Network Pharmacology Approach Reveals the Potential Immune Function Activation and Tumor Cell Apoptosis Promotion of Xia Qi Decoction in Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of XQD Chemical Ingredients
2.2. Target Selection and Herb-Ingredient-Target Network Construction of XQD
2.3. Collection of Therapeutic Targets for LC
2.4. Protein-Protein Interaction Data
2.5. Herb-Ingredient-LC Therapeutic Target Network Analysis
2.6. Pathway Enrichment Analysis
2.7. Relationship Analysis Between LC Targets and Immunological Targets Affected by XQD
3. Results
3.1. OB Prediction and DL Calculation
3.2. Target Selection and Herb-Ingredient-Target Network Construction of XQD
3.3. Construction of PPI Network in XQD
3.4. Constructing the Network of Herb-Ingredient-Target-LC Therapeutic Target
3.5. Pathway Analysis of XQD Acting on LC Therapeutic Targets
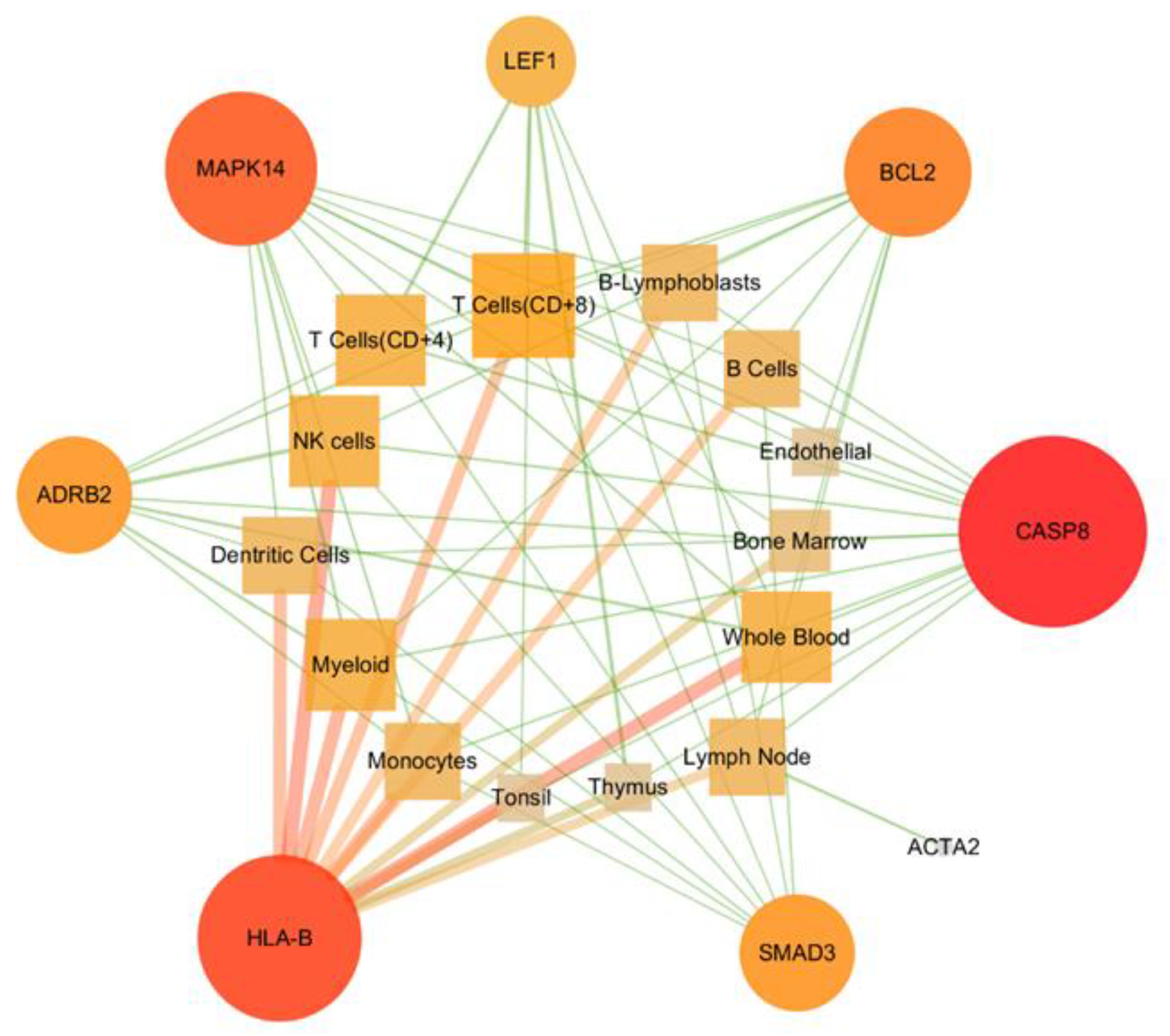
3.6. Constructing Target-Immune Tissues and Cell Network
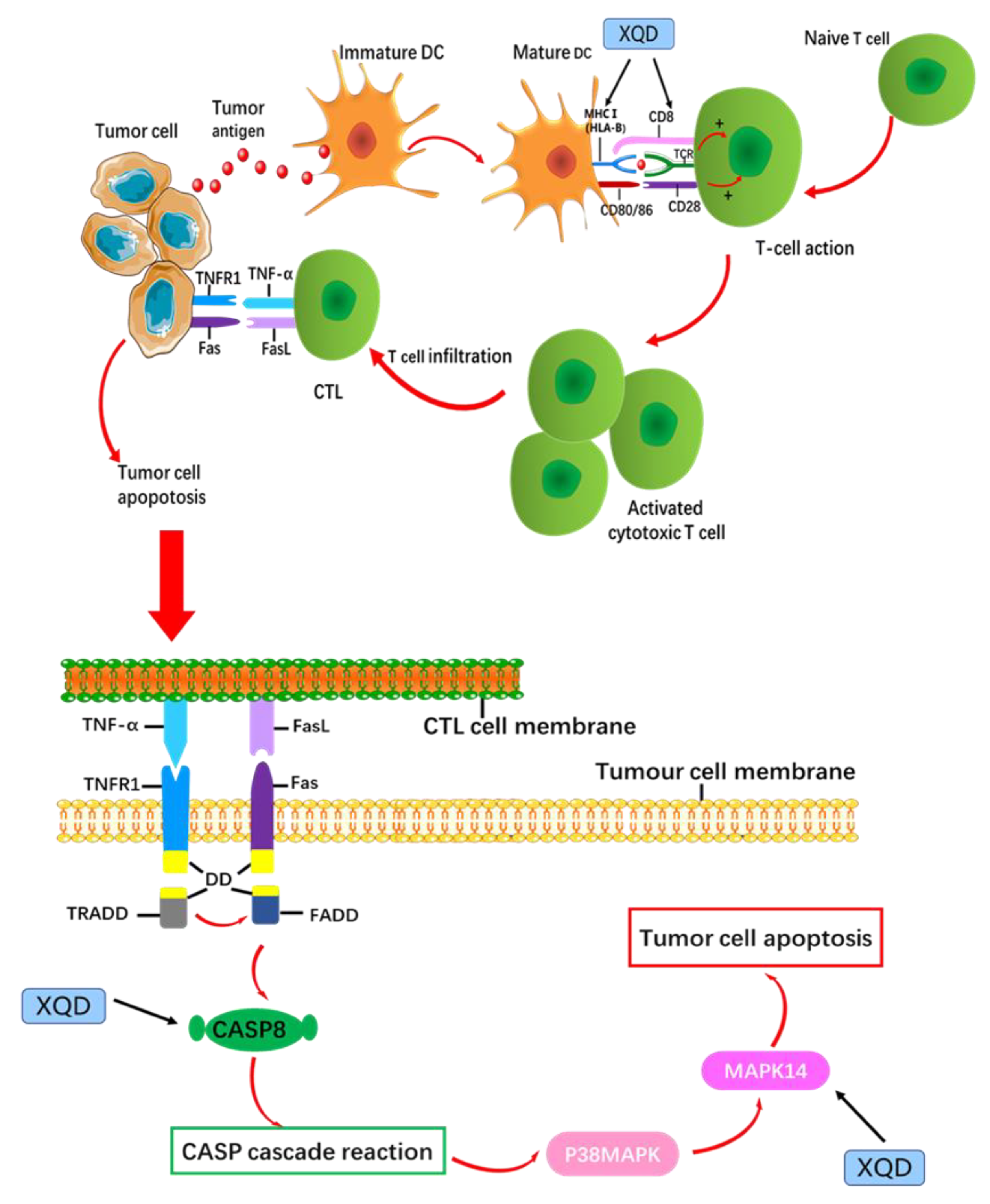
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stumbryte, A.; Gudleviciene, Z.; Kundrotas, G.; Dabkeviciene, D.; Kunickaite, A.; Cicenas, S. Individual and combined effect of TP53; MDM2; MDM4; MTHFR.; CCR5; and CASP8 gene polymorphisms in lung cancer. Oncotarget 2018, 9, 3214–3229. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics: 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Dai, M.; Chen, W.Q.; Li, N. Cancer trends in China. Jpn. J. Clin. Oncol. 2010, 40, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Zheng, R.S.; Baade, P.D.; Zhang, S.W.; Zeng, H.M.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China: 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics: 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- De-Freitas, A.C.; Gurgel, A.P.; De-Lima, E.G.; Sao-Marcos, B.D.; Do-Amaral, C.M.M. Human papillomavirus and lung carcinogenesis: An overview. J. Cancer Res. Clin. Oncol. 2016, 142, 2415–2427. [Google Scholar] [CrossRef]
- Cheung, F. TCM: Made in China. Nature 2011, 480, S82–S83. [Google Scholar] [CrossRef]
- Jiang, W.Y. Therapeutic wisdom in traditional Chinese medicine: A perspective from modern science. Trends Pharmacol. Sci. 2005, 26, 558–563. [Google Scholar] [CrossRef]
- Zimmermann, G.R.; Lehar, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Aloudat, S.; Singh, S.A. systematic review of patient and health system characteristics associated with late referral in chronic kidney disease. BMC Nephrol. 2008, 9, 3. [Google Scholar] [CrossRef]
- Shi, S.H.; Cai, Y.P.; Cai, X.J.; Zheng, X.Y.; Cao, D.S.; Ye, F.Q.; Xiang, Z. A network pharmacology approach to understanding the mechanisms of action of traditional medicine: Bushenhuoxue formula for treatment of chronic kidney disease. PLoS ONE. 2014, 9, e89123. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.J.; Zhang, X.; Wang, S.R.; Li, X. Experience of Professor Li Xian in the Application of Xia Qi Decoction. Chin. Med. Mod. Distance Educ. China 2017, 15, 61–62. [Google Scholar]
- Du, C.; Lin, J. Xia Qi Decoction clinical application and experience. Guangming J. Chin. Med. 2012, 27, 1262–1263. [Google Scholar]
- Lin, Y.; Huang, S.; Huang, Z.X. Clinical observation of xieqi decoction in the treatment of acute exacerbation of chronic bronchitis. Guangming J. Chin. Med. 2019, 34, 2163–2165. [Google Scholar]
- Gao, H.L.; Chen, G.F.; Wu, R.T.; Li, S.J. Observation on the effect of XQD plus or minus in the treatment of lung cancer with obstructive inflammation. Da Jia Jian Kang (Acad. Version) 2013, 7, 21–22. [Google Scholar]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2017, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Zhang, B.X.; Luo, S.J.; Yan, J.; Gu, H.; Luo, J.; Zhang, Y.L.; Tao, O.; Wang, Y. Construction of automatic elucidation platform for mechanism of traditional Chinese medicine. Chin. J. Chin. Mater. Med. 2015, 40, 3697–3702. [Google Scholar]
- Barrett, T.; Troup, D.B.; Wilhite, S.E.; Ledoux, P.; Rudnev, D.; Evangelista, C.; Kim, I.F.; Soboleva, A.; Tomashevsky, M.; Edgar, R. NCBI GEO: Mining tens of millions of expression profiles—database and tools update. Nucleic Acids Res. 2007, 35, D760–D765. [Google Scholar] [CrossRef]
