Mechanisms Underlying the Inhibition of Tyrosine Kinase Inhibitor-Induced Anorexia and Fatigue by Royal Jelly in Renal Cell Carcinoma Patients and the Correlation between Macrophage Colony Stimulating Factor and Inflammatory Mediators
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patients and Study Protocol
2.3. Statistical Analyses
3. Results
3.1. Patient Demographics
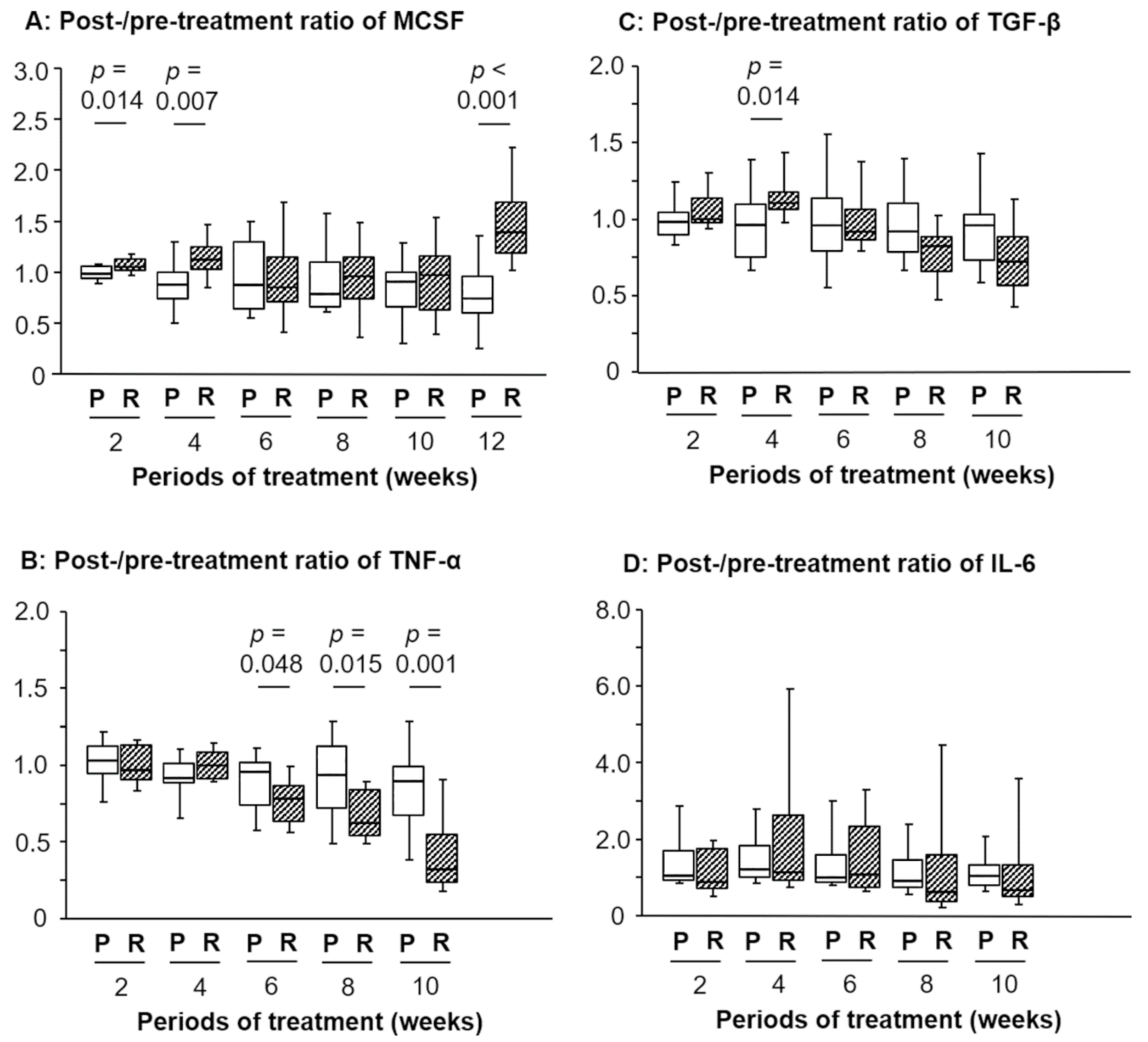
3.2. Changes in the Post-/Pretreatment Ratio of M-CSF after RJ Intake and Its Clinical Roles
3.3. Changes in the Post-/Pretreatment Ratio of Inflammation-Related Markers after RJ Intake
3.4. Correlation between the Ratios of Inflammation-Related Markers and Anorexia or Fatigue
3.5. Correlation between the Ratio of M-CSF and IL-6, TNF-α, or TGF-β Ratios
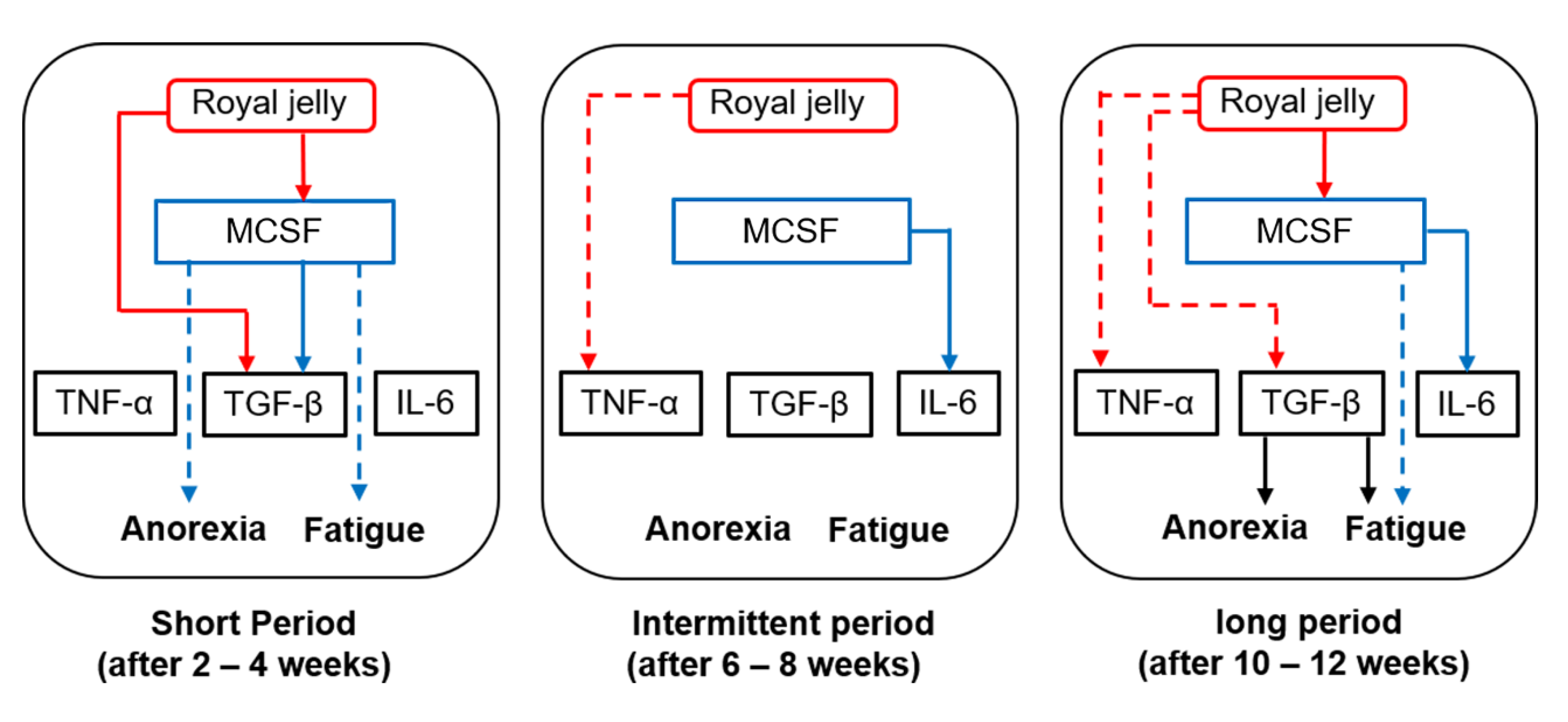
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 2018, 9, 2160. [Google Scholar] [CrossRef] [PubMed]
- Szkudlarek, A.; Wilk, M.; Maciążek-Jurczyk, M. In Vitro Investigations of Acetohexamide Binding to Glycated Serum Albumin in the Presence of Fatty Acid. Molecules 2020, 25, 2340. [Google Scholar] [CrossRef]
- Singh, R.; Mishra, M.K.; Aggarwal, H. Inflammation, Immunity, and Cancer. Mediat. Inflamm. 2017, 2017, 6027305. [Google Scholar] [CrossRef] [PubMed]
- Franzolin, G.; Tamagnone, L. Semaphorin Signaling in Cancer-Associated Inflammation. Int. J. Mol. Sci. 2019, 20, 377. [Google Scholar] [CrossRef] [PubMed]
- Ming-Hua, C.; Bao-Hua, Z.; Lei, Y. Mechanisms of Anorexia Cancer Cachexia Syndrome and Potential Benefits of Traditional Medicine and Natural Herbs. Curr. Pharm. Biotechnol. 2016, 17, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chu, S.; Gao, Y.; Ai, Q.; Liu, Y.; Li, X.; Chen, N. A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis. Cells 2019, 8, 738. [Google Scholar] [CrossRef]
