Zinc and Traumatic Brain Injury: From Chelation to Supplementation
Abstract
1. Introduction
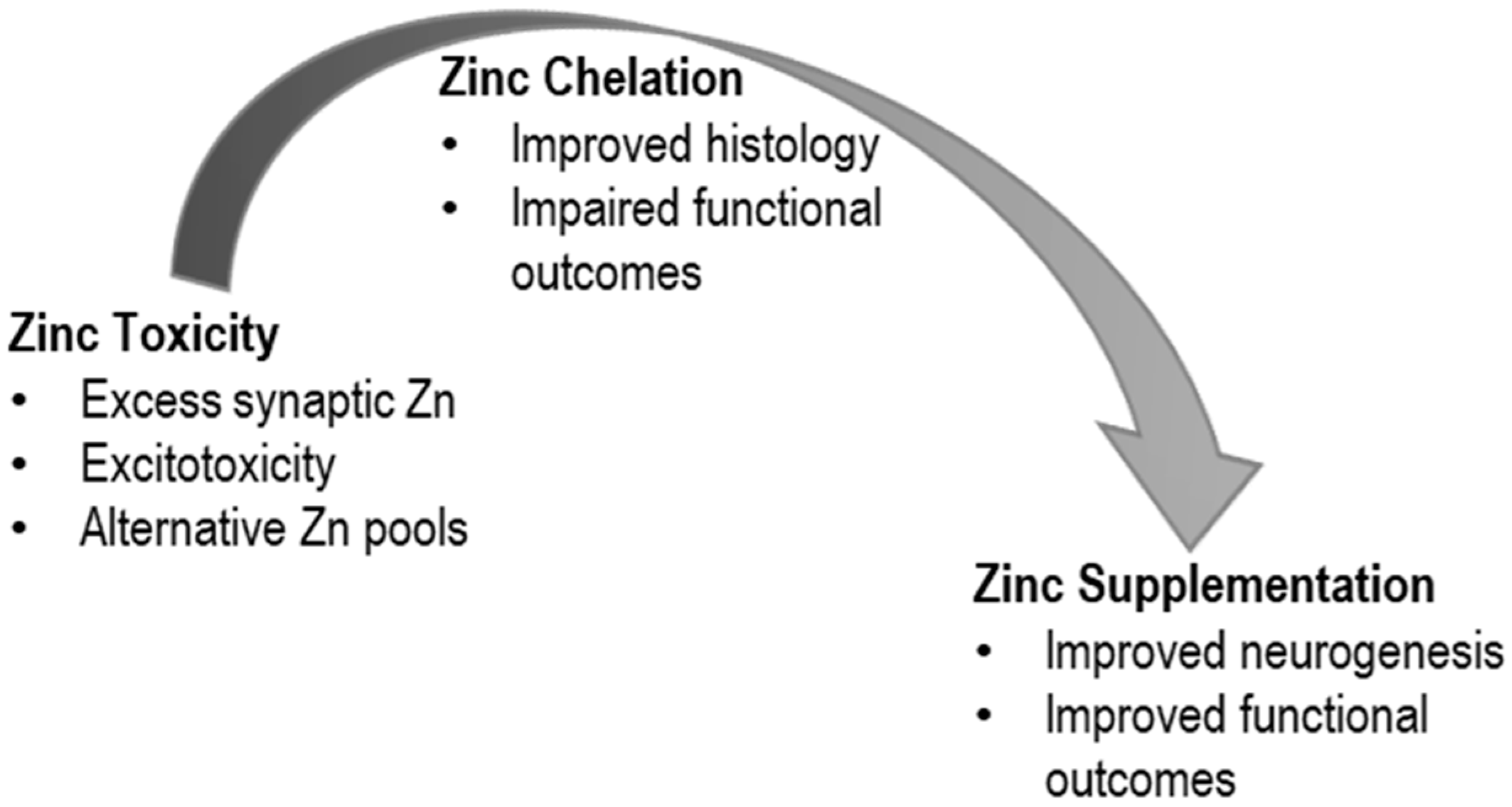
2. TBI and Zinc Toxicity
2.1. Zinc Sources
2.2. Mechanisms of Toxicity
3. TBI and Zinc Restriction
3.1. TBI and Zinc Chelation
3.2. TBI and Zinc Deficiency
4. TBI and Zinc Supplementation
4.1. Clinical Trial
4.2. Animal Studies
4.3. Role of TBI and Zinc in Neurogenesis
5. Conclusions
Funding
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the Global Incidence of Traumatic Brain Injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Salmond, C.H.; Sahakian, B.J. Cognitive Outcome in Traumatic Brain Injury Survivors. Curr. Opin. Crit. Care 2005, 11, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.E.; Starkstein, S.E. Pathophysiologic Aspects of Major Depression Following Traumatic Brain Injury. J. Head Trauma. Rehabil. 2005, 20, 475–487. [Google Scholar] [CrossRef]
- Levin, H.S.; McCauley, S.R.; Josic, C.P.; Boake, C.; Brown, S.A.; Goodman, H.S.; Merritt, S.G.; Brundage, S.I. Predicting Depression Following Mild Traumatic Brain Injury. Arch. Gen. Psychiatry 2005, 62, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W.; Koh, J.Y. Zinc and Brain Injury. Annu. Rev. Neurosci. 1998, 21, 347–375. [Google Scholar] [CrossRef]
- Suh, S.W.; Chen, J.W.; Motamedi, M.; Bell, B.; Listiak, K.; Pons, N.F.; Danscher, G.; Frederickson, C.J. Evidence that Synaptically-Released Zinc Contributes to Neuronal Injury after Traumatic Brain Injury. Brain Res. 2000, 852, 268–273. [Google Scholar] [CrossRef]
- Portbury, S.D.; Hare, D.J.; Sgambelloni, C.; Finkelstein, D.I.; Adlard, P.A. A Time-Course Analysis of Changes in Cerebral Metal Levels Following a Controlled Cortical Impact. Metallomics 2016, 8, 193–200. [Google Scholar] [CrossRef]
- Hellmich, H.L.; Eidson, K.A.; Capra, B.A.; Garcia, J.M.; Boone, D.R.; Hawkins, B.E.; Uchida, T.; Dewitt, D.S.; Prough, D.S. Injured Fluoro-Jade-Positive Hippocampal Neurons Contain High Levels of Zinc after Traumatic Brain Injury. Brain Res. 2007, 1127, 119–126. [Google Scholar] [CrossRef]
- Morris, D.R.; Levenson, C.W. Neurotoxicity of Zinc. Adv. Neurobiol. 2017, 18, 303–312. [Google Scholar]
- Koh, J.Y.; Choi, D.W. Zinc Alters Excitatory Amino Acid Neurotoxicity on Cortical Neurons. J. Neurosci. 1988, 8, 2164–2171. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cole, T.B.; Palmiter, R.D.; Koh, J.Y. Accumulation of Zinc in Degenerating Hippocampal Neurons of ZnT3-Null Mice after Seizures: Evidence against Synaptic Vesicle Origin. J. Neurosci. 2000, 20, RC79. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Maret, W.; Cuajungco, M.P. Zinc and Excitotoxic Brain Injury: A New Model. Neuroscientist 2004, 10, 18–25. [Google Scholar] [CrossRef]
