Treatment of C3 Glomerulopathy in Adult Kidney Transplant Recipients: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategies
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis and Statistical Analysis
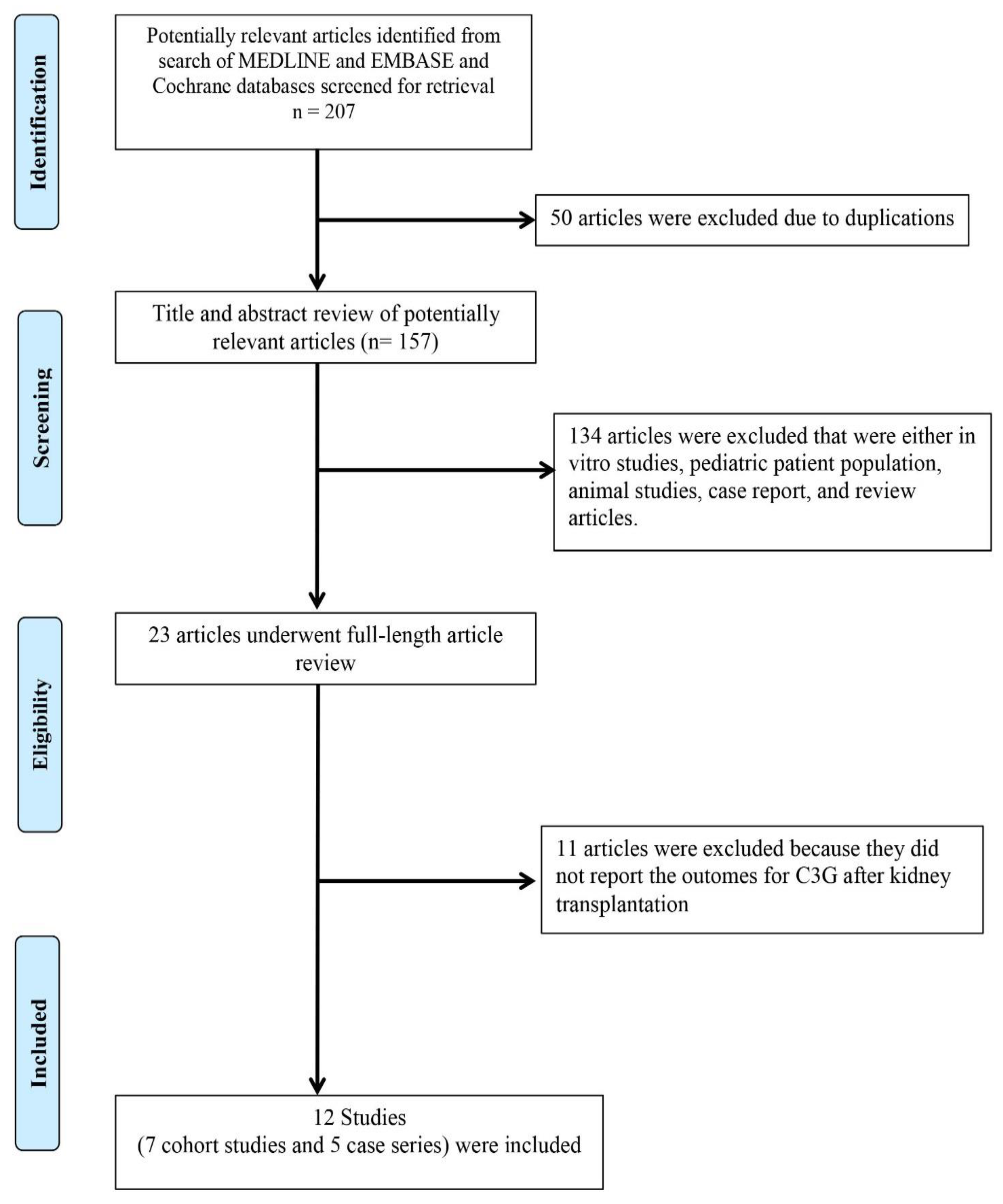
3. Result
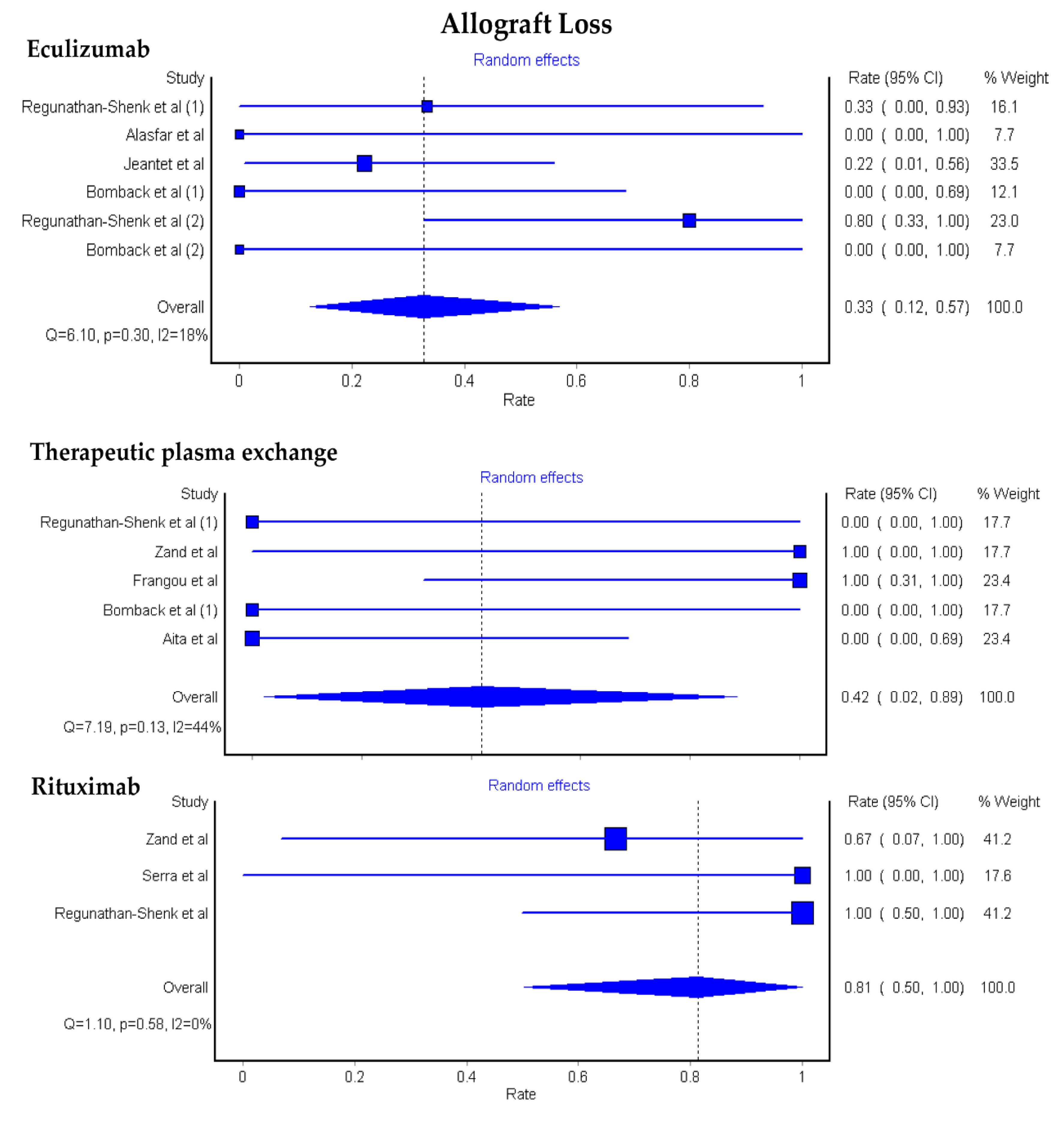
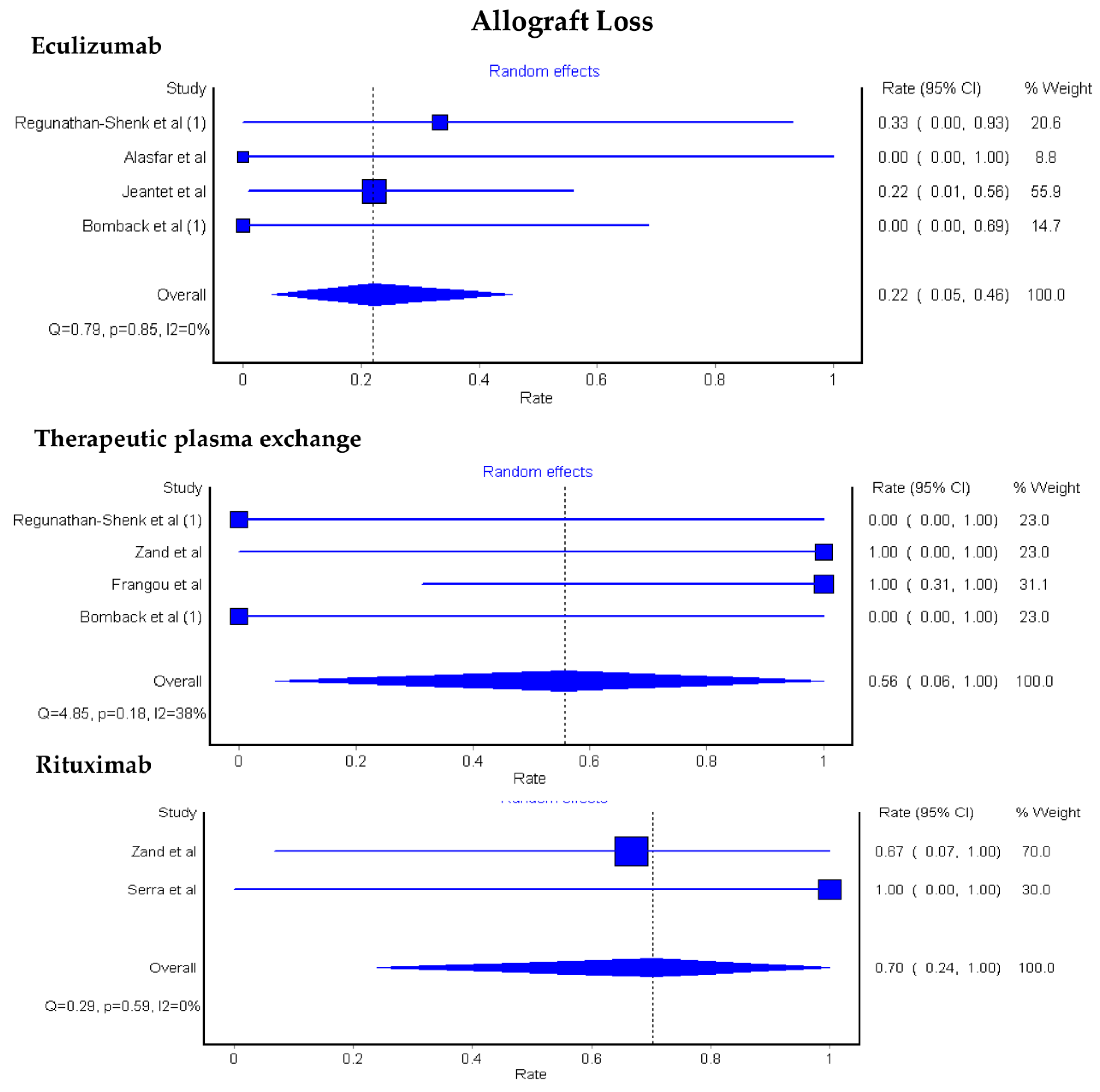
3.1. Allograft Loss among KTx Patients with C3G
3.2. Allograft Loss among KTx Patients with C3GN and DDD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pickering, M.C.; D’Agati, V.D.; Nester, C.M.; Smith, R.J.; Haas, M.; Appel, G.B.; Alpers, C.E.; Bajema, I.M.; Bedrosian, C.; Braun, M.; et al. C3 glomerulopathy: Consensus report. Kidney Int. 2013, 84, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Goodship, T.H.; Cook, H.T.; Fakhouri, F.; Fervenza, F.C.; Frémeaux-Bacchi, V.; Kavanagh, D.; Nester, C.M.; Noris, M.; Pickering, M.C.; Rodríguez de Córdoba, S.; et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017, 91, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.B.; Bomback, A.S. C3 Glomerulopathy: Pathogenesis and Treatment. Adv. Chronic Kidney Dis. 2020, 27, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.; Wani, A.S.; Gupta, A.; Agrawal, V. Pediatric C3 glomerulopathy: A 12-year single-center experience. Pediatr. Nephrol. 2020. [Google Scholar] [CrossRef]
- Khandelwal, P.; Bhardwaj, S.; Singh, G.; Sinha, A.; Hari, P.; Bagga, A. Therapy and outcomes of C3 glomerulopathy and immune-complex membranoproliferative glomerulonephritis. Pediatr. Nephrol. 2020. [Google Scholar] [CrossRef]
- Peleg, Y.; Appel, G.B. Mycophenolate Mofetil Treatment of C3 Glomerulopathy. Clin. J. Am. Soc. Nephrol. 2020, 15, 1234–1236. [Google Scholar] [CrossRef]
- Caravaca-Fontán, F.; Díaz-Encarnación, M.M.; Lucientes, L.; Cavero, T.; Cabello, V.; Ariceta, G.; Quintana, L.F.; Marco, H.; Barros, X.; Ramos, N.; et al. Mycophenolate Mofetil in C3 Glomerulopathy and Pathogenic Drivers of the Disease. Clin. J. Am. Soc. Nephrol. 2020, 15, 1287–1298. [Google Scholar] [CrossRef]
- Hanna, R.M.; Hou, J.; Hasnain, H.; Arman, F.; Selamet, U.; Wilson, J.; Olanrewaju, S.; Zuckerman, J.E.; Barsoum, M.; Yabu, J.M.; et al. Diverse Clinical Presentations of C3 Dominant Glomerulonephritis. Front. Med. 2020, 7, 293. [Google Scholar] [CrossRef]
- Puri, P.; Walters, G.D.; Fadia, M.N.; Konia, M.; Gibson, K.A.; Jiang, S.H. The impact of reclassification of C3 predominant glomerulopathies on diagnostic accuracy, outcome and prognosis in patients with C3 glomerulonephritis. BMC Nephrol. 2020, 21, 265. [Google Scholar] [CrossRef]
- Fakhouri, F.; Le Quintrec, M.; Frémeaux-Bacchi, V. Practical management of C3 glomerulopathy and immunoglobulin-mediated MPGN: Facts and uncertainties. Kidney Int. 2020. [Google Scholar] [CrossRef]
- Perkins, S.J. Genetic and Protein Structural Evaluation of Atypical Hemolytic Uremic Syndrome and C3 Glomerulopathy. Adv. Chronic Kidney Dis. 2020, 27, 120–127.e124. [Google Scholar] [CrossRef] [PubMed]
- Caravaca-Fontán, F.; Lucientes, L.; Cavero, T.; Praga, M. Update on C3 Glomerulopathy: A Complement-Mediated Disease. Nephron 2020, 144, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Bomback, A.S.; Appel, G.B. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat. Rev. Nephrol. 2012, 8, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.H.; Appel, G.B.; Blom, A.M.; Cook, H.T.; D’Agati, V.D.; Fakhouri, F.; Fremeaux-Bacchi, V.; Józsi, M.; Kavanagh, D.; Lambris, J.D.; et al. C3 glomerulopathy—Understanding a rare complement-driven renal disease. Nat. Rev. Nephrol. 2019, 15, 129–143. [Google Scholar] [CrossRef] [PubMed]