- Li, Y.H.; Yu, C.Y.; Li, X.X.; Zhang, P.; Tang, J.; Yang, Q.; Fu, T.; Zhang, X.; Cui, X.; Tu, G.; et al. Therapeutic target database update 2018: Enriched resource for facilitating bench-to-clinic research of targeted therapeutics. Nucleic Acids Res. 2017, 46, D1121–D1127. [Google Scholar]
- Hamosh, A.; Scott, A.F.; Amberger, J.; Bocchini, C.; Valle, D.; Mckusick, V.A. Online Mendelian inheritance in man (OMIM): A knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005, 33, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.W.; Ho, M.Y.; Shui, H.A.; Tsai, C.B.; Tseng, M.J. Analysis of dermal papilla cell interactome using STRING database to profile the ex vivo hair growth inhibition effect of a vinca alkaloid drug, colchicine. Int. J. Mol. Sci. 2015, 16, 3579–3598. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Y.; Luo, S.J.; Qiao, Y.J. TCM grammar systems: An approach to aid the interpretation of the molecular interactions in Chinese herbal medicine. J. Ethnopharmacol. 2011, 137, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Alpi, E.; Bely, B.; Bingley, M.; Britto, R.; Bursteinas, B.; Busiello, G. UniProt, a worldwide hub of protein knowledge. Nucleic Acids Res. 2009, 47, D506–D515. [Google Scholar]
- Yu, G.; Wang, W.; Wang, X.; Xu, M.; Zhang, L.; Ding, L.; Guo, R.; Shi, Y. Network pharmacology-based strategy to investigate pharmacological mechanisms of zuojinwan for treatment of gastritis. BMC Complement. Altern. Med. 2018, 18, 292. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny-Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The human gene integrator. Database 2010, 2010, 1–16. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 2012, 13, 6964–6982. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Tan, H.Y.; Cheung, F.; Wang, N.; Huang, J.; Feng, Y. A network-based pharmacology study of the herb-induced liver injury potential of traditional hepatoprotective Chinese herbal medicines. Molecules 2017, 22, 632. [Google Scholar] [CrossRef]
- Bin-Sayeed, M.S.; Ameen, S.S. Beta-Sitosterol: A Promising but Orphan Nutraceutical to Fight Against Cancer. Nutr. Cancer 2015, 67, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Tran, E.; Ong, C.K.; Lee, S.K.; Do, P.T.; Huynh, T.T.; Nguyen, T.H.; Lee, J.J.; Tan, Y.; et al. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J. Cell Physiol. 2003, 197, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rankin, G.O.; Li, Z.L.; DePriest, L.; Chen, Y.C. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011, 128, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Ahn, W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2008, 2, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Chen, X.B.; Wang, S.L.; Li, Y. Expression of ER and AR in lung cancer. Chin. J. Lung Cancer 2008, 11, 126–129. [Google Scholar]
- Wang, Y.; Romigh, T.; He, X.; Tan, M.H.; Orloff, M.S.; Silverman, R.H. Differential regulation of PTEN expression by androgen receptor in prostate and breast cancers. Oncogene 2011, 30, 4327–4338. [Google Scholar] [CrossRef] [PubMed]
- Stabile, L.P.; Lyker, J.S.; Gubish, C.T.; Zhang, W.P.; Grandis, J.R.; Siegfried, J.M. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005, 65, 1459–1470. [Google Scholar] [CrossRef]
- Yuk, J.E.; Woo, J.S.; Yun, C.Y.; Lee, J.S.; Kim, J.H.; Song, G.Y.; Yang, E.J.; Hur, I.K.; Kim, I.S. Effects of lactose-β-sitosterol and β-sitosterol on ovalbumin-induced lung inflammation in actively sensitized mice. Int. Immunopharmacol. 2007, 7, 1517–1527. [Google Scholar] [CrossRef]
- Fraile, L.; Crisci, E.; Lorena, C.; María, A.; Navarro, J.O.; María, M. Immunomodulatory properties of beta-sitosterol in pig immune responses. Int. Immunopharmacol. 2012, 13, 316–321. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Hu, M.; Wei, P.; Zhu, W. BAMBI overexpression together with β-sitosterol ameliorates NSCLC via inhibiting autophagy and inactivating TGF-β/Smad2/3 pathway. Oncol. Rep. 2017, 37, 3046–3054. [Google Scholar] [CrossRef]