- Miyata, Y.; Araki, K.; Ohba, K.; Matsuo, T.; Nakamura, Y.; Yuno, T.; Mukae, Y.; Otsubo, A.; Mistunari, K.; Mochizuki, Y.; et al. Oral intake of royal jelly improves anti-cancer effects and suppresses adverse events of molecular targeted therapy by regulating TNF-α and TGF-β in renal cell carcinoma: A preliminary study based on a randomized double-blind clinical trial. Mol. Clin. Oncol. 2020, 13, 29. [Google Scholar]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bensalah, K.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur. Urol. 2019, 75, 799–810. [Google Scholar] [CrossRef]
- Nakayama, T.; Saito, K.; Kumagai, J.; Nakajima, Y.; Kijima, T.; Yoshida, S.; Kihara, K.; Fujii, Y. Higher Serum C-reactive Protein Level Represents the Immunosuppressive Tumor Microenvironment in Patients with Clear Cell Renal Cell Carcinoma. Clin. Genitourin. Cancer 2018, 16, e1151–e1158. [Google Scholar] [CrossRef]
- Wang, T.; Lu, R.; Kapur, P.; Jaiswal, B.S.; Hannan, R.; Zhang, Z.; Pedrosa, I.; Luke, J.J.; Zhang, H.; Goldstein, L.D.; et al. An Empirical Approach Leveraging Tumorgrafts to Dissect the Tumor Microenvironment in Renal Cell Carcinoma Identifies Missing Link to Prognostic Inflammatory Factors. Cancer Discov. 2018, 8, 1142–1155. [Google Scholar] [CrossRef]
- Kovaleva, O.V.; Samoilova, D.V.; Shitova, M.S.; Gratchev, A. Tumor Associated Macrophages in Kidney Cancer. Anal. Cell Pathol. 2016, 2016, 9307549. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Miyata, Y.; Mochizuki, Y.; Yasuda, T.; Nakamura, Y.; Araki, K.; Sagara, Y.; Matsuo, T.; Ohba, K.; Sakai, H. Pathological Significance and Prognostic Roles of Densities of CD57+ Cells, CD68+ Cells, and Mast Cells, and Their Ratios in Clear Cell Renal Cell Carcinoma. Hum. Pathol. 2018, 79, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Schnetz, M.; Meier, J.K.; Rehwald, C.; Mertens, C.; Urbschat, A.; Tomat, E.; Akam, E.A.; Baer, P.; Roos, F.C.; Brüne, B.; et al. The Disturbed Iron Phenotype of Tumor Cells and Macrophages in Renal Cell Carcinoma Influences Tumor Growth. Cancers 2020, 12, 530. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, L.; Wang, Y.; Jiang, Q.; Liu, Z.; Zhang, J.; Zhou, Q.; Zeng, H.; Tong, S.; Wang, T.; et al. Tumor-associated Macrophage-derived Interleukin-23 Interlinks Kidney Cancer Glutamine Addiction With Immune Evasion. Eur. Urol. 2019, 75, 752–763. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Ushach, I.; Zlotnik, A. Biological Role of Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF) and Macrophage Colony-Stimulating Factor (M-CSF) on Cells of the Myeloid Lineage. J. Leukoc. Biol. 2016, 100, 481–489. [Google Scholar] [CrossRef]
- Ohashi, E.; Kohno, K.; Arai, N.; Harashima, A.; Ariyasu, T.; Ushio, S. Adenosine N1-Oxide Exerts Anti-inflammatory Effects Through the PI3K/Akt/GSK-3β Signaling Pathway and Promotes Osteogenic and Adipocyte Differentiation. Biol. Pharm. Bull. 2019, 42, 968–976. [Google Scholar] [CrossRef]
- Fan, P.; Han, B.; Hu, H.; Wei, Q.; Zhang, X.; Meng, L.; Nie, J.; Tang, X.; Tian, X.; Zhang, L.; et al. Proteome of Thymus and Spleen Reveals That 10-hydroxydec-2-enoic Acid Could Enhance Immunity in Mice. Expert Opin. Ther. Targets 2020, 24, 267–279. [Google Scholar] [CrossRef]
- Araki, K.; Miyata, Y.; Ohba, K.; Nakamura, Y.; Matsuo, T.; Mochizuki, Y.; Sakai, H. Oral Intake of Royal Jelly Has Protective Effects Against Tyrosine Kinase Inhibitor-Induced Toxicity in Patients with Renal Cell Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Trial. Medicines 2018, 6, 2. [Google Scholar] [CrossRef]
- Karaca, T.; Uz, Y.H.; Demirtas, S.; Karaboga, I.; Can, G. Protective Effect of Royal Jelly in 2,4,6 Trinitrobenzene Sulfonic Acid-Induced Colitis in Rats. Iran J. Basic Med. Sci. 2015, 18, 370–379. [Google Scholar]
- Sun, Y.; Han, M.; Shen, Z.; Huang, H.; Miao, X. Anti-hypertensive and Cardioprotective Effects of a Novel Apitherapy Formulation via Upregulation of Peroxisome Proliferator-Activated Receptor-α and -γ in Spontaneous Hypertensive Rats. Saudi J. Biol. Sci. 2018, 25, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; El-Sayed, Y.S.; Eldaim, M.A.; Ibrahim, A. Nephroprotective efficacy of ceftriaxone against cisplatin-induced