- D’Agati, V.D.; Bomback, A.S. C3 glomerulopathy: what’s in a name? Kidney Int. 2012, 82, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Appel, G.B.; Cook, H.T.; Hageman, G.; Jennette, J.C.; Kashgarian, M.; Kirschfink, M.; Lambris, J.D.; Lanning, L.; Lutz, H.U.; Meri, S.; et al. Membranoproliferative glomerulonephritis type II (dense deposit disease): An update. J. Am. Soc. Nephrol. 2005, 16, 1392–1403. [Google Scholar] [CrossRef]
- Smith, R.J.; Alexander, J.; Barlow, P.N.; Botto, M.; Cassavant, T.L.; Cook, H.T.; de Córdoba, S.R.; Hageman, G.S.; Jokiranta, T.S.; Kimberling, W.J.; et al. New approaches to the treatment of dense deposit disease. J. Am. Soc. Nephrol. 2007, 18, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Gamez, J.D.; Vrana, J.A.; Theis, J.D.; Bergen, H.R., 3rd; Zipfel, P.F.; Dogan, A.; Smith, R.J. Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int. 2009, 75, 952–960. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Sethi, S.; Van Praet, J.; Nath, K.A.; Fervenza, F.C. Kidney Disease Caused by Dysregulation of the Complement Alternative Pathway: An Etiologic Approach. J. Am. Soc. Nephrol. 2015, 26, 2917–2929. [Google Scholar] [CrossRef]
- Thurman, J.M. Complement and the Kidney: An Overview. Adv. Chronic Kidney Dis. 2020, 27, 86–94. [Google Scholar] [CrossRef]
- Bomback, A.S.; Santoriello, D.; Avasare, R.S.; Regunathan-Shenk, R.; Canetta, P.A.; Ahn, W.; Radhakrishnan, J.; Marasa, M.; Rosenstiel, P.E.; Herlitz, L.C.; et al. C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int. 2018, 93, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Servais, A.; Noël, L.-H.; Roumenina, L.; Le Quintrec, M.; Ngo, S.; Dragon-Durey, M.-A.; Macher, M.-A.; Zuber, J.; Karras, A.; Provot, F.; et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012, 82, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Medjeral-Thomas, N.R.; O’Shaughnessy, M.M.; O’Regan, J.A.; Traynor, C.; Flanagan, M.; Wong, L.; Teoh, C.W.; Awan, A.; Waldron, M.; Cairns, T.; et al. C3 Glomerulopathy: Clinicopathologic Features and Predictors of Outcome. Clin. J. Am. Soc. Nephrol. 2014, 9, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Okuda, Y.; Ishikura, K.; Hamada, R.; Harada, R.; Sakai, T.; Hamasaki, Y.; Hataya, H.; Fukuzawa, R.; Ogata, K.; Honda, M. Membranoproliferative glomerulonephritis and C3 glomerulonephritis: Frequency, clinical features, and outcome in children. Nephrology 2015, 20, 286–292. [Google Scholar] [CrossRef]
- Caliskan, Y.; Torun, E.S.; Tiryaki, T.O.; Oruc, A.; Ozluk, Y.; Akgul, S.U.; Temurhan, S.; Oztop, N.; Kilicaslan, I.; Sever, M.S. Immunosuppressive Treatment in C3 Glomerulopathy: Is it Really Effective? Am. J. Nephrol. 2017, 46, 96–107. [Google Scholar] [CrossRef]
- Wani, A.S.; Zahir, Z.; Gupta, A.; Agrawal, V. Clinicopathological Significance and Renal Outcomes of Light Microscopic Patterns in Complement Component 3 Glomerulopathy. Nephron 2020, 144, 228–235. [Google Scholar] [CrossRef]
- Ravindran, A.; Fervenza, F.C.; Smith, R.J.H.; Sethi, S. C3 glomerulopathy associated with monoclonal Ig is a distinct subtype. Kidney Int. 2018, 94, 178–186. [Google Scholar] [CrossRef]