- Rajavel, T.; Mohankumar, R.; Archunan, G.; Ruckmani, K.; Devi, K.P. Beta sitosterol and Daucosterol (phytosterols identified in Grewia tiliaefolia) perturbs cell cycle and induces apoptotic cell death in A549 cells. Sci. Rep. 2017, 7, 3418. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, H.; Li, W.; Wang, Y.; Mu, Q.; Wang, X.; He, Z.; Yao, H. Protective effect of cavidine on acetic acid-induced murine colitis via regulating antioxidant, cytokine profile and NF-kB signal transduction pathways. Chem. Biol. Interact. 2015, 239, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, H.; Li, W.; Mu, Q.; Yao, H.; Wang, Y. Anti-inflammatory effects of Cavidine in Vitro and in Vivo, a Selective COX-2 Inhibitor in LPS-Induced Peritoneal Macrophages of Mouse. Inflammation 2015, 38, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Jin, L.T.; Lu, L.L.; Lu, X.Y.; Zhang, C.L.; Zhang, F.Y.; Liang, W. Naringenin reduces lung metastasis in a breast cancer resection model. Protein Cell 2011, 2, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic Acid Decorated Naringenin Nanoparticles, Appraisal of Chemopreventive and Curative Potential for Lung Cancer. Pharmaceutics 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.Y.; Park, C.; Hwang, H.J.; Kim, G.Y.; Choi, B.T.; Kim, W.J. Naringenin up-regulates the expression of death receptor 5 and enhances TRAIL-induced apoptosis in human lung cancer A549 cell. Mol. Nutr. Food Res. 2011, 55, 300–309. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Ai, X.H.; Cheng, B.J.; Lu, S. Formononetin suppresses the proliferation of human non-small cell lung cancer through induction of cell cycle arrest and apoptosis. Int. J. Clin. Exp. Pathol. 2014, 7, 8453–8461. [Google Scholar]
- Cathcart, M.C.; Useckaite, Z.; Drakeford, C.; Semik, V.; Lysaght, J.; Gately, K.; O’Byrne, K.J.; Pidgeon, G.P. Anti-cancer effects of baicalein in non-small cell lung cancer in-vitro and in-vivo. BMC Cancer 2016, 6, 707. [Google Scholar] [CrossRef]
- Lee, H.Z.; Leung, H.W.C.; Lai, M.Y.; Wu, C.H. Baicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma ch27 cells. Anticancer. Res. 2015, 25, 959–964. [Google Scholar]
- Ohdaira, H.; Sekiguchi, M.; Miyata, K.; Yoshida, K. MicroRNA-494 suppresses cell proliferation and induces senescence in A549 lung cancer cells. Cell Prolif. 2012, 45, 32–38. [Google Scholar] [CrossRef]
- Roberson, R.S.; Kussick, S.J.; Vallieres, E.; Chen, S.Y.J.; Wu, D.Y. Escape from Therapy-I Induced Accelerated Cellular Senescence in p53-Null Lung Cancer Cells and in Human Lung Cancers. Cancer Res. 2005, 65, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Arden, K.C. FoXO: Linking New Signaling Pathways. Mol. Cell 2004, 14, 416–418. [Google Scholar] [CrossRef]
- Li, H.; Ouyang, R.Y.; Wang, Z.; Zhou, W.H.; Chen, H.Y.; Jiang, Y.W.; Zhang, Y.; Li, H.; Liao, M.; Wang, W.; et al. MiR-150 promotes cellular metastasis in non-small cell lung cancer by targeting FOXO4. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, L.; Zhao, F.; Yang, G.; Wang, J.J. Tetrandrine suppresses lung cancer growth and induces apoptosis, potentially via the VEGF/HIF-1α/ICAM-1 signaling pathway. Oncol Lett. 2018, 15, 7433–7437. [Google Scholar] [CrossRef]
- Xu, R.T.; Shu, Y.Q. Estrogen and its signaling pathway in non-small cell lung cancer (NSCLC). J. Nanjing Med. Univ. 2009, 23, 217–223. [Google Scholar] [CrossRef]
- Marquez-Garban, D.C.; Pietras, R.J. Estrogen-Signaling Pathways in Lung Cancer. Adv. Exp. Med. Biol. 2008, 617, 281–289. [Google Scholar]
- Bikkavilli, R.K.; Avasarala, S.; Van Scoyk, M.; Arcaroli, J.; Brzezinski, C.; Zhang, W.; Edwards, M.G.; Rathinam, M.K.; Zhou, T.; Tauler, J. Wnt7a is a novel inducer of β-catenin-independent tumor-suppressive cellular senescence in lung cancer. Oncogene 2015, 34, 5317–5328. [Google Scholar] [CrossRef]
- Mohd, F.; Haitao, W.; Uma, G.; Little, P.J.; Xu, J.P.; Zheng, W.H. FOXO Signaling Pathways as Therapeutic Targets in Cancer. Int. J. Biol. Sci. 2017, 13, 815–827. [Google Scholar]
- Liu, W.; Chen, X.; He, Y.; Tian, Y.; Xu, L.; Ma, Y.; Hu, P.; Su, K.; Luo, Z.; Wei, L.; et al. TNF α inhibits xenograft tumor formation by A549 lung cancer cells in nude mice via the HIF 1α/VASP signaling pathway. Oncol. Rep. 2019, 41, 2418–2430. [Google Scholar] [CrossRef]