- Sensi, S.L.; Jeng, J.M. Rethinking the Excitotoxic Ionic Milieu: The Emerging role of Zn(2+) in Ischemic Neuronal Injury. Curr. Mol. Med. 2004, 4, 87–111. [Google Scholar] [CrossRef] [PubMed]
- Doering, P.; Stoltenberg, M.; Penkowa, M.; Rungby, J.; Larsen, A.; Danscher, G. Chemical Blocking of Zinc Ions in CNS Increases Neuronal Damage Following Traumatic Brain Injury (TBI) in Mice. PLoS ONE 2010, 5, e10131. [Google Scholar] [CrossRef] [PubMed]
- Yeiser, E.C.; Lerant, A.A.; Casto, R.M.; Levenson, C.W. Free Zinc Increases at the Site of Injury after Cortical Stab Wounds in Mature but not Immature Rat Brain. Neurosci. Lett. 1999, 277, 75–78. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.H.; Palmiter, R.D.; Koh, J.Y. Zinc Released from Metallothionein-iii may Contribute to Hippocampal CA1 and Thalamic Neuronal Death Following Acute Brain Injury. Exp. Neurol. 2003, 184, 337–347. [Google Scholar] [CrossRef]
- Lee, S.J.; Koh, J.Y. Roles of Zinc and Metallothionein-3 in Oxidative Stress-Induced Lysosomal Dysfunction, Cell Death, and Autophagy in Neurons and Astrocytes. Mol. Brain 2010, 3, 30–39. [Google Scholar] [CrossRef]
- Jiang, D.; Sullivan, P.G.; Sensi, S.L.; Steward, O.; Weiss, J.H. Zn(2+) Induces Permeability Transition Pore Opening and Release of Pro-Apoptotic Peptides from Neuronal Mitochondria. J. Biol. Chem. 2001, 276, 47524–47529. [Google Scholar] [CrossRef]
- Sensi, S.L.; Ton-That, D.; Weiss, J.H. Mitochondrial Sequestration and Ca(2+)-Dependent Release of Cytosolic Zn(2+) Loads in Cortical Neurons. Neurobiol. Dis. 2002, 10, 100–108. [Google Scholar] [CrossRef]
- Stork, C.J.; Li, Y.V. Elevated Cytoplasmic Free Zinc and Increased Reactive Oxygen Species Generation in the Context of Brain Injury. Acta Neurochir. Suppl. 2016, 121, 347–353. [Google Scholar]
- Isaev, N.K.; Stelmashook, E.V.; Genrikhs, E.E. Role of Zinc and Copper Ions in the Pathogenetic Mechanisms of Traumatic Brain Injury and Alzheimer’s Disease. Rev. Neurosci. 2020, 31, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Sheline, C.T.; Behrens, M.M.; Choi, D.W. Zinc-induced Cortical Neuronal Death: Contribution of Energy Failure Attributable to Loss of NAD(+) and Inhibition of Glycolysis. J. Neurosci. 2000, 20, 3139–3146. [Google Scholar] [CrossRef]
- Sheline, C.T.; Cai, A.L.; Zhu, J.; Shi, C. Serum or Target Deprivation-Induced Neuronal Death Causes Oxidative Neuronal Accumulation of Zn2+ And Loss of NAD+. Eur. J. Neurosci. 2010, 32, 894–904. [Google Scholar] [CrossRef]