- Beck, L.; Bomback, A.S.; Choi, M.J.; Holzman, L.B.; Langford, C.; Mariani, L.H.; Somers, M.J.; Trachtman, H.; Waldman, M. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am. J. Kidney Dis. 2013, 62, 403–441. [Google Scholar] [CrossRef]
- Ravindran, A.; Fervenza, F.C.; Smith, R.J.H.; De Vriese, A.S.; Sethi, S. C3 Glomerulopathy: Ten Years’ Experience at Mayo Clinic. Mayo Clin. Proc. 2018, 93, 991–1008. [Google Scholar] [CrossRef]
- Rabasco, C.; Cavero, T.; Román, E.; Rojas-Rivera, J.; Olea, T.; Espinosa, M.; Cabello, V.; Fernández-Juarez, G.; González, F.; Ávila, A.; et al. Effectiveness of mycophenolate mofetil in C3 glomerulonephritis. Kidney Int. 2015, 88, 1153–1160. [Google Scholar] [CrossRef]
- Besbas, N.; Gulhan, B.; Gucer, S.; Korkmaz, E.; Ozaltin, F. A novel CFHR5 mutation associated with C3 glomerulonephritis in a Turkish girl. J. Nephrol. 2014, 27, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Bomback, A.S.; Smith, R.J.; Barile, G.R.; Zhang, Y.; Heher, E.C.; Herlitz, L.; Stokes, M.B.; Markowitz, G.S.; D’Agati, V.D.; Canetta, P.A.; et al. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin. J. Am. Soc. Nephrol. 2012, 7, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Herlitz, L.C.; Bomback, A.S.; Markowitz, G.S.; Stokes, M.B.; Smith, R.N.; Colvin, R.B.; Appel, G.B.; D’Agati, V.D. Pathology after Eculizumab in Dense Deposit Disease and C3 GN. J. Am. Soc. Nephrol. 2012, 23, 1229–1237. [Google Scholar] [CrossRef]
- Bonucchi, D.; Leonelli, M.; Damiano, F.; Granito, M.; Ghiandai, G.; De Amicis, S.; Americo, C.; Ligabue, G.; Albertazzi, V.; Cappelli, G. Post-transplant recurrence of glomerulonephritis: A complex clinical case. G. Ital. Nefrol. 2010, 27 (Suppl. S52), S82–S84. [Google Scholar] [PubMed]
- Daina, E.; Noris, M.; Remuzzi, G. Eculizumab in a Patient with Dense-Deposit Disease. N. Engl. J. Med. 2012, 366, 1161–1163. [Google Scholar] [CrossRef]
- Garnier, A.S.; Augusto, J.F.; Pellier, I.; Subra, J.F.; Sayegh, J. Successful long-term outcome of kidney transplantation in a patient with X-linked thrombocytopenia: 9-year follow-up. Transplantation 2014, 98, e57–e58. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, S.; Fyfe, B.; Weiss, L.; Xiao, X.; Zhang, Y.; Smith, R.J. Eculizumab and recurrent C3 glomerulonephritis. Pediatr. Nephrol. 2013, 28, 1975–1981. [Google Scholar] [CrossRef]
- Inman, M.; Prater, G.; Fatima, H.; Wallace, E. Eculizumab-induced reversal of dialysis-dependent kidney failure from C3 glomerulonephritis. Clin. Kidney J. 2015, 8, 445–448. [Google Scholar] [CrossRef]
- Kerns, E.; Rozansky, D.; Troxell, M.L. Evolution of immunoglobulin deposition in C3-dominant membranoproliferative glomerulopathy. Pediatr. Nephrol. 2013, 28, 2227–2231. [Google Scholar] [CrossRef]
- McCaughan, J.A.; O’Rourke, D.M.; Courtney, A.E. Recurrent dense deposit disease after renal transplantation: An emerging role for complementary therapies. Am. J. Transplant. 2012, 12, 1046–1051. [Google Scholar] [CrossRef]