- Zhong, G.W.; Chen, X.; Fang, X.; Wang, D.S.; Xie, M.X.; Chen, Q. Fra-1 is upregulated in lung cancer tissues and inhibits the apoptosis of lung cancer cells by the P53 signaling pathway. Oncol. Rep. 2015, 35, 447–453. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yang, W.; Lu, J.B. Knockdown of GluA2 induces apoptosis in non-small-cell lung cancer A549 cells through the p53 signaling pathway. Oncol. Lett. 2017, 14, 1005–1010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoque, M.O.; Brait, M.; Rosenbaum, E.; Poeta, M.L.; Pal, P.; Begum, S.; Dasgupta, S.; Carvalho, A.L.; Ahrendt, S.A.; Westra, W.H.; et al. Genetic and Epigenetic Analysis of erbB Signaling Pathway Genes in Lung Cancer. J. Thorac. Oncol. 2010, 5, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.T.; Li, J.; Wang, J.H.; Wu, Q.; Yang, P.A.; Hsu, H.C.; Smythies, L.E.; Mountz, J.D. IL-17 Activates the Canonical NF-kB Signaling Pathway in Autoimmune B Cells of BXD2 Mice To Upregulate the Expression of Regulators of G-Protein Signaling 16. J. Immunol. 2010, 184, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Li, Y.Y.; Wang, J.M.; Manthari, R.K.; Wang, J.D. Fluoride induces apoptosis and autophagy through the IL-17 signaling pathway in mice hepatocytes. Arch. Toxicol. 2018, 92, 3277–3289. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.; Kitajewski, J. VEGF and Delta-Notch, interacting signalling pathways in tumor angiogenesis. Br. J. Cancer. 2008, 99, 1204–1209. [Google Scholar] [CrossRef]
- Stacker, S.A.; Achen, M.G. The VEGF signaling pathway in cancer, the road ahead. Chin. J. Cancer 2013, 32, 297–302. [Google Scholar]
- Cao, X.T.; He, W. Medical Immunology; People’s Medical Publishing House: Beijing, China, 2015; p. 133. [Google Scholar]
- Geng, J.; Altman, J.D.; Krishnakumar, S.; Raghavan, M. Empty conformers of HLA-I preferentially bind CD8 and regulate CD8+ T cell function. ELife Sci. 2018, 7, 1–20. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.J.; Shi, G.L.; Ni, L.; Song, C.X.; Zhang, Z.X.; Xu, S.F. Analysis of HLA-A, HLA-B and HLA-DRB1 alleles in Chinese patients with lung cancer. Genet Mol. Res. 2010, 9, 750–755. [Google Scholar] [CrossRef]
- Ji, G.H.; Li, M.; Cui, Y.; Wang, J.F. The relationship of CASP8 polymorphism and cancer susceptibility, a meta-analysis. Cell Mol. Biol. 2014, 6, 20–28. [Google Scholar]
- Zhou, C.C.; Wu, Y.L.; Fei, K. Targeted Therapy for Lung Cancer; People’s Medical Publishing House: Beijing, China, 2016; p. 18. [Google Scholar]
- Scatena, R.; Bottoni, P.; Botta, G.; Martorana, G.E.; Giardina, B. The role of mitochondria in pharmaco-toxicology: A reevaluation of an old, newly emerging topic. AJP Cell Physiol. 2007, 293, C12–C21. [Google Scholar] [CrossRef]
- Hacker, G.; Paschen, S.A. Therapeutic targets in the mitochondrial apoptotic pathway. Expert. Opin. Targets 2007, 11, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.; Landvik, N.E.; Lind, H.; Skaug, V.; Haugen, A.; Zienolddiny, S. A combination of functional polymorphisms in the CASP8, MMP1, IL10 and SEPS1 genes affects risk of non-small cell lung cancer. Lung Cancer 2011, 71, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Maelfait, J.; Vercammen, E.; Janssens, S.; Schotte, P.; Haegman, M.; Magez, S.; Beyaert, R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J. Exp. Med. 2008, 205, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Zheng, L.; Ahmad, M.; Wang, J.; Speirs, C.K.; Siegel, R.M.; Dale, J.K.; Puck, J.; Davis, J.; Hall, C.G.; et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 2002, 419, 395–399. [Google Scholar] [CrossRef]
- Rébé, C.; Cathelin, S.; Launay, S.; Filomenko, R.; Prévotat, L.; L’Ollivier, C.; Gyan, E.; Micheau, O.; Grant, S.; Dubart-Kupperschmitt, A.; et al. Caspase-8 prevents sustained activation of NF-kB in monocytes undergoing macrophagic differentiation. Blood 2007, 109, 1442–1450. [Google Scholar] [CrossRef]
- Galluzzi, L.; Joza, N.; Tasdemir, E.; Maiuri, M.C.; Hengartner, M.; Abrams, J.M.; Tavernarakis, N.; Penninger, J.; Madeo, F.; Kroemer, G. No death without life: Vital functions of apoptotic effectors. Cell Death Differ. 2008, 15, 1113–1123. [Google Scholar] [CrossRef]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef]
- Desideri, E.; Vegliante, R.; Cardaci, S.; Nepravishta, R.; Paci, M.; Ciriolo, M.R. MAPK14/p38α-dependent modulation of glucose metabolism affects ROS levels and autophagy during starvation. Autophagy 2014, 10, 1652–1665. [Google Scholar] [CrossRef]
- Valladares, A.; Alvarez, A.M.; Ventura, J.J.; Roncero, C.; Benito, M.; Porras, A. p38 Mitogen-Activated Protein Kinase Mediates Tumor Necrosis Factor-α-Induced Apoptosis in Rat Fetal Brown Adipocytes. Endocrinology 2000, 141, 4383–4395. [Google Scholar] [CrossRef]
