Paracellular Filtration Secretion Driven by Mechanical Force Contributes to Small Intestinal Fluid Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Epithelial Monolayers Using Freshly Harvested Mouse Enterocytes
2.2. Culture of Caco-2 and T84 Cells and Fibroblasts
2.3. Measurement of Fluid Flow across the Epithelia
2.4. Preparation of Sacs from Intact Mouse Small Intestine for Measurement of Fluid Flow
2.5. Rat Intestine Mounted in the Ussing Chamber System
2.6. Statistics
3. Results
3.1. Study 1. Epithelia of Mouse Enterocytes Establish Hydrostatic Pressure Gradients when Exposed to Vigorous Aeration
3.2. Findings
3.3. Study 2. The Movement of Fluid through Epithelia Is Unidirectional
3.4. Findings
3.5. Study 3. Tight Junctions Are Required for the Paracellular Movement of Fluid
3.6. Findings
3.7. Study 4. Aeration Drives Fluid Flow Independent of Ion Movements or Gradients
3.8. Findings
3.9. Study 5. Fluid Flow Driven by Mechanical Force Is Not Directly Dependent on Cellular Metabolism
3.10. Findings
3.11. Study 6. Zinc Inhibits Fluid Flow by Epithelia
3.12. Findings
3.13. Study 7. Agitating Sacs of Mouse Small Intestine Causes Fluid Flow
3.14. Findings
3.15. Study 8. Fluid Flow by Intact Rat Small Intestine Mounted in Ussing Chambers
3.16. Findings
4. Discussion
4.1. The Evidence for Ion-Coupled Fluid Secretion
4.2. Fluid Secretion Can Be Independent of Ion Secretion
4.3. Mechanical Force Contributes to Small Intestinal Fluid Secretion
“…some of us, who had stayed longer on the (vibrating) platform, felt an unspeakable and pressing necessity which had to be promptly satisfied.”(Nicolas Tesla and Mechanical Therapy, 1896).
4.4. A Combination of Ion Secretion and Mechanical Force Contribute to Small Intestinal Fluid Secretion
4.5. The Role of the Tight Junction in Unidirectional Fluid Flow
4.6. Zinc, the Tight Junction, and Fluid Flow
4.7. A New Model of High-Volume Intestinal Fluid Secretion Driven by Mechanical Force
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | cystic fibrosis |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| FITC-dextran | fluorescein isothiocyanate conjugated with dextran |
References
- Adelman, J.L.; Sheng, Y.; Choe, S.; Abramson, J.; Wright, E.M.; Rosenberg, J.M.; Grabe, M. Structural Determinants of Water Permeation through the Sodium-Galactose Transporter vSGLT. Biophys. J. 2014, 106, 1280–1289. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Murtazina, R.; Cha, B.; Chakraborty, M.; Sarker, R.; Chen, T.; Lin, Z.; Hogema, B.M.; De Jonge, H.R.; Seidler, U.; et al. D-Glucose Acts via Sodium/Glucose Cotransporter 1 to Increase NHE3 in Mouse Jejunal Brush Border by a Na+/H+ Exchange Regulatory Factor 2–Dependent Process. Gastroenterology 2011, 140, 560–571. [Google Scholar] [CrossRef] [Green Version]
- Leiper, J.B. Fate of ingested fluids: Factors affecting gastric emptying and intestinal absorption of beverages in humans. Nutr. Rev. 2015, 73 (Suppl. 2), 57–72. [Google Scholar] [CrossRef] [Green Version]
- Frizzell, R.A.; Hanrahan, J.W. Physiology of Epithelial Chloride and Fluid Secretion. Cold Spring Harb. Perspect. Med. 2012, 2, a009563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murek, M.; Kopic, S.; Geibel, J. Evidence for intestinal chloride secretion. Exp. Physiol. 2010, 95, 471–478. [Google Scholar] [CrossRef]
- Lucas, M. An alternative explanation for the occurrence of short circuit current increases in the small intestine following challenge by bacterial enterotoxins. Med. Hypotheses 2013, 81, 601–606. [Google Scholar] [CrossRef]
- Lucas, M. Diarrhoeal disease through enterocyte secretion: A doctrine untroubled by proof. Exp. Physiol. 2010, 95, 479–484. [Google Scholar] [CrossRef]
- Masyuk, A.I.; Marinelli, R.A.; LaRusso, N.F. Water transport by epithelia of the digestive tract. Gastroenterology 2002, 122, 545–562. [Google Scholar] [CrossRef]