- Le Quintrec, M.; Lionet, A.; Kandel, C.; Bourdon, F.; Gnemmi, V.; Colombat, M.; Goujon, J.M.; Frémeaux-Bacchi, V.; Fakhouri, F. Eculizumab for treatment of rapidly progressive C3 glomerulopathy. Am. J. Kidney Dis. 2015, 65, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Lunn, A.; Kirschfink, M.; Thorner, P.; Hebert, D.; Langlois, V.; Pluthero, F.; Licht, C. Eculizumab and Refractory Membranoproliferative Glomerulonephritis. N. Engl. J. Med. 2012, 366, 1165–1166. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Pasini, A.; Emma, F. Eculizumab for the treatment of dense-deposit disease. N. Engl. J. Med. 2012, 366, 1163–1165. [Google Scholar] [CrossRef]
- Sánchez-Moreno, A.; De la Cerda, F.; Cabrera, R.; Fijo, J.; López-Trascasa, M.; Bedoya, R.; Rodríguez de Córdoba, S.; Ybot-González, P. Eculizumab in dense-deposit disease after renal transplantation. Pediatr. Nephrol. 2014, 29, 2055–2059. [Google Scholar] [CrossRef] [PubMed]
- Zuber, J.; Fakhouri, F.; Roumenina, L.T.; Loirat, C.; Frémeaux-Bacchi, V. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat. Rev. Nephrol. 2012, 8, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Suarez, M.L.; Thongprayoon, C.; Mao, M.A.; Leeaphorn, N.; Bathini, T.; Cheungpasitporn, W. Outcomes of Kidney Transplant Patients with Atypical Hemolytic Uremic Syndrome Treated with Eculizumab: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 919. [Google Scholar] [CrossRef] [PubMed]
- Bomback, A.S.; Markowitz, G.S.; Appel, G.B. Complement-Mediated Glomerular Diseases: A Tale of 3 Pathways. Kidney Int. Rep. 2016, 1, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Regunathan-Shenk, R.; Avasare, R.S.; Ahn, W.; Canetta, P.A.; Cohen, D.J.; Appel, G.B.; Bomback, A.S. Kidney Transplantation in C3 Glomerulopathy: A Case Series. Am. J. Kidney Dis. 2019, 73, 316–323. [Google Scholar] [CrossRef]
- Cosio, F.G.; Cattran, D.C. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int. 2017, 91, 304–314. [Google Scholar] [CrossRef]
- Zand, L.; Lorenz, E.C.; Cosio, F.G.; Fervenza, F.C.; Nasr, S.H.; Gandhi, M.J.; Smith, R.J.; Sethi, S. Clinical findings, pathology, and outcomes of C3GN after kidney transplantation. J. Am. Soc. Nephrol. 2014, 25, 1110–1117. [Google Scholar] [CrossRef]
- Coppola, S.; Singh, N.; Onyirimba, J. C3 Glomerulopathy de novo in a kidney transplant patient. Am. J. Kidney Dis. 2019, 73, 666. [Google Scholar] [CrossRef]
- Gundlapalli, S.; Mondhe, S.D. Transplantation in C3 glomerulopathy—Damned if you do, damned if you don’t. Indian J. Transplant. 2019, 13, 154–155. [Google Scholar] [CrossRef]
- Kumar, A.; Bharati, J.; Nada, R.; Singh, S.; Sharma, A.; Gupta, K.L.; Ramachandran, R. Utility of plasma exchange in early recurrent C3 glomerulopathy. Indian J. Transplant. 2019, 13, 122–126. [Google Scholar] [CrossRef]
- Kumar, A.; Nada, R.; Ramachandran, R.; Rawat, A.; Tiewsoh, K.; Das, R.; Rayat, C.S.; Gupta, K.L.; Vasishta, R.K. Outcome of C3 glomerulopathy patients: Largest single-centre experience from South Asia. J. Nephrol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pinarbasi, A.S.; Dursun, I.; Poyrazoglu, M.H.; Akgun, H.; Bozpolat, A.; Dusunsel, R. Evaluation of the children with C3 glomerulopathy. Saudi J. Kidney Dis. Transpl. 2020, 31, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Schena, F.P.; Esposito, P.; Rossini, M. A Narrative Review on C3 Glomerulopathy: A Rare Renal Disease. Int. J. Mol. Sci. 2020, 21, 525. [Google Scholar] [CrossRef]
- Willows, J.; Brown, M.; Sheerin, N.S. The role of complement in kidney disease. Clin. Med. (Lond.) 2020, 20, 156–160. [Google Scholar] [CrossRef]