subchronic renal fibrosis in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Aslan, Z.; Aksoy, L. Anti-inflammatory Effects of Royal Jelly on Ethylene Glycol Induced Renal Inflammation in Rats. Int. Braz. J. Urol. 2015, 41, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Zargar, H.R.; Hemmati, A.A.; Ghafourian, M.; Arzi, A.; Rezaie, A.; Javad-Moosavi, S.A. Long-term treatment with royal jelly improves bleomycin-induced pulmonary fibrosis in rats. Can. J. Physiol. Pharmacol. 2017, 95, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Almeer, R.S.; Soliman, D.; Kassab, R.B.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Metwally, D.; Abdel Moneim, A.E. Royal Jelly Abrogates Cadmium-Induced Oxidative Challenge in Mouse Testes: Involvement of the Nrf2 Pathway. Int. J. Mol. Sci. 2018, 19, 3979. [Google Scholar] [CrossRef]
- Park, H.M.; Hwang, E.; Lee, K.G.; Han, S.M.; Cho, Y.; Kim, S.Y. Royal Jelly Protects Against Ultraviolet B-induced Photoaging in Human Skin Fibroblasts via Enhancing Collagen Production. J. Med. Food 2011, 14, 899–906. [Google Scholar] [CrossRef]
- Koya-Miyata, S.; Okamoto, I.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Biosci. Biotechnol. Biochem. 2004, 68, 767–773. [Google Scholar] [CrossRef]
- Majtan, J.; Kumar, P.; Majtan, T.; Walls, A.F.; Klaudiny, J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp. Dermatol. 2010, 19, e73–e79. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Bianchini, R.; Roth-Walter, F.; Ohradanova-Repic, A.; Flicker, S.; Hufnagl, K.; Fischer, M.B.; Stockinger, H.; Jensen-Jarolim, E. IgG4 Drives M2a Macrophages to a Regulatory M2b-like Phenotype: Potential Implication in Immune Tolerance. Allergy 2019, 74, 483–494. [Google Scholar] [CrossRef]
- Trus, E.; Basta, S.; Gee, K. Who’s in Charge Here? Macrophage Colony Stimulating Factor and Granulocyte Macrophage Colony Stimulating Factor: Competing Factors in Macrophage Polarization. Cytokine 2020, 127, 154939. [Google Scholar] [CrossRef] [PubMed]