- Hakim, A.; Lifson, N. Effects of pressure on water and solute transport by dog intestinal mucosa in vitro. Am. J. Physiol. Content 1969, 216, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Yablonski, M.E.; Lifson, N. Mechanism of production of intestinal secretion by elevated venous pressure. J. Clin. Investig. 1976, 57, 904–915. [Google Scholar] [CrossRef] [Green Version]
- Hakim, A.A.; Papeleux, C.B.; Lane, J.B.; Lifson, N.; Yablonski, M.E. Mechanism of production of intestinal secretion by negative luminal pressure. Am. J. Physiol. Metab. 1977, 233, E416. [Google Scholar] [CrossRef]
- Hemlin, M.; Sjövall, H. Evidence for sympathetic regulation of the hydraulic conductance of rat jejunal mucosa in vivo. Acta Physiol. Scand. 1989, 135, 459–467. [Google Scholar] [CrossRef]
- Lee, M.K.; Coupar, I.M. Inhibition by morphine of dibutyryl cyclic AMP-induced fluid secretion from the rat jejunum. J. Pharm. Pharmacol. 1983, 35, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Gayer, C.P.; Basson, M.D. The effects of mechanical forces on intestinal physiology and pathology. Cell. Signal. 2009, 21, 1237–1244. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, B.; Davison, J.S. The relationship between gastrointestinal motility and secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 1987, 252, G1–G7. [Google Scholar] [CrossRef]
- Kimura, Y.; Van Der Merwe, M.; Bering, S.B.; Penmatsa, H.; Conoley, V.G.; Vegge, A.; Naren, A.P.; Buddington, R.K. Glucose transport by epithelia prepared from harvested enterocytes. Cytotechnology 2013, 67, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Tani, K.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Model for the Architecture of Claudin-Based Paracellular Ion Channels through Tight Junctions. J. Mol. Biol. 2015, 427, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Jakab, R.L.; Collaco, A.M.; Ameen, N. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G82–G98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buccigrossi, V.; Laudiero, G.; Russo, C.; Miele, E.; Sofia, M.; Monini, M.; Ruggeri, F.M.; Guarino, A. Chloride Secretion Induced by Rotavirus Is Oxidative Stress-Dependent and Inhibited by Saccharomyces boulardii in Human Enterocytes. PLoS ONE 2014, 9, e99830. [Google Scholar] [CrossRef] [Green Version]
- Pusch, M.; Zifarelli, G. Thermal Sensitivity of CLC and TMEM16 Chloride Channels and Transporters. Curr. Top. Membr. 2014, 74, 213–231. [Google Scholar] [CrossRef]
- Buzzi, N.; Bilbao, P.S.; Boland, R.; De Boland, A.R. Extracellular ATP activates MAP kinase cascades through a P2Y purinergic receptor in the human intestinal Caco-2 cell line. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 1651–1659. [Google Scholar] [CrossRef]
- Pa, C.; Verga, M.-E.; Kouame, K.S.A.; Pittet, A.; Rey-Bellet, C.G.; Fontaine, O.; Di Paolo, E.R.; Gehri, M. Demonstration of the effectiveness of zinc in diarrhoea of children living in Switzerland. Eur. J. Pediatr. 2015, 174, 1061–1067. [Google Scholar] [CrossRef]
- Gefeller, E.M.; Martens, H.; Aschenbach, J.R.; Klingspor, S.; Twardziok, S.; Wrede, P.; Pieper, R.; Lodemann, U. Effects of age and zinc supplementation on transport properties in the jejunum of piglets. J. Anim. Physiol. Anim. Nutr. 2015, 99, 542–552. [Google Scholar] [CrossRef]
- Patel, A. Zinc for Acute Diarrhea and Amoxicillin for Pneumonia, Do They Work? Delivered at the AIIMS, IJP Excellence Award for the year 2013 on 7th September 2014. Indian J. Pediatr. 2015, 82, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, M.Y.; Theodoratou, E.; Jabeen, A.; Imdad, A.; Eisele, T.P.; Ferguson, J.; Jhass, A.; Rudan, I.; Campbell, H.; Black, R.E.; et al. Preventive zinc supplementation in developing countries: Impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011, 11 (Suppl. 3), S23. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs330/en/ (accessed on 10 March 2018).