- Drake, K.A.; Ellington, N.; Gattineni, J.; Torrealba, J.R.; Hendricks, A.R. Clinicopathological features of C3 glomerulopathy in children: A single-center experience. Pediatr. Nephrol. 2020, 35, 153–162. [Google Scholar] [CrossRef]
- Gulleroglu, K.; Baskin, E.; Ozdemir, H.; Moray, G.; Haberal, M. Recurrence and Outcomes of Complement-Related Renal Disease After Pediatric Renal Transplantation. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2020, 18, 82–83. [Google Scholar] [CrossRef]
- Kim, J.S.; Foster, K.W.; Westphal, S.G. Eculizumab in post-transplant C3 glomerulonephritis caused by a C3 mutation. Clin. Nephrol. 2020, 93, 51–56. [Google Scholar] [CrossRef]
- Levine, A.P.; Chan, M.M.Y.; Sadeghi-Alavijeh, O.; Wong, E.K.S.; Cook, H.T.; Ashford, S.; Carss, K.; Christian, M.T.; Hall, M.; Harris, C.L.; et al. Large-scale whole-genome sequencing reveals the genetic architecture of primary membranoproliferative GN and C3 glomerulopathy. J. Am. Soc. Nephrol. 2020, 31, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; El Kossi, M.; Kim, J.J.; Shaheen, I.S.; Sharma, A.; Halawa, A. Complement-mediated renal diseases after kidney transplantation—Current diagnostic and therapeutic options in de novo and recurrent diseases. World J. Transplant. 2018, 8, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Angelo, J.R.; Bell, C.S.; Braun, M.C. Allograft failure in kidney transplant recipients with membranoproliferative glomerulonephritis. Am. J. Kidney Dis. 2011, 57, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Iatropoulos, P.; Noris, M.; Mele, C.; Piras, R.; Valoti, E.; Bresin, E.; Curreri, M.; Mondo, E.; Zito, A.; Gamba, S.; et al. Complement gene variants determine the risk of immunoglobulin-associated MPGN and C3 glomerulopathy and predict long-term renal outcome. Mol. Immunol. 2016, 71, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Nasr, S.H.; Farris, A.B.; Schinstock, C.A.; Alexander, M.P.; Cornell, L.D. Eculizumab deposition in renal transplants. Am. J. Transplant. 2019, 19, 361. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Lim, W.H.; Shingde, M.; Wong, G. Recurrent and de novo Glomerulonephritis After Kidney Transplantation. Front. Immunol. 2019, 10, 1944. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Controll. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Easterbrook, P.J.; Berlin, J.A.; Gopalan, R.; Matthews, D.R. Publication bias in clinical research. Lancet 1991, 337, 867–872. [Google Scholar] [CrossRef]
- Frangou, E.; Varnavidou-Nicolaidou, A.; Petousis, P.; Soloukides, A.; Theophanous, E.; Savva, I.; Michael, N.; Toumasi, E.; Georgiou, D.; Stylianou, G.; et al. Clinical course and outcome after kidney transplantation in patients with C3 glomerulonephritis due to CFHR5 nephropathy. Nephrol. Dial. Transplant. 2019. [Google Scholar] [CrossRef] [PubMed]
- Serra, N.; Facundo, C.; Canal, C.; Arce, Y.; Ayasreh, N.; Vila, A.; Bardaji, B.; Silva, I.; Lopez, V.; Benito, S.; et al. Three cases of monoclonal gammopathy of renal significance after kidney transplantation. De novo C3 glomerulopathy. Nefrologia 2019, 39, 198–201. [Google Scholar] [CrossRef]