- Ohba, K.; Miyata, Y.; Yasuda, T.; Asai, A.; Mitsunari, K.; Matsuo, T.; Mochizuki, Y.; Matsunaga, N.; Sakai, H. Efficacy and Safety of Sunitinib Alternate Day Regimen in Patients With Metastatic Renal Cell Carcinoma in Japan: Comparison With Standard 4/2 Schedule. Asia Pac. J. Clin. Oncol. 2018, 14, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, R.L.; Paunu, N.; Turpeenniemi-Hujanen, T.; Reunamo, T.; Jekunen, A.; Kataja, V.; Sintonen, H.; Purmonen, T.; Kellokumpu-Lehtinen, P.L. Sunitinib First-line Treatment in Metastatic Renal Cell Carcinoma: Costs and Effects. Anticancer Res. 2019, 39, 5559–5564. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Takagi, T.; Kondo, T.; Fukuda, H.; Tachibana, H.; Yoshida, K.; Iizuka, J.; Okumi, M.; Ishida, H.; Tanabe, K. Efficacy of Axitinib After Nivolumab Failure in Metastatic Renal Cell Carcinoma. In Vivo 2020, 34, 1541–1546. [Google Scholar] [CrossRef]
- Sharif, S.N.; Darsareh, F. Effect of royal jelly on menopausal symptoms: A randomized placebo-controlled clinical trial. Complement Ther. Clin. Pract. 2019, 37, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Daugėlaitė, G.; Užkuraitytė, K.; Jagelavičienė, E.; Filipauskas, A.; Daugėlaitė, G. Prevention and Treatment of Chemotherapy and Radiotherapy Induced Oral Mucositis. Medicina 2019, 55, 25. [Google Scholar] [CrossRef]
- Laviron, M.; Boissonnas, A. Ontogeny of tumor-associated macrophages. Front. Immunol. 2019, 10, 1799. [Google Scholar] [CrossRef]
| Weeks (W) | Anorexia | p Value | Fatigue | p Value | ||
|---|---|---|---|---|---|---|
| Absence | Presence | Absence | Presence | |||
| 2 W | 1.07/1.03–1.12 | 1.00/0.94–1.04 | 0.004 | 1.05/1.02–1.13 | 0.97/0.93–1.06 | 0.006 |
| 4 W | 1.14/0.85–1.25 | 0.96/0.84–1.08 | 0.137 | 1.14/0.77–1.25 | 0.92/0.77–1.09 | 0.027 |
| 6 W | 0.83/0.68–1.10 | 0.87/0.65–1.26 | 0.772 | 0.83/0.74–1.14 | 0.88/0.65–1.21 | 0.941 |
| 8 W | 0.89/0.69–1.22 | 0.92/0.66–1.13 | 0.971 | 0.95/0.68–1.14 | 0.84/0.68–1.17 | 0.632 |
| 10 W | 0.93/0.47–1.09 | 0.93/0.68–1.01 | 0.885 | 0.98/0.58–1.09 | 0.92/0.71–1.02 | 0.883 |