- Canani, R.B.; Secondo, A.; Passariello, A.; Buccigrossi, V.; Canzoniero, L.M.; Ruotolo, S.; Puzone, C.; Porcaro, F.; Pensa, M.; Braucci, A.; et al. Zinc inhibits calcium-mediated and nitric oxide-mediated ion secretion in human enterocytes. Eur. J. Pharmacol. 2010, 626, 266–270. [Google Scholar] [CrossRef]
- Bzik, V.A.; Medani, M.; Baird, A.W.; Winter, D.C.; Brayden, D.J. Mechanisms of action of zinc on rat intestinal epithelial electrogenic ion secretion: Insights into its antidiarrhoeal actions. J. Pharm. Pharmacol. 2012, 64, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.; Sehested, J.; Feng, Z.; Poulsen, H. Serosal zinc attenuate serotonin and vasoactive intestinal peptide induced secretion in piglet small intestinal epithelium in vitro. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 149, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hoque, K.M.; Rajendran, V.M.; Binder, H.J. Zinc inhibits cAMP-stimulated Cl secretion via basolateral K-channel blockade in rat ileum. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G956–G963. [Google Scholar] [CrossRef] [Green Version]
- Medani, M.; Bzik, V.A.; Rogers, A.C.; Collins, D.; Kennelly, R.; Winter, D.C.; Brayden, D.J.; Baird, A.W. Zinc sulphate attenuates chloride secretion in Human colonic mucosae in vitro. Eur. J. Pharmacol. 2012, 696, 166–171. [Google Scholar] [CrossRef]
- Valenzano, M.C.; DiGuilio, K.; Mercado, J.; Teter, M.; To, J.; Ferraro, B.; Mixson, B.; Manley, I.; Baker, V.; Moore, B.A.; et al. Remodeling of Tight Junctions and Enhancement of Barrier Integrity of the CACO-2 Intestinal Epithelial Cell Layer by Micronutrients. PLoS ONE 2015, 10, e0133926. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Valenzano, M.C.; Mercado, J.M.; Zurbach, E.P.; Mullin, J.M. Zinc Supplementation Modifies Tight Junctions and Alters Barrier Function of CACO-2 Human Intestinal Epithelial Layers. Dig. Dis. Sci. 2012, 58, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Lundin, P.D.; Weström, B.R.; Pantzar, N.; Karlsson, B.W. Bidirectional small intestinal permeability changes to different-sized mol-ecules after HCl-induced injury in the rat. Dig. Dis. Sci. 1997, 42, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Zhang, W.; Thomas, H.G.; Barish, A.O.; Berry, S.; Kiel, J.S.; Naren, A.P. A Tannic Acid-Based Medical Food, Cesinex®, Exhibits Broad-Spectrum Antidiarrheal Properties: A Mechanistic and Clinical Study. Dig. Dis. Sci. 2011, 57, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, S.E.; Brigman, K.N.; Koller, B.H.; Boucher, R.C.; Stutts, M.J. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 1994, 266, 107–109. [Google Scholar] [CrossRef]