- Wong, L.; Moran, S.; Lavin, P.J.; Dorman, A.M.; Conlon, P.J. Kidney transplant outcomes in familial C3 glomerulopathy. Clin. Kidney J. 2016, 9, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Alasfar, S.; Carter-Monroe, N.; Rosenberg, A.Z.; Montgomery, R.A.; Alachkar, N. Membranoproliferative glomerulonephritis recurrence after kidney transplantation: Using the new classification. BMC Nephrol. 2016, 17, 7. [Google Scholar] [CrossRef]
- Jeantet, G.; Bertrand, D.; Anglicheau, D.; Jourde-Chiche, N.; Gatault, P.; Cassuto, E.; Fakhouri, F.; Fremeaux-Bacchi, V.; Sellier-Leclerc, L.; Lionet, A. Eculizumab in C3 glomerulopathy recurrence after kidney transplant. In Transplant International; Wiley-Blackwell: Hoboken, NJ, USA, 2017; p. 5. [Google Scholar]
- Aita, K.; Ito, S.; Tanabe, K.; Toma, H.; Yamaguchi, Y.; Nagata, M. Early recurrence of dense deposit disease with marked endocapillary proliferation after renal transplantation. Pathol. Int. 2006, 56, 101–109. [Google Scholar] [CrossRef]
- Le Quintrec, M.; Rabant, M.; Marinozzi, C.; Kamar, N.; Buchler, M.; Mousson, C.; de Ligny, H.B.; Bridoux, F.; Olagne, J.; Frimat, M. uncontrolled Pathways Activation After Renal Transplantation And C5b9 Deposits On Graft Predict Graft Outcome In Adult Renal Transplant With C3 Glomerulopathy: O36. Transpl. Int. 2013, 26, 9. [Google Scholar]
- Andresdottir, M.B.; Assmann, K.J.; Hoitsma, A.J.; Koene, R.A.; Wetzels, J.F. Renal transplantation in patients with dense deposit disease: Morphological characteristics of recurrent disease and clinical outcome. Nephrol. Dial. Transplant. 1999, 14, 1723–1731. [Google Scholar] [CrossRef]
- Droz, D.; Nabarra, B.; Noel, L.H.; Leibowitch, J.; Crosnier, J. Recurrence of dense deposits in transplanted kidneys: I. Sequential survey of the lesions. Kidney Int. 1979, 15, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Koopman, J.J.E.; de Vries, A.P.J.; Bajema, I.M. C3 glomerulopathy. Nephrol. Dial. Transplant. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Nester, C.M.; Smith, R.J. Membranoproliferative glomerulonephritis and C3 glomerulopathy: Resolving the confusion. Kidney Int. 2012, 81, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.; Frémeaux-Bacchi, V.; Noël, L.H.; Cook, H.T.; Pickering, M.C. C3 glomerulopathy: A new classification. Nat. Rev. Nephrol. 2010, 6, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Fervenza, F.C. Membranoproliferative glomerulonephritis: Pathogenetic heterogeneity and proposal for a new classification. Semin. Nephrol. 2011, 31, 341–348. [Google Scholar] [CrossRef]
- Sethi, S.; Fervenza, F.C.; Zhang, Y.; Zand, L.; Vrana, J.A.; Nasr, S.H.; Theis, J.D.; Dogan, A.; Smith, R.J. C3 glomerulonephritis: Clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012, 82, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Cook, H.T. C3 glomerulopathy. F1000Res 2017, 6, 248. [Google Scholar] [CrossRef] [PubMed]
- Gale, D.P.; de Jorge, E.G.; Cook, H.T.; Martinez-Barricarte, R.; Hadjisavvas, A.; McLean, A.G.; Pusey, C.D.; Pierides, A.; Kyriacou, K.; Athanasiou, Y.; et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 2010, 376, 794–801. [Google Scholar] [CrossRef]
- Dragon-Durey, M.A.; Blanc, C.; Marinozzi, M.C.; van Schaarenburg, R.A.; Trouw, L.A. Autoantibodies against complement components and functional consequences. Mol. Immunol. 2013, 56, 213–221. [Google Scholar] [CrossRef]