- Edlund, S.; Bu, S.; Schuster, N.; Aspenström, P.; Heuchel, R.; Heldin, N.E.; Dijke, P.; Heldin, C.H.; Landström, M. Transforming growth factor-beta 1 (TGF-beta)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-beta-activated kinase 1 and mitogen-activated protein kinase 3. Mol. Bio. Cell 2003, 14, 529–544. [Google Scholar] [CrossRef]
- Zhuang, S.; Demirs, J.T.; Kochevar, I.E. p38 Mitogen-activated Protein Kinase Mediates Bid Cleavage, Mitochondrial Dysfunction, and Caspase-3 Activation during Apoptosis Induced by Singlet Oxygen but Not by Hydrogen Peroxide. J. Bio. Chem. 2000, 275, 25939–25948. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Chen, H.P.; Cheng, Y.J.; Lin, Y.H.; Liu, K.W.; Chen, Y.J.; Hou, M.F.; Wu, Y.C.; Lee, Y.C.; Yuan, S.S. The synthetic flavonoid WYC02-9 inhibits colorectal cancer cell growth through ROS-mediated activation of MAPK14 pathway. Life Sci. 2013, 92, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.T.; Lin, S.S.; Wang, C.K.; Lee, Y.B.; Chen, K.S.; Fong, Y.; Shih, Y.W. Acacetin inhibits the invasion and migration of human NSCLC a549 cells by suppressing the p38α MAPK signaling pathway. Mol. Cell Biochem. 2011, 350, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Juo, P.; Kuo, C.J.; Reynolds, S.E.; Konz, R.F.; Raingeaud, J.; Davis, R.J.; Ulevitch, R.J.; Nemerow, G.R.; Han, J. Fas activation of the p38 mitogen-activated protein kinase signaling pathway requires ICE CED-3 family proteases. Mol. Cell Biol. 1997, 17, 24–35. [Google Scholar] [CrossRef]
- Pogozelski, A.; Li, P.; Jiang, H.P.; Yan, Z. p38 gamma MAPK is required for exercise-induced Pgc-1alpha expression and mitochondrial biogenesis in skeletal muscle. FASEB J. 2008, 22, 754–767. [Google Scholar]
- Toshiyuki, O.; Glenn, E.; Michael, B. Yaffe1MAP kinase pathways activated by stress, the p38MAPK pathway. Crit. Care Med. 2000, 28, 67–71. [Google Scholar]
- Saitou, Y.; Shiraki, K.; Yamanaka, T.; Miyashita, K.; Inoue, T.; Yamanaka, Y.; Yamaguchi, Y.; Enokimura, N.; Yamamoto, N.; Itou, K.; et al. Augmentation of tumor necrosis factor family-Induced apoptosis by E3330 in human hepatocellular carcinoma cell lines via inhibition of NF kappa B. World J. Gastroenterol. 2005, 11, 6258–6261. [Google Scholar] [CrossRef]
- Pearl-Yafe, M.; Yolcu, E.S.; Stein, J.; Kaplan, O.; Yaniv, I.; Shirwan, H.; Askenasy, N. Fas ligand enhances hematopoietic cell engraftment through abrogation of alloimmune responses and nonimmunogenic interactions. Stem. Cells 2007, 25, 1448–1455. [Google Scholar] [CrossRef]
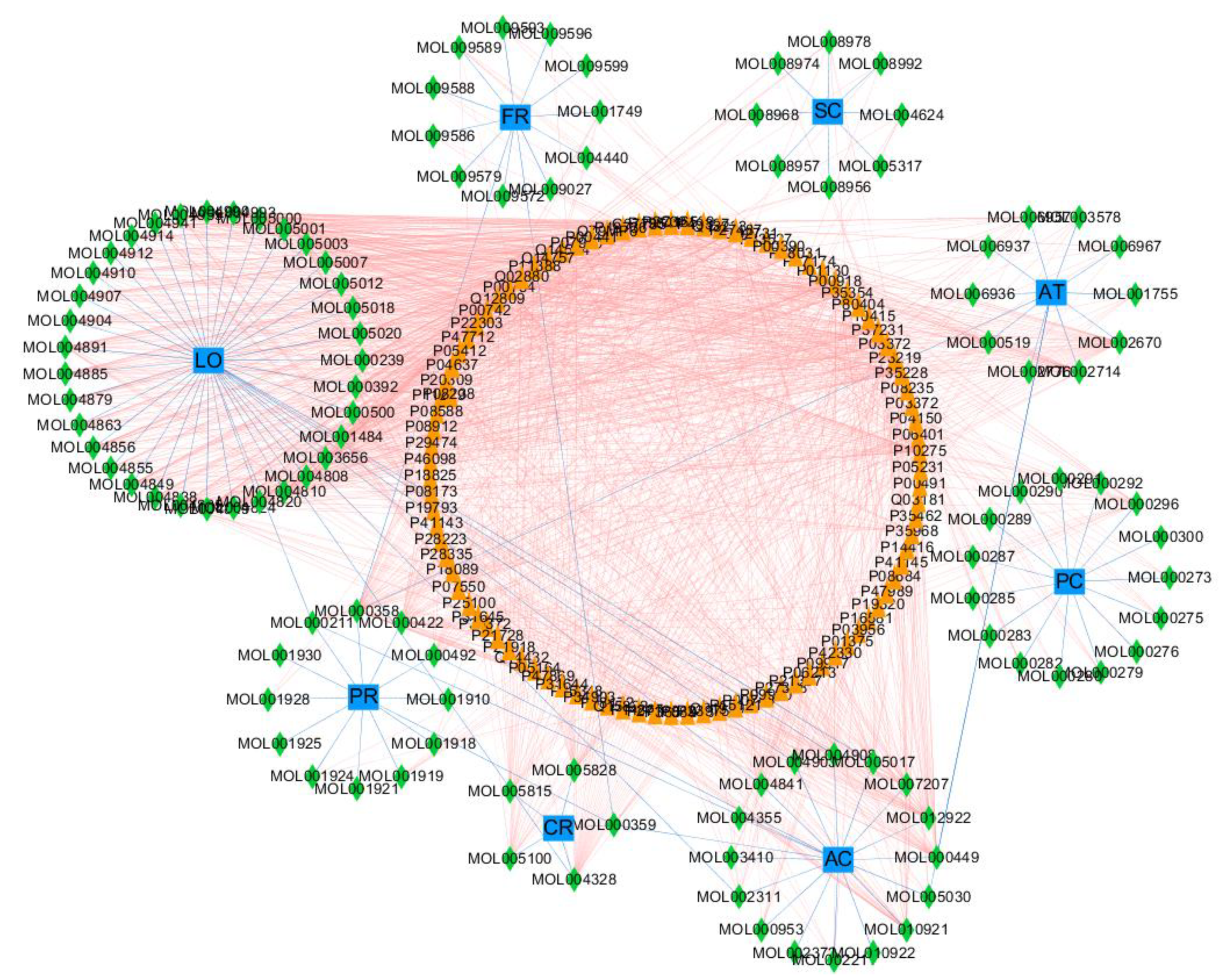
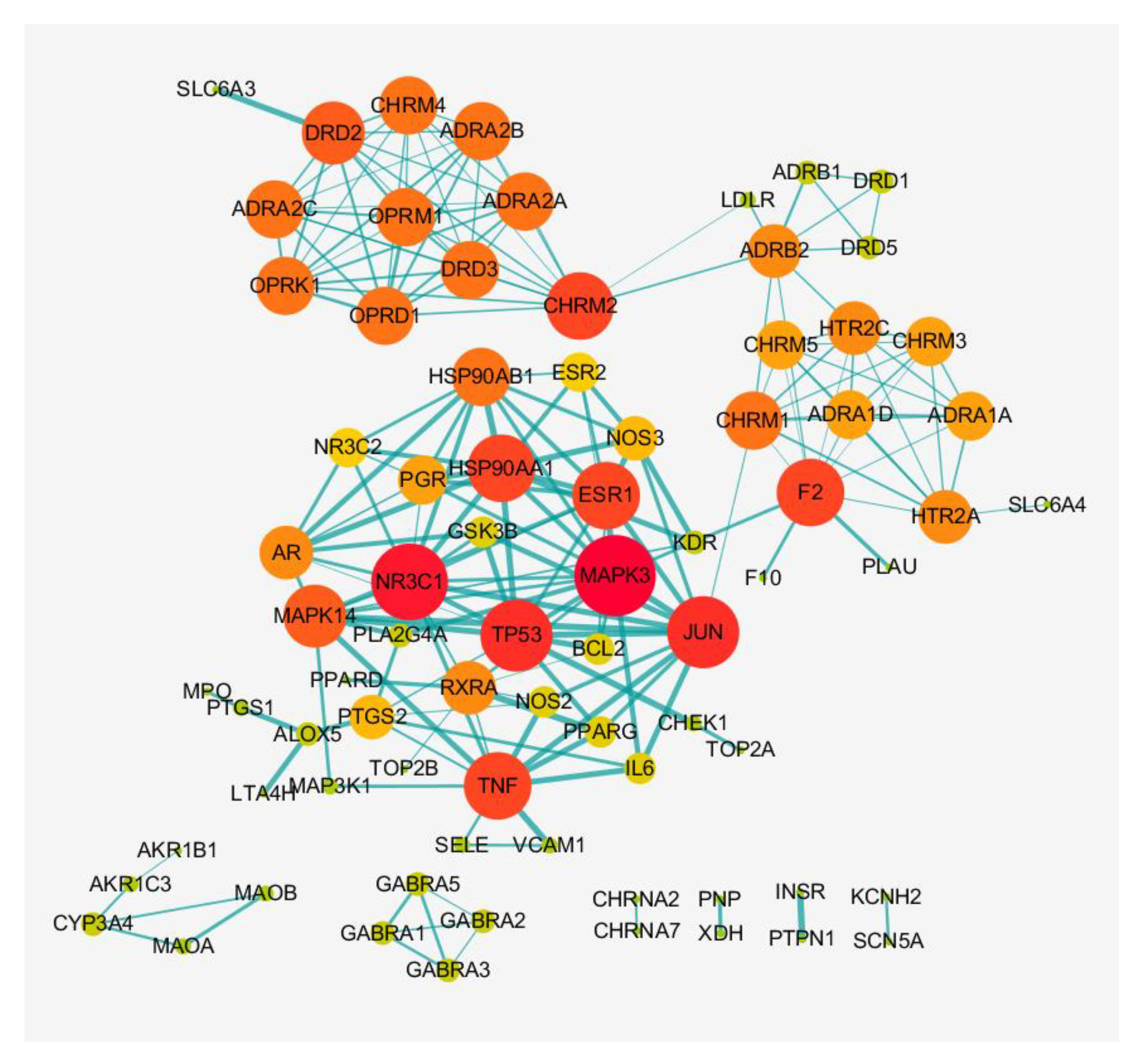
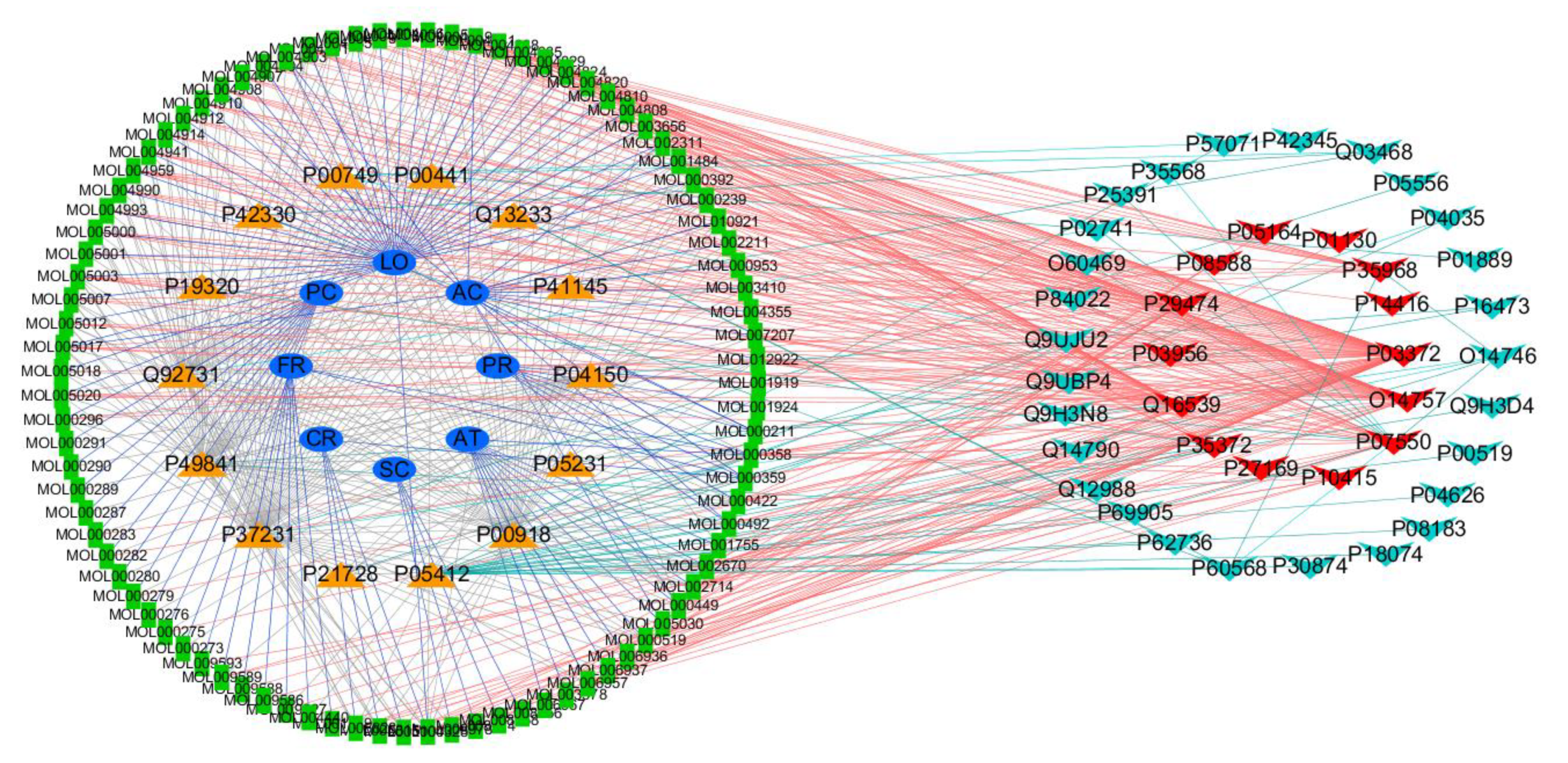

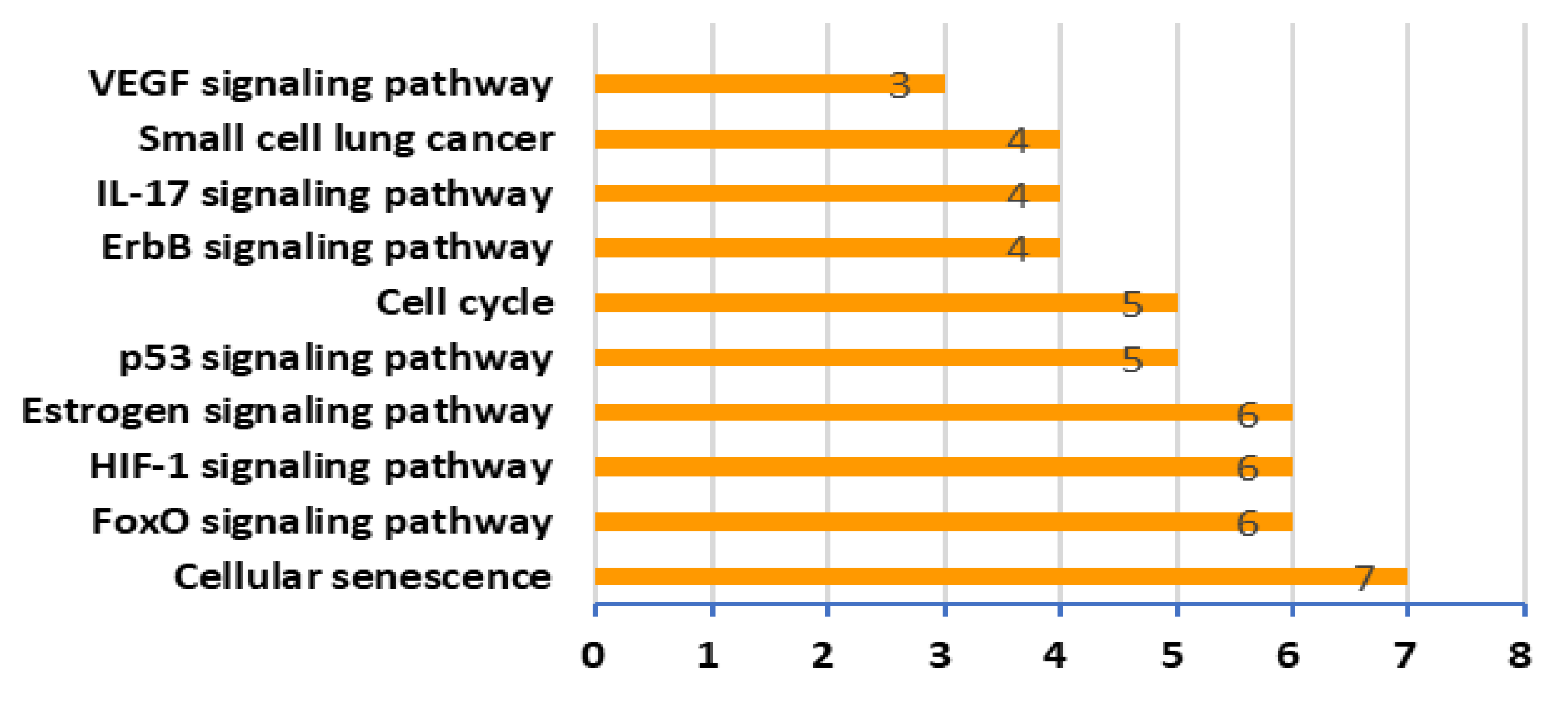
| ID | MOL ID | Molecule Name | OB/% | DL | Herb |
|---|---|---|---|---|---|
| ID001 | MOL000359 | sitosterol | 36.91 | 0.75 | CR, PR, FR, AC |
| ID002 | MOL004328 | naringenin | 59.29 | 0.21 | CR, LO |
| ID003 | MOL005100 | 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one | 47.74 | 0.27 | CR |
| ID004 | MOL005815 | Citromitin | 86.9 | 0.51 | CR |
| ID005 | MOL005828 | nobiletin | 61.67 | 0.52 | CR |
| ID006 | MOL001755 | 24-Ethylcholest-4-en-3-one | 36.08 | 0.76 | AT |
| ID007 | MOL002670 | Cavidine | 35.64 | 0.81 | AT |
| ID008 | MOL002714 | baicalein | 33.52 | 0.21 | AT |
| ID009 | MOL002776 | Baicalin | 40.12 | 0.75 | AT |
| ID010 | MOL000358 | beta-sitosterol | 36.91 | 0.75 | AT, FR, PR |
| ID011 | MOL000449 | Stigmasterol | 43.83 | 0.76 | AT, AC |
| ID012 | MOL005030 | gondoic acid | 30.7 | 0.2 | AT, AC |
| ID013 | MOL000519 | coniferin | 31.11 | 0.32 | AT |
| ID014 | MOL006936 | 10,13-eicosadienoic | 39.99 | 0.2 | AT |
| ID015 | MOL006937 | 12,13-epoxy-9-hydroxynonadeca-7,10-dienoic acid | 42.15 | 0.24 | AT |
| ID016 | MOL006957 | (3S,6S)-3-(benzyl)-6-(4-hydroxybenzyl)piperazine-2,5-quinone | 46.89 | 0.27 | AT |
| ID017 | MOL003578 | Cycloartenol | 38.69 | 0.78 | AT |
| ID018 | MOL006967 | beta-D-Ribofuranoside, xanthine-9 | 44.72 | 0.21 | AT |
| ID019 | MOL001910 | 11alpha,12alpha-epoxy-3beta-23-dihydroxy-30-norolean-20-en-28,12beta-olide | 64.77 | 0.38 | PR |
| ID020 | MOL001918 | paeoniflorgenone | 87.59 | 0.37 | PR |
