Quick orienting versus slow disengagement and two versions of the dot probe task. Behav. Res. Ther. 2007, 45, 607–615. [Google Scholar] [CrossRef]
- Cisler, J.M.; Koster, E.H. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clin. Psychol. Rev. 2010, 30, 203–216. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]

| SAD | Control | t/Chi-square (df) | p | |
|---|---|---|---|---|
| Age M (SD) | 27.97 (11.73) | 28.88 (9.82) | −0.319 (57) | 0.751 |
| Gender (% male) | 45.5% | 42.3% | 0.058 (1) | 0.809 |
| Education (% >junior college) | 39.4% | 69.2% | 5.192 (1) | 0.023 ** |
| LSAS a | 83.27 (20.92) | 14.35 (11.06) | 15.19 (57) | 0.000 *** |
| PHQ-9 b | 10.13 (6.43) | 2.12 (2.17) | 6.06 (54) | 0.000 *** |
| QOLS c | 67.03 (10.85) | 91.27 (10.76) | −8.43 (55) | 0.000 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigurjónsdóttir, Ó.; Bjornsson, A.S.; Wessmann, I.D.; Kristjánsson, Á. Measuring Biases of Visual Attention: A Comparison of Four Tasks. Behav. Sci. 2020, 10, 28. https://doi.org/10.3390/bs10010028
Sigurjónsdóttir Ó, Bjornsson AS, Wessmann ID, Kristjánsson Á. Measuring Biases of Visual Attention: A Comparison of Four Tasks. Behavioral Sciences. 2020; 10(1):28. https://doi.org/10.3390/bs10010028
Chicago/Turabian StyleSigurjónsdóttir, Ólafía, Andri S. Bjornsson, Inga D. Wessmann, and Árni Kristjánsson. 2020. "Measuring Biases of Visual Attention: A Comparison of Four Tasks" Behavioral Sciences 10, no. 1: 28. https://doi.org/10.3390/bs10010028
APA StyleSigurjónsdóttir, Ó., Bjornsson, A. S., Wessmann, I. D., & Kristjánsson, Á. (2020). Measuring Biases of Visual Attention: A Comparison of Four Tasks. Behavioral Sciences, 10(1), 28. https://doi.org/10.3390/bs10010028