- Schwank, G.; Koo, B.-K.; Sasselli, V.; Dekkers, J.F.; Heo, I.; Demircan, T.; Sasaki, N.; Boymans, S.; Cuppen, E.; Van Der Ent, C.K.; et al. Functional Repair of CFTR by CRISPR/Cas9 in Intestinal Stem Cell Organoids of Cystic Fibrosis Patients. Cell Stem Cell 2013, 13, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Yang, J.; Chen, T.E.; Zachos, N.C.; Kovbasnjuk, O.; Verkman, A.S.; Donowitz, M. Translating molecular physiology of intes-tinal transport into pharmacologic treatment of diarrhea: Stimulation of Na+ absorption. Clin. Gastroenterol. Hepatol. 2014, 12, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Thiagarajah, J.R.; Ko, E.A.; Tradtrantip, L.; Donowitz, M.; Verkman, A.S. Discovery and development of antisecretory drugs for treating diarrheal diseases. Clin. Gastroenterol. Hepatol. 2014, 12, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Donowitz, M.; Keusch, G.T.; Binder, H.J. Effect of Shigella Enterotoxin on Electrolyte Transport in Rabbit Ileum. Gastroenterology 1975, 69, 1230–1237. [Google Scholar] [CrossRef]
- Kandel, G.; Donohue-Rolfe, A.; Donowitz, M.; Keusch, G.T. Pathogenesis of Shigella diarrhea. XVI. Selective targetting of Shiga toxin to villus cells of rabbit jejunum explains the effect of the toxin on intestinal electrolyte transport. J. Clin. Investig. 1989, 84, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Andersen, Y.S.; Gillin, F.D.; Eckmann, L. Adaptive Immunity-Dependent Intestinal Hypermotility Contributes to Host Defense against Giardia spp. Infect. Immun. 2006, 74, 2473–2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.-J.; Wei, T.-S.; Chou, Y.-H.; Yang, C.-P.; Wu, C.-L.; Chen, Y.-C.; Liu, S.-Y. Whole-body vibration for functional constipation: A single-centre, single-blinded, randomized controlled trial. Color. Dis. 2012, 14, e779–e785. [Google Scholar] [CrossRef] [PubMed]
- Ron, Y.; Halpern, Z.; Safadi, R.; Dickman, R.; Dekel, R.; Sperber, A.D. Safety and efficacy of the vibrating capsule, an innovative non-pharmacological treatment modality for chronic constipation. Neurogastroenterol. Motil. 2015, 27, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.D.; Camilleri, M.; Acosta, A.; Boldingh, A.; Busciglio, I.; Burton, D.; Ryks, M.; Zinsmeister, A.R. A single-center, prospective, double-blind, sham-controlled, randomized study of the effect of a vibrating capsule on colonic transit in patients with chronic constipation. Neurogastroenterol. Motil. 2017, 29, e13034. [Google Scholar] [CrossRef]
- Stuempfle, K.J.; Hoffman, M.D. Gastrointestinal distress is common during a 161-km ultramarathon. J. Sports Sci. 2015, 33, 1–8. [Google Scholar] [CrossRef]
- De Oliveira, E.P.; Burini, R.C. The impact of physical exercise on the gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care. 2009, 12, 533–538. [Google Scholar] [CrossRef]