- Bridoux, F.; Desport, E.; Frémeaux-Bacchi, V.; Chong, C.F.; Gombert, J.M.; Lacombe, C.; Quellard, N.; Touchard, G. Glomerulonephritis with isolated C3 deposits and monoclonal gammopathy: A fortuitous association? Clin. J. Am. Soc. Nephrol. 2011, 6, 2165–2174. [Google Scholar] [CrossRef]
- Avasare, R.S.; Canetta, P.A.; Bomback, A.S.; Marasa, M.; Caliskan, Y.; Ozluk, Y.; Li, Y.; Gharavi, A.G.; Appel, G.B. Mycophenolate Mofetil in Combination with Steroids for Treatment of C3 Glomerulopathy: A Case Series. Clin. J. Am. Soc. Nephrol. 2018, 13, 406–413. [Google Scholar] [CrossRef]
- Blosser, C.D.; Bloom, R.D. Recurrent glomerular disease after kidney transplantation. Curr. Opin. Nephrol. Hypertens 2017, 26, 501–508. [Google Scholar] [CrossRef]
- Habbig, S.; Mihatsch, M.J.; Heinen, S.; Beck, B.; Emmel, M.; Skerka, C.; Kirschfink, M.; Hoppe, B.; Zipfel, P.F.; Licht, C. C3 deposition glomerulopathy due to a functional factor H defect. Kidney Int. 2009, 75, 1230–1234. [Google Scholar] [CrossRef]
- Nester, C.M.; Smith, R.J. Treatment options for C3 glomerulopathy. Curr. Opin. Nephrol. Hypertens. 2013, 22, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Nester, C.M.; Smith, R.J. Complement inhibition in C3 glomerulopathy. Semin. Immunol. 2016, 28, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Oosterveld, M.J.; Garrelfs, M.R.; Hoppe, B.; Florquin, S.; Roelofs, J.J.; van den Heuvel, L.P.; Amann, K.; Davin, J.C.; Bouts, A.H.; Schriemer, P.J.; et al. Eculizumab in Pediatric Dense Deposit Disease. Clin. J. Am. Soc. Nephrol. 2015, 10, 1773–1782. [Google Scholar] [CrossRef]
- Zipfel, P.F.; Wiech, T.; Rudnick, R.; Afonso, S.; Person, F.; Skerka, C. Complement Inhibitors in Clinical Trials for Glomerular Diseases. Front. Immunol. 2019, 10, 2166. [Google Scholar] [CrossRef] [PubMed]
| C3GN among KTx Recipients | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Type of Study | Patients (n) | Age at Time of Diagnosis/Transplant, Median (Years) | Females (n) | Time to Dialysis or KTX, Median (Months) | Type of KTX | Complement Abnormality | Median Follow-Up (Months) | Median Time from KTx to Recurrence (Months) | Recurrence | Rituximab, Graft Failure (n) | Eculizumab, Graft Failure (n) | PLEX + Steroids, Graft Failure (n) | No Therapy for Recurrence, Graft Failure (n) | Graft Failure, Total (n) |
| Regunathan-Shenk et al., 2019 [48] | Cohort | 12 | 22 | 3 | 48 | LRKTx, 7 LUKTx, 3 DDKTx, 2 | CD46, 1 C3Nef, 2 C5Nef, 1 None, 1 Not done, 7 | 76 | - | 8, yes 2, probable2, no | 0 | 3, 1 (1 treated with Eculizumab + PLEX) | 1, 0 | 9, 2 | 3 |
| Zand et al., 2014 [50] | Cohort | 21 | 20.8 | 9 | 42.3 | LKTx, 17 DDKTx, 4 | - | 73.9 | 28 | 14, yes 7, no | 3, 2 | 0,0 | 1 + plus autologous peripheral stem cell transplant, 0 1 treated with steroids alone, 1 | 10, 0 | 7 |
| Frangou et al., 2019 [71] | Cohort | 17 | 46.7 | 4 | - | LRKTx, 3 LUKTx, 3 DDKTx, 11 | CFHR5, 17 | 157 | 37 | 3, yes 9, probable | 0,0 | 0,0 | 2, 2 | 14, 3 | 5 |
| Serra et al., 2018 [72] | Case series | 3 (de novo) | 66 | 1 | - | DDKTx, 3 | None, 2 Anti-CFH ab, 1 | - | 72 | 3 de novo | 1, 1 | 0 | 0 | 2, 2 | 3 |
| Wong et al., 2016 [73] | Case series | 4 (familial) | 26.5 | 2 | - | DDKTx, 4 | - | - | 97 | 2, yes | 0 | 0 | 0 | 2, 1 | 1 |
| Alasfar et al., 2016 [74] | Cohort | 5 | 37.4 | 3 | - | DDKTx,1 LUKTx, 1 | - | 63.6 | - | 2, yes | 0 | 1,0 | 0 | 1, 1 | 1 |
| Jeantet et al., 2017 [75] | Cohort | 9 | - | - | - | - | - | - | 1.5 | 9, yes | 0 | 9, 2 | 0 | 0 | 2 |