- Morley, S.N.; Power, J.M.; Coulson, E.J.; Bartlett, P.F. Zinc-Mediated Neuronal Death is Dependent on Trk Activation. Exp. Neurol. 2007, 205, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Sensi, S.L.; Yin, H.Z.; Weiss, J.H. AMPA/Kainate Receptor-Triggered Zn2+ Entry into Cortical Neurons Induces Mitochondrial Zn2+ Uptake and Persistent Mitochondrial Dysfunction. Eur. J. Neurosci. 2000, 12, 3813–3818. [Google Scholar] [CrossRef]
- Kim, Y.H.; Koh, J.Y. The Role of NADPH Oxidase and Neuronal Nitric Oxide Synthase in Zinc-Induced Poly(ADP-Ribose) Polymerase Activation and Cell Death in Cortical Culture. Exp. Neurol. 2002, 177, 407–418. [Google Scholar] [CrossRef]
- Sun, K.J.; Zhu, L.; Wang, H.D.; Ji, X.J.; Pan, H.; Chen, M.; Lu, T.J.; Fan, Y.W.; Cheng, H.L.; Hang, C.H.; et al. Zinc as Mediator of Ubiquitin Conjugation Following Traumatic Brain Injury. Brain Res. 2013, 1506, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, E.Y.; Gwag, B.J.; Sohn, S.; Koh, J.Y. Zinc-Induced Cortical Neuronal Death with Features of Apoptosis and Necrosis: Mediation by Free Radicals. Neuroscience 1999, 89, 175–182. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Ma, S.; Zhang, Y.; Liang, P. TPEN Attenuates Neural Autophagy Induced by Synaptically-Released Zinc Translocation and Improves Histological Outcomes after Traumatic Brain Injury in Rats. Ann. Clin. Lab. Sci. 2018, 48, 446–452. [Google Scholar] [PubMed]
- Hellmich, H.L.; Frederickson, C.J.; DeWitt, D.S.; Saban, R.; Parsley, M.O.; Stephenson, R.; Velasco, M.; Uchida, T.; Shimamura, M.; Prough, D.S. Protective Effects of Zinc Chelation in Traumatic Brain Injury Correlate with Upregulation of Neuroprotective Genes in Rat Brain. Neurosci. Lett. 2004, 355, 221–225. [Google Scholar] [CrossRef]
- Domínguez, M.I.; Blasco-Ibáñez, J.M.; Crespo, C.; Marqués-Marí, A.I.; Martínez-Guijarro, F.J. Zinc Chelation during Non-Lesioning Overexcitation Results in Neuronal Death in the Mouse Hippocampus. Neuroscience 2003, 116, 791–806. [Google Scholar] [CrossRef]
- Hellmich, H.L.; Eidson, K.; Cowart, J.; Crookshanks, J.; Boone, D.K.; Shah, S.; Uchida, T.; DeWitt, D.S.; Prough, D.S. Chelation of Neurotoxic Zinc Levels does not Improve Neurobehavioral Outcome after Traumatic Brain Injury. Neurosci. Lett. 2008, 440, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hawkins, B.E.; DeWitt, D.S.; Prough, D.S.; Maret, W. The Relationship between Transient Zinc Ion Fluctuations and Redox Signaling in the Pathways of Secondary Cellular Injury: Relevance to Traumatic Brain Injury. Brain Res. 2010, 1330, 131–141. [Google Scholar] [CrossRef] [PubMed]