| 12 W | 1.26/0.95–1.51 | 0.86/0.71–1.37 | 0.219 | 1.37/1/20–1.57 | 0.86/0.66–1.10 | 0.017 |
| Variables | Anorexia | p Value | Fatigue | p Value | ||
|---|---|---|---|---|---|---|
| Absence | Presence | Absence | Presence | |||
| TNF-α | ||||||
| 2W | 1.03/0.91–1.16 | 0.99/0.90–1.10 | 0.170 | 1.03/0.92–1.24 | 1.00/0.91–1.10 | 0.740 |
| 4W | 0.95/0.92–1.07 | 0.95/0.89–1.05 | 0.104 | 0.95/0.92–1.07 | 0.97/0.90–1.08 | 0.713 |
| 6W | 0.79/0.61–0.99 | 0.89/0.76–1.00 | 0.470 | 0.79/0.64–0.98 | 0.89/0.72–1.01 | 0.320 |
| 8W | 0.67/0.55–0.96 | 0.85/0.58–0.95 | 0.294 | 0.78/0.54–0.90 | 0.80/0.61–1.00 | 0.302 |
| 10W | 0.33/0.27–0.85 | 0.81/0.46–0.95 | 0.104 | 0.33/0.27–0.85 | 0.81/0.46–0.95 | 0.105 |
| 12W | 0.53/0.10–0.97 | 0.79/0.24–0.87 | 0.805 | 0.27/0.04–0.90 | 0.86/0.46–0.91 | 0.294 |
| TGF-β | ||||||
| 2W | 1.02/0.98–1.05 | 0.98/0.91–1.06 | 0.448 | 1.03/0.98–1.14 | 0.98/0.92–1.04 | 0.151 |
| 4W | 1.07/0.99–1.19 | 1.01/0.89–1.12 | 0.347 | 1.11/1.04–1.16 | 1.01/0.82–1.10 | 0.097 |
| 6W | 0.91/0.80–0.98 | 0.99/0.91–1.14 | 0.159 | 0.91/0.81–1.00 | 0.97/0.83–1.14 | 0.320 |
| 8W | 0.87/0.65–0.92 | 0.88/0.75–1.03 | 0.588 | 0.82/0.63–0.90 | 0.89/0.80–1.10 | 0.083 |
| 10W | 0.74/0.56–0.96 | 0.88/0.68–1.04 | 0.233 | 0.68/0.55–0.81 | 0.94/0.78–1.04 | 0.020 |
| 12W | 0.61/0.46–0.76 | 0.90/0.77–0.96 | 0.030 | 0.60/0.40–0.83 | 0.88/0.63–0.99 | 0.030 |
| IL-6 | ||||||
| 2W | 0.91/0.72–1.91 | 1.06/0.91–1.36 | 0.588 | 1.13/0.80–1.82 | 0.98/0.83–1.54 | 0.768 |
| 4W | 1.04/0.90–2.44 | 1.32/1.08–1.91 | 0.347 | 1.89/1.05–2.62 | 1.18/0.88–1.85 | 0.286 |
| 6W | 1.06/0.85–2.74 | 1.00/0.86–1.30 | 0.664 | 1.11/0.99–1.67 | 0.95/0.82–1.95 | 0.238 |
| 8W | 0.70/0.54–1.79 | 0.95/0.71–1.30 | 0.857 | 0.71/0.49–1.17 | 0.90/0.69–1.65 | 0.397 |
| 10W | 0.69/0.57–1.06 | 0.98/0.75–1.87 | 0.148 | 0.81/0.56–1.38 | 0.89/0.63–1.35 | 0.632 |
| 12W | 0.81/0.53–1.12 | 1.01/0.44–1.63 | 0.426 | 0.73/0.44–1.18 | 1.01/0.50–1.63 | 0.854 |
| Variables | 2 Weeks | 4 Weeks | 6 Weeks | 8 Weeks | 10 Weeks | 12 Weeks |