- Lucas, M.L.; Morrison, J.D. An investigation into the relationship between small intestinal fluid secretion and systemic arterial blood pressure in the anesthetized rat. Physiol. Rep. 2015, 3, e12407. [Google Scholar] [CrossRef]
- De Luca, A.; Coupar, I.M. Insights into opioid action in the intestinal tract. Pharmacol. Ther. 1996, 69, 103–115. [Google Scholar] [CrossRef]
- Saraví, F.D.; Cincunegui, L.M.; Saldeña, T.A.; Carra, G.E.; Ibáñez, J.E. Increased oxygen consumption caused by cAMP- and Ca(2+)-mediated chloride secretion in rat distal colon. Acta Gastroenterol. Latinoam. 2005, 35, 13–18. [Google Scholar]
- Corinaldesi, R.; Stanghellini, V.; Barbara, G.; Tomassetti, P.; De Giorgio, R. Clinical approach to diarrhea. Intern. Emerg. Med. 2012, 7 (Suppl. 3), 255–262. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Ko, E.-A.; Verkman, A.S. Antidiarrheal Efficacy and Cellular Mechanisms of a Thai Herbal Remedy. PLoS Negl. Trop. Dis. 2014, 8, e2674. [Google Scholar] [CrossRef]
- Vandebrouck, C.; Melin, P.; Norez, C.; Robert, R.; Guibert, C.; Mettey, Y.; Becq, F. Evidence that CFTR is expressed in rat tracheal smooth muscle cells and contributes to bronchodilation. Respir. Res. 2006, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- De Lisle, R.C.; Meldi, L.; Mueller, R. Intestinal smooth muscle dysfunction develops postnatally in cystic fibrosis mice. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 689–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risse, P.-A.; Kachmar, L.; Matusovsky, O.S.; Novali, M.; Gil, F.R.; Javeshghani, S.; Keary, R.; Haston, C.K.; Michoud, M.-C.; Martin, J.G.; et al. Ileal smooth muscle dysfunction and remodeling in cystic fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1–G8. [Google Scholar] [CrossRef] [Green Version]
- Kelly, T.; Buxbaum, J. Gastrointestinal Manifestations of Cystic Fibrosis. Dig. Dis. Sci. 2015, 60, 1903–1913. [Google Scholar] [CrossRef]
- Borowitz, D.; Gelfond, D. Intestinal complications of cystic fibrosis. Curr. Opin. Pulm. Med. 2013, 19, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Beyder, A.; Farrugia, G. Ion channelopathies in functional GI disorders. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G581–G586. [Google Scholar] [CrossRef] [Green Version]
- Fung, C.; Ellis, M.; Bornstein, J.C. Luminal Cholera Toxin Alters Motility in Isolated Guinea-Pig Jejunum via a Pathway Inde-pendent of 5-HT(3) Receptors. Front. Neurosci. 2010, 4, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialowas, S.; Hagbom, M.; Nordgren, J.; Karlsson, T.; Sharma, S.; Magnusson, K.-E.; Svensson, L. Rotavirus and Serotonin Cross-Talk in Diarrhoea. PLoS ONE 2016, 11, e0159660. [Google Scholar] [CrossRef] [Green Version]
- Spiller, R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: Alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol. Motil. 2007, 19, 25–31. [Google Scholar] [CrossRef]