- McClain, C.J.; Twyman, D.L.; Ott, L.G.; Rapp, R.P.; Tibbs, P.A.; Norton, J.A.; Kasarskis, E.J.; Dempsey, R.J.; Young, B. Serum and Urine Zinc Response in Head-Injured Patients. J. Neurosurg. 1986, 64, 224–230. [Google Scholar] [CrossRef]
- Corniola, R.S.; Tassabehji, N.M.; Hare, J.; Sharma, G.; Levenson, C.W. Zinc Deficiency Impairs Neuronal Precursor Cell Proliferation and Induces Apoptosis via P53-Mediated Mechanisms. Brain Res. 2008, 1237, 52–61. [Google Scholar] [CrossRef]
- Adamo, A.M.; Zago, M.P.; Mackenzie, G.G.; Aimo, L.; Keen, C.L.; Keenan, A.; Oteiza, P.I. The Role of Zinc in the Modulation of Neuronal Proliferation and Apoptosis. Neurotox. Res. 2010, 17, 1–14. [Google Scholar] [CrossRef]
- Seth, R.; Corniola, R.S.; Gower-Winter, S.D.; Morgan, T.J., Jr.; Bishop, B.; Levenson, C.W. Zinc Deficiency Induces Apoptosis Via Mitochondrial P53- and Caspase-Dependent Pathways in Human Neuronal Precursor Cells. J. Trace Elem. Med. Biol. 2015, 30, 59–65. [Google Scholar] [CrossRef]
- Suh, S.W.; Won, S.J.; Hamby, A.M.; Yoo, B.H.; Fan, Y.; Sheline, C.T.; Tamano, H.; Takeda, A.; Liu, J. Decreased Brain Zinc Availability Reduces Hippocampal Neurogenesis in Mice and Rats. J. Cereb. Blood Flow Metab. 2009, 29, 1579–1588. [Google Scholar] [CrossRef]
- Gao, H.L.; Zheng, W.; Xin, N.; Chi, Z.H.; Wang, Z.Y.; Chen, J.; Wang, Z.Y. Zinc Deficiency Reduces Neurogenesis Accompanied by Neuronal Apoptosis through Caspase-Dependent and -Independent Signaling Pathways. Neurotox. Res. 2009, 16, 416–425. [Google Scholar] [CrossRef]
- Cope, E.C.; Morris, D.R.; Scrimgeour, A.G.; VanLandingham, J.W.; Levenson, C.W. Zinc Supplementation Provides Behavioral Resiliency in a Rat Model of Traumatic Brain Injury. Physiol. Behav. 2011, 104, 942–947. [Google Scholar] [CrossRef]
- Abdul-Muneer, P.M.; Pfister, B.J.; Haorah, J.; Chandra, N. Role of Matrix Metalloproteinases in the Pathogenesis of Traumatic Brain Injury. Mol. Neurobiol. 2016, 53, 6106–6123. [Google Scholar] [CrossRef] [PubMed]
- Scrimgeour, A.G.; Carrigan, C.T.; Condlin, M.L.; Urso, M.L.; Van den Berg, R.M.; van Helden, H.P.M.; Montain, S.J.; Joosen, M.J.A. Dietary Zinc Modulates Matrix Metalloproteinases in Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2495–2506. [Google Scholar] [CrossRef]