|---|---|---|---|---|---|---|
| TNF-α | 0.19/0.301 | 0.13/0.461 | 0.13/0.475 | 0.05/0.774 | 0.07/0.685 | 0.11/0.532 |
| TGF-β | 0.04/0.813 | 4.03/0.020 | 0.15/0.385 | 0.11/0.553 | 0.22/0.219 | 0.28/0.115 |
| IL-6 | 0.23/0.204 | 0.16/0.366 | 0.13/0.461 | 0.54/0.001 | 0.62/<0.001 | 0.33/0.061 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuno, T.; Miyata, Y.; Mukae, Y.; Otsubo, A.; Mitsunari, K.; Matsuo, T.; Ohba, K.; Sakai, H. Mechanisms Underlying the Inhibition of Tyrosine Kinase Inhibitor-Induced Anorexia and Fatigue by Royal Jelly in Renal Cell Carcinoma Patients and the Correlation between Macrophage Colony Stimulating Factor and Inflammatory Mediators. Med. Sci. 2020, 8, 43. https://doi.org/10.3390/medsci8040043
Yuno T, Miyata Y, Mukae Y, Otsubo A, Mitsunari K, Matsuo T, Ohba K, Sakai H. Mechanisms Underlying the Inhibition of Tyrosine Kinase Inhibitor-Induced Anorexia and Fatigue by Royal Jelly in Renal Cell Carcinoma Patients and the Correlation between Macrophage Colony Stimulating Factor and Inflammatory Mediators. Medical Sciences. 2020; 8(4):43. https://doi.org/10.3390/medsci8040043
Chicago/Turabian StyleYuno, Tsutomu, Yasuyoshi Miyata, Yuta Mukae, Asato Otsubo, Kensuke Mitsunari, Tomohiro Matsuo, Kojiro Ohba, and Hideki Sakai. 2020. "Mechanisms Underlying the Inhibition of Tyrosine Kinase Inhibitor-Induced Anorexia and Fatigue by Royal Jelly in Renal Cell Carcinoma Patients and the Correlation between Macrophage Colony Stimulating Factor and Inflammatory Mediators" Medical Sciences 8, no. 4: 43. https://doi.org/10.3390/medsci8040043
APA StyleYuno, T., Miyata, Y., Mukae, Y., Otsubo, A., Mitsunari, K., Matsuo, T., Ohba, K., & Sakai, H. (2020). Mechanisms Underlying the Inhibition of Tyrosine Kinase Inhibitor-Induced Anorexia and Fatigue by Royal Jelly in Renal Cell Carcinoma Patients and the Correlation between Macrophage Colony Stimulating Factor and Inflammatory Mediators. Medical Sciences, 8(4), 43. https://doi.org/10.3390/medsci8040043