- Kellum, J.M.; Albuquerque, F.C.; Stoner, M.C.; Harris, R.P. Stroking human jejunal mucosa induces 5-HT release and Cl- secretion via afferent neurons and 5-HT4 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 277, G515–G520. [Google Scholar] [CrossRef]
- Cil, O.; Phuan, P.W.; Lee, S.; Tan, J.; Haggie, P.M.; Levin, M.H.; Sun, L.; Thiagarajah, J.R.; Ma, T.; Verkman, A.S. CFTR activator increases intestinal fluid secretion and normalizes stool output in a mouse model of constipation. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, A.; Camilleri, M.; Kolar, G.; Erwin, P.; West, C.P.; Murad, M.H. Systematic review with meta-analysis: Highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment. Pharmacol. Ther. 2013, 39, 239–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, J.D. The use of novel promotility and prosecretory agents for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation. Adv. Ther. 2009, 26, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Zhu, J.X.; Chan, H.C. Regulation of Ion Transport by 5-Hydroxytryptamine in Rat Colon. Clin. Exp. Pharmacol. Physiol. 2004, 31, 424–428. [Google Scholar] [CrossRef]
- Jakab, R.L.; Collaco, A.M.; Ameen, N. Lubiprostone Targets Prostanoid Signaling and Promotes Ion Transporter Trafficking, Mucus Exocytosis, and Contractility. Dig. Dis. Sci. 2012, 57, 2826–2845. [Google Scholar] [CrossRef]
- Jiang, C.; Xu, Q.; Wen, X.; Sun, H.-B. Current developments in pharmacological therapeutics for chronic constipation. Acta Pharm. Sin. B 2015, 5, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Thayalasekeran, S.; Ali, H.; Tsai, H.H. Novel therapies for constipation. World J. Gastroenterol. 2013, 19, 8247–8251. [Google Scholar] [CrossRef]
- Kim, N.H.; Park, J.H.; Park, J.-S.; Joung, Y.-H. The Effect of Deoxycholic Acid on Secretion and Motility in the Rat and Guinea Pig Large Intestine. J. Neurogastroenterol. Motil. 2017, 23, 606–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, E.S.; Chokhavatia, S. Targeting Small Bowel Receptors to Treat Constipation and Diarrhea. Curr. Gastroenterol. Rep. 2017, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.X. Calcium-sensing receptor: A new target for therapy of diarrhea. World J. Gastroenterol. 2016, 22, 2711–2724. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Askwith, C.; Javed, N.H.; Cooke, H.J. Autonomic nervous system and secretion across the intestinal mucosal surface. Auton. Neurosci. 2007, 133, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, H.J.; Xue, J.; Yu, J.G.; Wunderlich, J.; Wang, Y.-Z.; Guzmán, J.; Javed, N.; Christofi, F.L. Mechanical stimulation releases nucleotides that activate P2Y1 receptors to trigger neural reflex chloride secretion in guinea pig distal colon. J. Comp. Neurol. 2003, 469, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Günzel, D.; Theune, D.; Czichos, C.; Schulzke, J.-D.; Fromm, M. Water channels and barriers formed by claudins. Ann. N. Y. Acad. Sci. 2017, 1397, 100–109. [Google Scholar] [CrossRef]