- Choi, B.Y.; Kim, J.H.; Kim, H.J.; Lee, B.E.; Kim, I.Y.; Sohn, M.; Suh, S.W. Zinc Chelation Reduces Traumatic Brain Injury-Induced Neurogenesis in the Subgranular Zone of the Hippocampal Dentate Gyrus. J. Trace Elem. Med. Biol. 2014, 28, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Ott, L.; Kasarskis, E.; Rapp, R.; Moles, K.; Dempsey, R.J.; Tibbs, P.A.; Kryscio, R.; McClain, C. Zinc Supplementation is Associated with Improved Neurologic Recovery Rate and Visceral Protein Levels of Patients with Severe Closed Head Injury. J. Neurotrauma 1996, 13, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Khazdouz, M.; Mazidi, M.; Ehsaei, M.R.; Ferns, G.; Kengne, A.P.; Norouzy, A.R. Impact of Zinc Supplementation on the Clinical Outcomes of Patients with Severe Head Trauma: A Double-Blind Randomized Clinical Trial. J. Diet. Suppl. 2018, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cope, E.C.; Morris, D.R.; Scrimgeour, A.G.; Levenson, C.W. Use of Zinc as a Treatment for Traumatic Brain Injury in the Rat: Effects on Cognitive and Behavioral Outcomes. Neurorehabilit. Neural Repair 2012, 26, 907–913. [Google Scholar] [CrossRef]
- Hill, A.S.; Sahay, A.; Hen, R. Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology 2015, 40, 2368–2378. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult Hippocampal Neurogenesis and Cognitive Flexibility—Linking Memory and Mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Micheli, L.; Ceccarelli, M.; D’Andrea, G.; Tirone, F. Depression and Adult Neurogenesis: Positive Effects of the Antidepressant Fluoxetine and of Physical Exercise. Brain Res. Bull. 2018, 143, 181–193. [Google Scholar] [CrossRef]
- Tunc-Ozcan, E.; Peng, C.Y.; Zhu, Y.; Dunlop, S.R.; Contractor, A.; Kessler, J.A. Activating Newborn Neurons Suppresses Depression and Anxiety-Like Behaviors. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Cope, E.C.; Morris, D.R.; Gower-Winter, S.D.; Brownstein, N.C.; Levenson, C.W. Effect of Zinc Supplementation on Neuronal Precursor Proliferation in the Rat Hippocampus after Traumatic Brain Injury. Exp. Neurol. 2016, 279, 96–103. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levenson, C.W. Zinc and Traumatic Brain Injury: From Chelation to Supplementation. Med. Sci. 2020, 8, 36. https://doi.org/10.3390/medsci8030036
Levenson CW. Zinc and Traumatic Brain Injury: From Chelation to Supplementation. Medical Sciences. 2020; 8(3):36. https://doi.org/10.3390/medsci8030036
Chicago/Turabian StyleLevenson, Cathy W. 2020. "Zinc and Traumatic Brain Injury: From Chelation to Supplementation" Medical Sciences 8, no. 3: 36. https://doi.org/10.3390/medsci8030036
APA StyleLevenson, C. W. (2020). Zinc and Traumatic Brain Injury: From Chelation to Supplementation. Medical Sciences, 8(3), 36. https://doi.org/10.3390/medsci8030036