| ID021 | MOL001919 | (3S,5R,8R,9R,10S,14S)-3,17-dihydroxy-4,4,8,10,14-pentamethyl-2,3,5,6,7,9-hexahydro-1H-cyclopenta[a]phenanthrene-15,16-dione | 43.56 | 0.53 | PR |
| ID022 | MOL001921 | Lactiflorin | 49.12 | 0.8 | PR |
| ID023 | MOL001924 | paeoniflorin | 53.87 | 0.79 | PR |
| ID024 | MOL001925 | paeoniflorin_qt | 68.18 | 0.4 | PR |
| ID025 | MOL001928 | albiflorin_qt | 66.64 | 0.33 | PR |
| ID026 | MOL001930 | benzoyl paeoniflorin | 31.27 | 0.75 | PR |
| ID027 | MOL000211 | Mairin | 55.38 | 0.78 | PR, LO, AC |
| ID028 | MOL000422 | kaempferol | 41.88 | 0.24 | PR |
| ID029 | MOL000492 | (+)-catechin | 54.83 | 0.24 | PR, AC |
| ID030 | MOL004624 | Longikaurin A | 47.72 | 0.53 | SC |
| ID031 | MOL005317 | Deoxyharringtonine | 39.27 | 0.81 | SC |
| ID032 | MOL008956 | Angeloylgomisin O | 31.97 | 0.85 | SC |
| ID033 | MOL008957 | Schizandrer B | 30.71 | 0.83 | SC |
| ID034 | MOL008968 | Gomisin-A | 30.69 | 0.78 | SC |
| ID035 | MOL008974 | Gomisin G | 32.68 | 0.83 | SC |
| ID036 | MOL008978 | Gomisin R | 34.84 | 0.86 | SC |
| ID037 | MOL008992 | Wuweizisu C | 46.27 | 0.84 | SC |
| ID038 | MOL001749 | ZINC03860434 | 43.59 | 0.35 | FR |
| ID039 | MOL004440 | Peimisine | 57.4 | 0.81 | FR |
| ID040 | MOL009027 | Cyclopamine | 55.42 | 0.82 | FR |
| ID041 | MOL009572 | Chuanbeinone | 41.07 | 0.71 | FR |
| ID042 | MOL009579 | ent-(16S)-atisan-13,17-oxide | 47.74 | 0.43 | FR |
| ID043 | MOL009586 | isoverticine | 48.23 | 0.67 | FR |
| ID044 | MOL009588 | Korseveriline | 35.16 | 0.68 | FR |
| ID045 | MOL009589 | Korseverinine | 53.51 | 0.71 | FR |
| ID046 | MOL009593 | verticinone | 60.07 | 0.67 | FR |
| ID047 | MOL009596 | sinpemine A | 46.96 | 0.71 | FR |
| ID048 | MOL009599 | songbeinone | 45.35 | 0.71 | FR |
| ID049 | MOL010921 | estrone | 53.56 | 0.32 | AC |
| ID050 | MOL010922 | Diisooctyl succinate | 31.62 | 0.23 | AC |
| ID051 | MOL002211 | 11,14-eicosadienoic acid | 39.99 | 0.2 | AC |
| ID052 | MOL002372 | (6Z,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene | 33.55 | 0.42 | AC |
| ID053 | MOL000953 | CLR | 37.87 | 0.68 | AC |
| ID054 | MOL002311 | Glycyrol | 90.78 | 0.67 | AC, LO |
| ID055 | MOL003410 | Ziziphin_qt | 66.95 | 0.62 | AC |
| ID056 | MOL004355 | Spinasterol | 42.98 | 0.76 | AC |
| ID057 | MOL004841 | Licochalcone B | 76.76 | 0.19 | AC, LO |
| ID058 | MOL004903 | liquiritin | 65.69 | 0.74 | AC, LO |
| ID059 | MOL004908 | Glabridin | 53.25 | 0.47 | AC, LO |
| ID060 | MOL005017 | Phaseol | 78.77 | 0.58 | AC, LO |
| ID061 | MOL007207 | Machiline | 79.64 | 0.24 | AC |
| ID062 | MOL012922 | l-SPD | 87.35 | 0.54 | AC |
| ID063 | MOL000273 | (2R)-2-[(3S,5R,10S,13R,14R,16R,17R)-3,16-dihydroxy-4,4,10,13,14-pentamethyl-2,3,5,6,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthren-17-yl]-6-methylhept-5-enoic acid | 30.93 | 0.81 | PC |
| ID064 | MOL000275 | trametenolic acid | 38.71 | 0.8 | PC |
| ID065 | MOL000276 | 7,9(11)-dehydropachymic acid | 35.11 | 0.81 | PC |
| ID066 | MOL000279 | Cerevisterol | 37.96 | 0.77 | PC |
| ID067 | MOL000280 | (2R)-2-[(3S,5R,10S,13R,14R,16R,17R)-3,16-dihydroxy-4,4,10,13,14-pentamethyl-2,3,5,6,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthren-17-yl]-5-isopropyl-hex-5-enoic acid | 31.07 | 0.82 | PC |
| ID068 | MOL000282 | ergosta-7,22E-dien-3beta-ol | 43.51 | 0.72 | PC |
| ID069 | MOL000283 | Ergosterol peroxide | 40.36 | 0.81 | PC |
| ID070 | MOL000285 | (2R)-2-[(5R,10S,13R,14R,16R,17R)-16-hydroxy-3-keto-4,4,10,13,14-pentamethyl-1,2,5,6,12,15,16,17-octahydrocyclopenta[a]phenanthren-17-yl]-5-isopropyl-hex-5-enoic acid | 38.26 | 0.82 | PC |
| ID071 | MOL000287 | 3beta-Hydroxy-24-methylene-8-lanostene-21-oic acid | 38.7 | 0.81 | PC |
| ID072 | MOL000289 | pachymic acid | 33.63 | 0.81 | PC |
| ID073 | MOL000290 | Poricoic acid A | 30.61 | 0.76 | PC |
| ID074 | MOL000291 | Poricoic acid B | 30.52 | 0.75 | PC |
| ID075 | MOL000292 | poricoic acid C | 38.15 | 0.75 | PC |
| ID076 | MOL000296 | hederagenin | 36.91 | 0.75 | PC |
| ID077 | MOL000300 | dehydroeburicoic acid | 44.17 | 0.83 | PC |
| ID078 | MOL005020 | dehydroglyasperins C | 53.82 | 0.37 | LO |
| ID079 | MOL005018 | Xambioona | 54.85 | 0.87 | LO |
| ID080 | MOL005012 | Licoagroisoflavone | 57.28 | 0.49 | LO |
| ID081 | MOL005007 | Glyasperins M | 72.67 | 0.59 | LO |
| ID082 | MOL005003 | Licoagrocarpin | 58.81 | 0.58 | LO |