| Bomback et al., 2012 [32] | Case series | 2 | 21 | 2 | - | - | C3Nef, 2 | - | 2.5 | 2 | - | 2, 0 (1 treated with Eculizumab + PLEX, steroids) | 1, 0 | 0 | 0 |
| DDD among KTx Recipients | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Type of Study | Patients, (n) | Age at Time of Diagnosis/Transplant, Median (Years) | Females (n) | Time to Dialysis or KTx, Median (Months) | Type of KTx | Complement Abnormality | Median Follow-Up (Months) | Median Time from KTx to Recurrence | Recurrence (n) | Rituximab, Graft Failure (n) | Eculizumab, Graft Failure (n) | PLEX, Graft Failure (n) | No Therapy, Graft Failure (n) | Graft Failure, Total (n) |
| Regunathan-Shenk, 2019 [48] | Cohort | 7 | 30 | 2 | - | LRKTx, 3 LUKTx, 2 DDKTx, 2 | C3Nef, 3 CFI, 1 Anti-CFH ab, 1 Not done, 2 | - | - | 5, true 3, Probable 1, no | 3, 3 (1 treated with rituximab + eculizumab) failed (1 treated with rituximab + PLEX), failed (1 treated with rituximab, eculizumab, PLEX), failed | 5, 4 (1 treated with eculizumab alone), survived (1 treated with eculizumab alone), failed (1 treated with eculizumab + PLEX) Failed (1 treated with rituximab, eculizumab, PLEX), failed (1 treated with rituximab + eculizumab) failed | 3,3 (1 treated with eculizumab + PLEX) Failed (1 treated with rituximab + PLEX), failed (1 treated with rituximab, eculizumab, PLEX), failed | 3, 2 | 7 |
| Aita et al., 2006 [76] | Case series | 2 | 25 | 0 | - | LRKTx, 2 | - | 6 | - | - | 0 | 0 | 2, 0 | 0 | 0 |
| LeQuintrec et al., 2013 [77] | Case series | 15 | - | - | - | - | - | - | - | 5 | 0 | 0 | 0 | 5, 3 | 3 |
| Andresdottir et al., 1999 [78] | Cohort | 13 | 23 | 7 | 84 | DDKTx, 12 LRKTx, 1 | - | 29 | 2.9 | 11 | 0 | 0 | 0 | 11, 8 | 8 |
| Droz et al., 1979 [79] | Cohort | 11 | - | - | - | DDKTx, 7 LRKTx, 4 | - | 30 | 4 | 9 | 0 | 0 | 0 | 9, 2 | 2 |
| Bomback et al., 2012 [32] | Case series | 1 | 42 | 1 | - | LRKTx, 1 | Negative | - | 20 | 1 | 0 | 1, 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Suarez, M.L.; Thongprayoon, C.; Hansrivijit, P.; Kovvuru, K.; Kanduri, S.R.; Aeddula, N.R.; Pivovarova, A.I.; Chewcharat, A.; Bathini, T.; Mao, M.A.; et al. Treatment of C3 Glomerulopathy in Adult Kidney Transplant Recipients: A Systematic Review. Med. Sci. 2020, 8, 44. https://doi.org/10.3390/medsci8040044
Gonzalez Suarez ML, Thongprayoon C, Hansrivijit P, Kovvuru K, Kanduri SR, Aeddula NR, Pivovarova AI, Chewcharat A, Bathini T, Mao MA, et al. Treatment of C3 Glomerulopathy in Adult Kidney Transplant Recipients: A Systematic Review. Medical Sciences. 2020; 8(4):44. https://doi.org/10.3390/medsci8040044
Chicago/Turabian StyleGonzalez Suarez, Maria L, Charat Thongprayoon, Panupong Hansrivijit, Karthik Kovvuru, Swetha R Kanduri, Narothama R Aeddula, Aleksandra I Pivovarova, Api Chewcharat, Tarun Bathini, Michael A Mao, and et al. 2020. "Treatment of C3 Glomerulopathy in Adult Kidney Transplant Recipients: A Systematic Review" Medical Sciences 8, no. 4: 44. https://doi.org/10.3390/medsci8040044
APA StyleGonzalez Suarez, M. L., Thongprayoon, C., Hansrivijit, P., Kovvuru, K., Kanduri, S. R., Aeddula, N. R., Pivovarova, A. I., Chewcharat, A., Bathini, T., Mao, M. A., Basu, A., & Cheungpasitporn, W. (2020). Treatment of C3 Glomerulopathy in Adult Kidney Transplant Recipients: A Systematic Review. Medical Sciences, 8(4), 44. https://doi.org/10.3390/medsci8040044