- Holmes, J.L.; Van Itallie, C.M.; Rasmussen, J.E.; Anderson, J.M. Claudin profiling in the mouse during postnatal intestinal develop-ment and along the gastrointestinal tract reveals complex expression patterns. Gene Expr. Patterns 2006, 6, 581–588. [Google Scholar] [CrossRef]
- Weber, C.R.; Turner, J.R. Dynamic modeling of the tight junction pore pathway. Ann. N. Y. Acad. Sci. 2017, 1397, 209–218. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Tietgens, A.J.; Anderson, J.M. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol. Biol. Cell 2017, 28, 524–534. [Google Scholar] [CrossRef]
- Cohen, L.; Sekler, I.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colono-cytes and thereby tight junction formation in the colon. Cell Death Dis. 2014, 5, e1307. [Google Scholar] [CrossRef] [Green Version]
- Sunuwar, L.; Medini, M.; Cohen, L.; Sekler, I.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Wolf, P.G.; Guo, S.; Guo, Y.; Gaskins, H.R.; Zhang, B. Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells. J. Nutr. Biochem. 2017, 43, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.X.; Lei, Z.; Wolf, P.G.; Gao, Y.; Guo, Y.M.; Zhang, B.K. Zinc Supplementation, via GPR39, Upregulates PKCζ to Protect Intestinal Barrier Integrity in Caco-2 Cells Challenged by Salmonella enterica Serovar Typhimurium. J. Nutr. 2017, 147, 1282–1289. [Google Scholar] [CrossRef] [Green Version]
- Mullin, J.M.; DiGuilio, K.M.; Valenzano, M.C.; Deis, R.; Thomas, S.; Zurbach, E.P.; Abdulhaqq, S.; Montaner, L.J. Zinc reduces epithelial barrier compromise induced by human seminal plasma. PLoS ONE 2017, 12, e0170306. [Google Scholar] [CrossRef] [Green Version]
- Lodemann, U.; Gefeller, E.M.; Aschenbach, J.R.; Martens, H.; Einspanier, R.; Bondzio, A. Dose Effects of Apical versus Basolateral Zinc Supplementation on Epithelial Resistance, Viability, and Metallothionein Expression in Two Intestinal Epithelial Cell Lines. J. Biochem. Mol. Toxicol. 2015, 29, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Mayo-Wilson, E.; Junior, J.A.; Imdad, A.; Dean, S.; Chan, X.H.S.; Chan, E.S.; Jaswal, A.; Bhutta, Z.A. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database Syst. Rev. 2014, CD009384. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.R.; Liang, G.H.; Wang, Y.; Das, S.; Shen, L.; Yu, A.S.L.; Nelson, D.J.; Turner, J.R. Claudin-2-dependent paracellular channels are dynamically gated. eLife 2015, 4, e09906. [Google Scholar] [CrossRef] [PubMed]
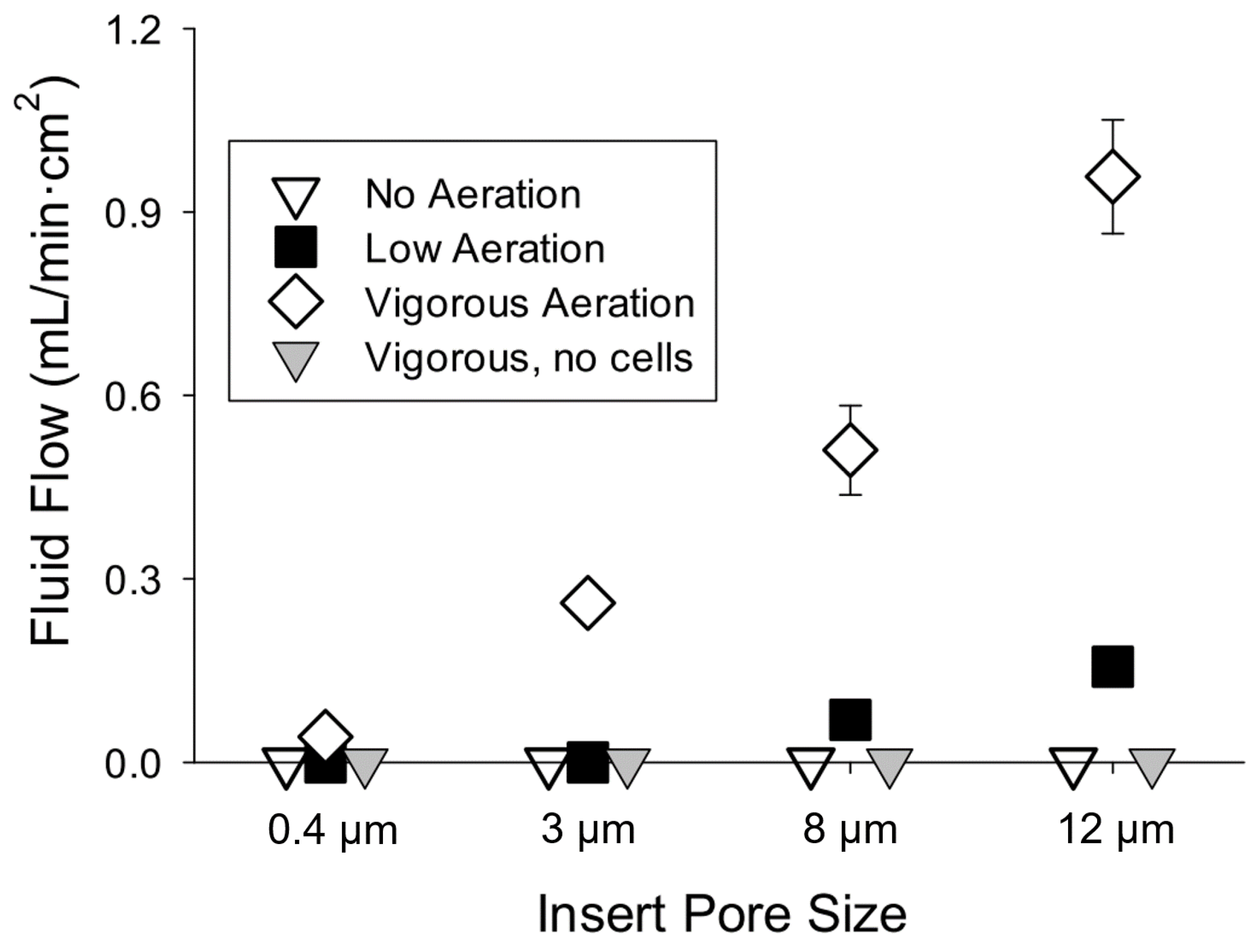