| ID083 | MOL005001 | Gancaonin H | 50.1 | 0.78 | LO |
| ID084 | MOL005000 | Gancaonin G | 60.44 | 0.39 | LO |
| ID085 | MOL004993 | 8-prenylated eriodictyol | 53.79 | 0.4 | LO |
| ID086 | MOL004990 | 7,2′,4′-trihydroxy-5-methoxy-3-arylcoumarin | 83.71 | 0.27 | LO |
| ID087 | MOL004959 | 1-Methoxyphaseollidin | 69.98 | 0.64 | LO |
| ID088 | MOL004941 | (2R)-7-hydroxy-2-(4-hydroxyphenyl)chroman-4-one | 71.12 | 0.18 | LO |
| ID089 | MOL004914 | 1,3-dihydroxy-8,9-dimethoxy-6-benzofurano[3,2-c]chromenone | 62.9 | 0.53 | LO |
| ID090 | MOL004912 | Glabrone | 52.51 | 0.5 | LO |
| ID091 | MOL004910 | Glabranin | 52.9 | 0.31 | LO |
| ID092 | MOL004907 | Glyzaglabrin | 61.07 | 0.35 | LO |
| ID093 | MOL004904 | licopyranocoumarin | 80.36 | 0.65 | LO |
| ID094 | MOL004891 | shinpterocarpin | 80.3 | 0.73 | LO |
| ID095 | MOL004885 | licoisoflavanone | 52.47 | 0.54 | LO |
| ID096 | MOL004879 | Glycyrin | 52.61 | 0.47 | LO |
| ID097 | MOL004863 | 3-(3,4-dihydroxyphenyl)-5,7-dihydroxy-8-(3-methylbut-2-enyl)chromone | 66.37 | 0.41 | LO |
| ID098 | MOL004856 | Gancaonin A | 51.08 | 0.4 | LO |
| ID099 | MOL004855 | Licoricone | 63.58 | 0.47 | LO |
| ID100 | MOL004849 | 3-(2,4-dihydroxyphenyl)-8-(1,1-dimethylprop-2-enyl)-7-hydroxy-5-methoxy-coumarin | 59.62 | 0.43 | LO |
| ID101 | MOL004838 | 8-(6-hydroxy-2-benzofuranyl)-2,2-dimethyl-5-chromenol | 58.44 | 0.38 | LO |
| ID102 | MOL004835 | Glypallichalcone | 61.6 | 0.19 | LO |
| ID103 | MOL004829 | Glepidotin B | 64.46 | 0.34 | LO |
| ID104 | MOL004824 | (2S)-6-(2,4-dihydroxyphenyl)-2-(2-hydroxypropan-2-yl)-4-methoxy-2,3-dihydrofuro[3,2-g]chromen-7-one | 60.25 | 0.63 | LO |
| ID105 | MOL004820 | kanzonols W | 50.48 | 0.52 | LO |
| ID106 | MOL004810 | glyasperin F | 75.84 | 0.54 | LO |
| ID107 | MOL004808 | glyasperin B | 65.22 | 0.44 | LO |
| ID108 | MOL003656 | Lupiwighteone | 51.64 | 0.37 | LO |
| ID109 | MOL001484 | Inermine | 75.18 | 0.54 | LO |
| ID110 | MOL000500 | Vestitol | 74.66 | 0.21 | LO |
| ID111 | MOL000392 | formononetin | 69.67 | 0.21 | LO |
| ID112 | MOL000239 | Jaranol | 50.83 | 0.29 | LO |
| Herb | MOL Name | MOL ID | Degree |
|---|---|---|---|
| FR PR AT | Beta-sitosterol | MOL000358 | 49 |
| PR | Kaempferol | MOL000422 | 44 |
| AC | L-SPD | MOL012922 | 44 |
| AT | Cavidine | MOL002670 | 43 |
| AC AT | Stigmasterol | MOL000449 | 39 |
| AC | Estrone | MOL010921 | 34 |
| AC | Machiline | MOL007207 | 32 |
| LO | Shinpterocarpin | MOL004891 | 30 |
| LO | Formononetin | MOL000392 | 30 |
| LO | Naringenin | MOL004328 | 29 |
| LO CR | 1-Methoxyphaseollidin | MOL004959 | 28 |
| LO | Licoagrocarpin | MOL005003 | 28 |
| Target ID | Gene Name | Protein Name | Degree |
|---|---|---|---|
| P10275 | AR | Androgen receptor | 83 |
| P03372 | ESR1 | Estrogen receptor | 74 |
| P00918 | CA2 | Carbonic anhydrase 2 | 60 |
| P37231 | PPARG | Peroxisome proliferator-activated receptor gamma | 56 |
| P35354 | PTGS2 | Prostaglandin G/H synthase 2 | 56 |
| P35228 | NOS2 | Nitric oxide synthase, inducible | 53 |
| P00734 | F2 | Prothrombin | 49 |
| P27487 | DPP4 | Dipeptidyl peptidase 4 | 49 |
| Q92731 | ESR2 | Estrogen receptor beta | 47 |
| Q07785 | CRK2 | Cell division control protein 2 homolog | 45 |
| P49841 | GSK3B | Glycogen synthase kinase-3 beta | 46 |
| O14757 | CHEK1 | Serine/threonine-protein kinase Chk1 | 45 |
| P18031 | PTPN1 | Tyrosine-protein phosphatase non-receptor type 1 | 43 |
| Q16539 | MAPK14 | Mitogen-activated protein kinase 14 | 39 |
| P07900 | HSP90AA1 | Heat shock protein HSP 90-alpha | 38 |
| P08238 | HSP90AB1 | Heat shock protein HSP 90-beta | 37 |
| P23219 | PTGS1 | Prostaglandin G/H synthase 1 | 32 |
| P00742 | F10 | Coagulation factor X | 31 |
| Q14524 | SCN5A | Sodium channel protein type 5 subunit alpha | 30 |
| Herb | MOL ID | MOL Name | Degree |
|---|---|---|---|
| FR PR AT | MOL000358 | Beta-sitosterol | 17 |
| PR | MOL000422 | Kaempferol | 14 |
| AT | MOL002670 | Cavidine | 13 |
| CR LO | MOL004328 | Naringenin | 12 |
| LO | MOL000392 | Formononetin | 11 |
| AC | MOL012922 | l-SPD | 11 |
| AT | MOL002714 | Baicalein | 10 |
| AT AC | MOL000449 | Stigmasterol | 10 |
| LO | MOL005003 | Licoagrocarpin | 10 |
| LO | MOL004959 | 1-Methoxyphaseollidin | 10 |
| LO AC | MOL004908 | Glabridin | 10 |
| LO | MOL004891 | Shinpterocarpin | 10 |
| AC | MOL004841 | Licochalcone B | 10 |
| LO | MOL001484 | Inermine | 10 |
| AC | MOL010921 | Estrone | 10 |
| AC PR | MOL000492 | (+)-catechin | 9 |
| CR | MOL005828 | Nobiletin | 9 |
| LO AC | MOL005017 | Phaseol | 9 |
| LO | MOL005000 | Gancaonin G | 9 |
| LO | MOL004941 | (2R)-7-hydroxy-2-(4-Hydroxyphenyl)chroman-4-one | 9 |
| LO | MOL004849 | 3-(2,4-dihydroxyphenyl)-8-(1,1-Dimethylprop-2-enyl)-7-hydroxy-5-methoxy-coumarin | 9 |
| LO | MOL004835 | Glypallichalcone | 9 |
| LO | MOL004829 | Glepidotin B | 9 |
| LO | MOL004824 | (2S)-6-(2,4-dihydroxyphenyl)-2-(2-hydroxypropan-2-yl)-4-methoxy-2,3-dihydrofuro[3,2-g]chromen-7-one | 9 |
| AC | MOL007207 | Machiline | 9 |
| Types of Action | Degree | Uniprot ID | Gene Name |
|---|---|---|---|
| Direct action | 75 | P03372 | ESR1 |
| Direct action | 45 | O14757 | CHEK1 |
| Direct action | 40 | Q16539 | MAPK14 |
| Direct action | 28 | P07550 | ADRB2 |
| Direct action | 16 | P29474 | NOS3 |
| Direct action | 14 | P35968 | KDR |
| Direct action | 11 | P35372 | OPRM1 |
| Direct action | 6 | P10415 | BCL2 |
| Direct action | 3 | P08588 | ADRB1 |
| Direct action | 1 | P27169 | PON1 |
| Direct action | 1 | P14416 | DRD2 |
| Direct action | 1 | P05164 | MPO |
| Direct action | 1 | P03956 | MMP1 |
| Direct action | 1 | P01130 | LDLR |
| Types of Action | Degree | Uniprot ID | Gene Name |
|---|---|---|---|
| Indirect action | 5 | P60568 | IL2 |
| Indirect action | 5 | O14746 | TERT |
| Indirect action | 4 | Q9UJU2 | LEF1 |
| Indirect action | 3 | Q03468 | ERCC6 |
| Indirect action | 3 | P69905 | HBA2 |
| Indirect action | 2 | P62736 | ACTA2 |
| Indirect action | 2 | P16473 | TSHR |
| Indirect action | 2 | P04035 | HMGCR |
| Indirect action | 2 | P02741 | CRP |
| Indirect action | 1 | Q9UBP4 | DKK3 |
| Indirect action | 1 | Q9H3N8 | HRH4 |
| Indirect action | 1 | Q9H3D4 | TP63 |
| Indirect action | 1 | Q14790 | CASP8 |
| Indirect action | 1 | Q12988 | HSPB3 |
| Indirect action | 1 | P84022 | SMAD3 |
| Indirect action | 1 | P57071 | PRDM15 |
| Indirect action | 1 | P42345 | MTOR |
| Indirect action | 1 | P35568 | IRS1 |
| Indirect action | 1 | P30874 | SSTR2 |
| Indirect action | 1 | P25391 | LAMA1 |
| Indirect action | 1 | P18074 | ERCC2 |
| Indirect action | 1 | P08183 | ABCB1 |
| Indirect action | 1 | P05556 | ITGB1 |
| Indirect action | 1 | P04626 | ERBB2 |
| Indirect action | 1 | P01889 | HLA-B |
| Indirect action | 1 | P00519 | ABL1 |
| Indirect action | 1 | O60469 | DSCAM |
| Name | HLA-B | CASP8 | MAPK14 | SMAD3 | ACTA2 | ADRB2 | BCL2 | LEF1 |
|---|---|---|---|---|---|---|---|---|
| Bone Marrow | 268 | 6 | 5 | 4 | 8 | 10 | 5 | 4 |
| Whole Blood | 689 | 12 | 13 | 4 | 9 | 25 | 6 | 9 |
| Lymph Node | 316 | 8 | 3 | 4 | 23 | 3 | 6 | 8 |
| Thymus | 229 | 6 | 3 | 4 | 17 | 2 | 4 | 35 |
| Tonsil | 302 | 6 | 3 | 4 | 11 | 3 | 5 | 6 |
| Myeloid | 630 | 11 | 21 | 5 | 9 | 22 | 6 | 4 |
| Monocytes | 577 | 9 | 13 | 5 | 8 | 9 | 5 | 4 |
| Dentritic Cells | 558 | 8 | 10 | 6 | 6 | 6 | 5 | 4 |
| NK cells | 716 | 13 | 10 | 5 | 8 | 41 | 7 | 5 |
| T Cells(CD+4) | 514 | 16 | 7 | 7 | 6 | 5 | 11 | 30 |
| T Cells(CD+8) | 512 | 15 | 7 | 5 | 7 | 14 | 8 | 27 |
| B-Lymphoblasts | 410 | 7 | 8 | 5 | 12 | 3 | 9 | 4 |
| B Cells | 411 | 8 | 5 | 8 | 7 | 3 | 11 | 4 |
| Endothelial | 154 | 6 | 5 | 4 | 5 | 3 | 6 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, Y. Network Pharmacology Approach Reveals the Potential Immune Function Activation and Tumor Cell Apoptosis Promotion of Xia Qi Decoction in Lung Cancer. Med. Sci. 2020, 8, 1. https://doi.org/10.3390/medsci8010001
Zhang S, Wang Y. Network Pharmacology Approach Reveals the Potential Immune Function Activation and Tumor Cell Apoptosis Promotion of Xia Qi Decoction in Lung Cancer. Medical Sciences. 2020; 8(1):1. https://doi.org/10.3390/medsci8010001
Chicago/Turabian StyleZhang, Song, and Yun Wang. 2020. "Network Pharmacology Approach Reveals the Potential Immune Function Activation and Tumor Cell Apoptosis Promotion of Xia Qi Decoction in Lung Cancer" Medical Sciences 8, no. 1: 1. https://doi.org/10.3390/medsci8010001
APA StyleZhang, S., & Wang, Y. (2020). Network Pharmacology Approach Reveals the Potential Immune Function Activation and Tumor Cell Apoptosis Promotion of Xia Qi Decoction in Lung Cancer. Medical Sciences, 8(1), 1. https://doi.org/10.3390/medsci8010001