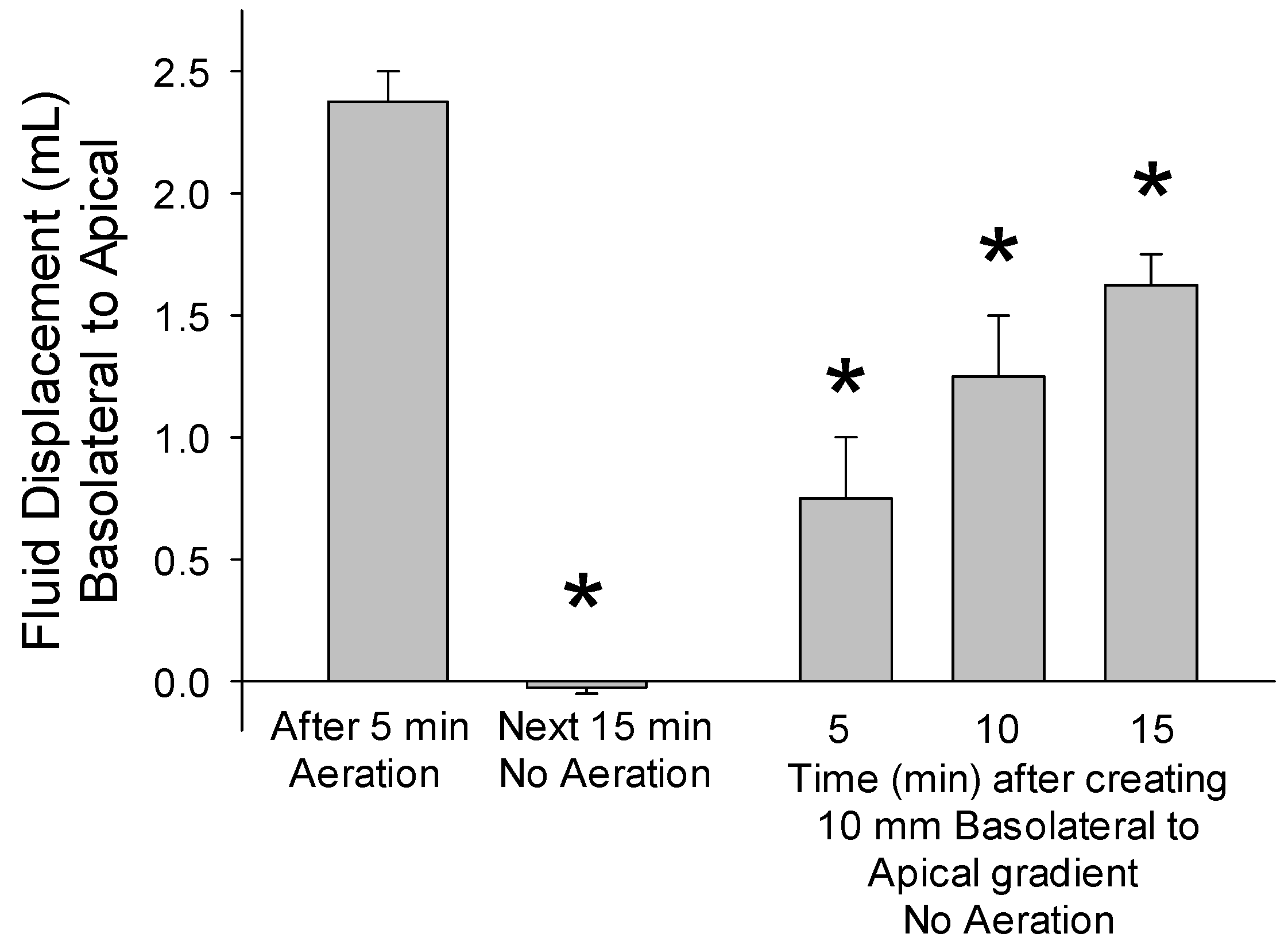

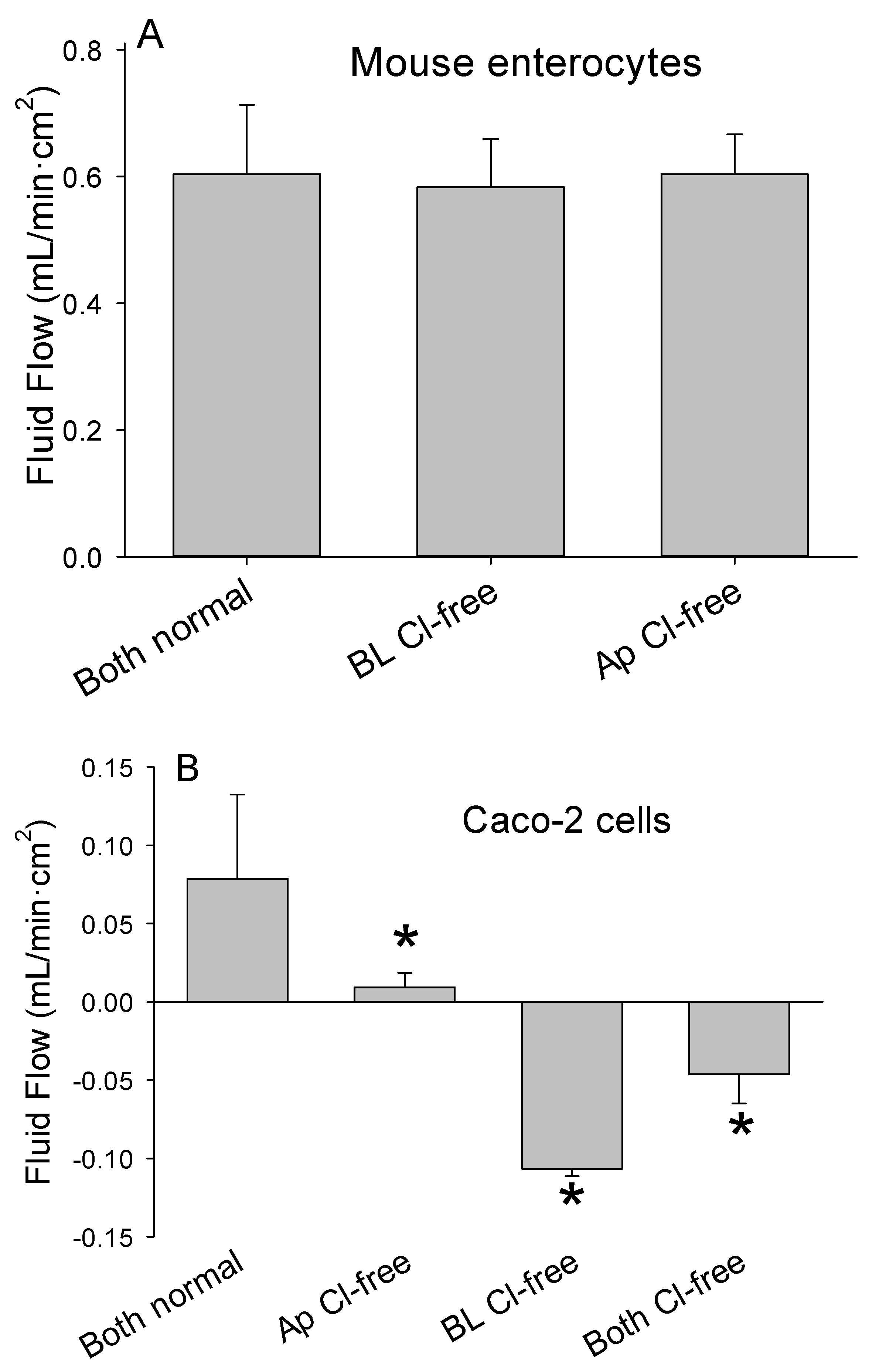






| Pore Size (µm) | Time to Equilibrate (min) |
|---|---|
| 0.4 | 15.35 ± 0.61 |
| 3 | 5.12 ± 0.09 |
| 8 | 1.88 ± 0.07 |
| 12 | 1.00 ± 0.04 |
| Compounds | Action | Conc. | Change in Flow |
|---|---|---|---|
| Glibenclamide | Inhibitor of CFTR and ATP-sensitive potassium channels (KATP) | 200 µM | None |
| CFTRinh-172 | Specific inhibitor of CFTR | 20 µM | None |
| Bumetanide | Inhibitor of the Na/K/2Cl cotransporter in the basolateral membrane (NKCC1) | 50 µM | None |
| Furosemide | Inhibitor of the Na/K/2Cl cotransporter | 200 µM | None |
| Nystatin | Permeabilizes both the apical and basolateral membranes to cations | 500 IU/mL | None |
| Ouabain | Inhibitor of Na-K-ATPase | 1 mM | None |
| Amilioride | Inhibitor of the epithelial sodium channel, ENaC | 50 µM | None |
| 4,4′-Diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS) | Inhibitor of the chloride–bicarbonate exchange AE1 | 100 µM | None |
| Tetraethylammonium | Potassium channel blocker | 5 mM | None |
| Orthovandadate | Generalized inhibitor of phosphatase | 100 µM | None |
| Approach | Evidence |
|---|---|
| Expose naked inserts to aeration | There is no flow or development of a hydrostatic pressure gradient |
| Reverse orientation of epithelia | Direction of flow remains basolateral to apical |
| Establish hydrostatic pressure gradients | Apical-to-basolateral gradient persists, basolateral-to-apical gradient gradually dissipates |
| Fluid flow with Caco-2 cells | Lower flow, reversal of flow with chloride gradient |
| Expose fibroblasts to paradigm | No flow or development of hydrostatic pressure gradient; tight junctions are necessary |
| Addition of zinc then citrate | Inhibition of flow by zinc is reversed by citrate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buddington, R.K.; Wong, T.; Howard, S.C. Paracellular Filtration Secretion Driven by Mechanical Force Contributes to Small Intestinal Fluid Dynamics. Med. Sci. 2021, 9, 9. https://doi.org/10.3390/medsci9010009
Buddington RK, Wong T, Howard SC. Paracellular Filtration Secretion Driven by Mechanical Force Contributes to Small Intestinal Fluid Dynamics. Medical Sciences. 2021; 9(1):9. https://doi.org/10.3390/medsci9010009
Chicago/Turabian StyleBuddington, Randal K., Thomas Wong, and Scott C. Howard. 2021. "Paracellular Filtration Secretion Driven by Mechanical Force Contributes to Small Intestinal Fluid Dynamics" Medical Sciences 9, no. 1: 9. https://doi.org/10.3390/medsci9010009
APA StyleBuddington, R. K., Wong, T., & Howard, S. C. (2021). Paracellular Filtration Secretion Driven by Mechanical Force Contributes to Small Intestinal Fluid Dynamics. Medical Sciences, 9(1), 9. https://doi.org/10.3390/medsci9010009





