Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention
Abstract
:1. Introduction
2. Epidemiology
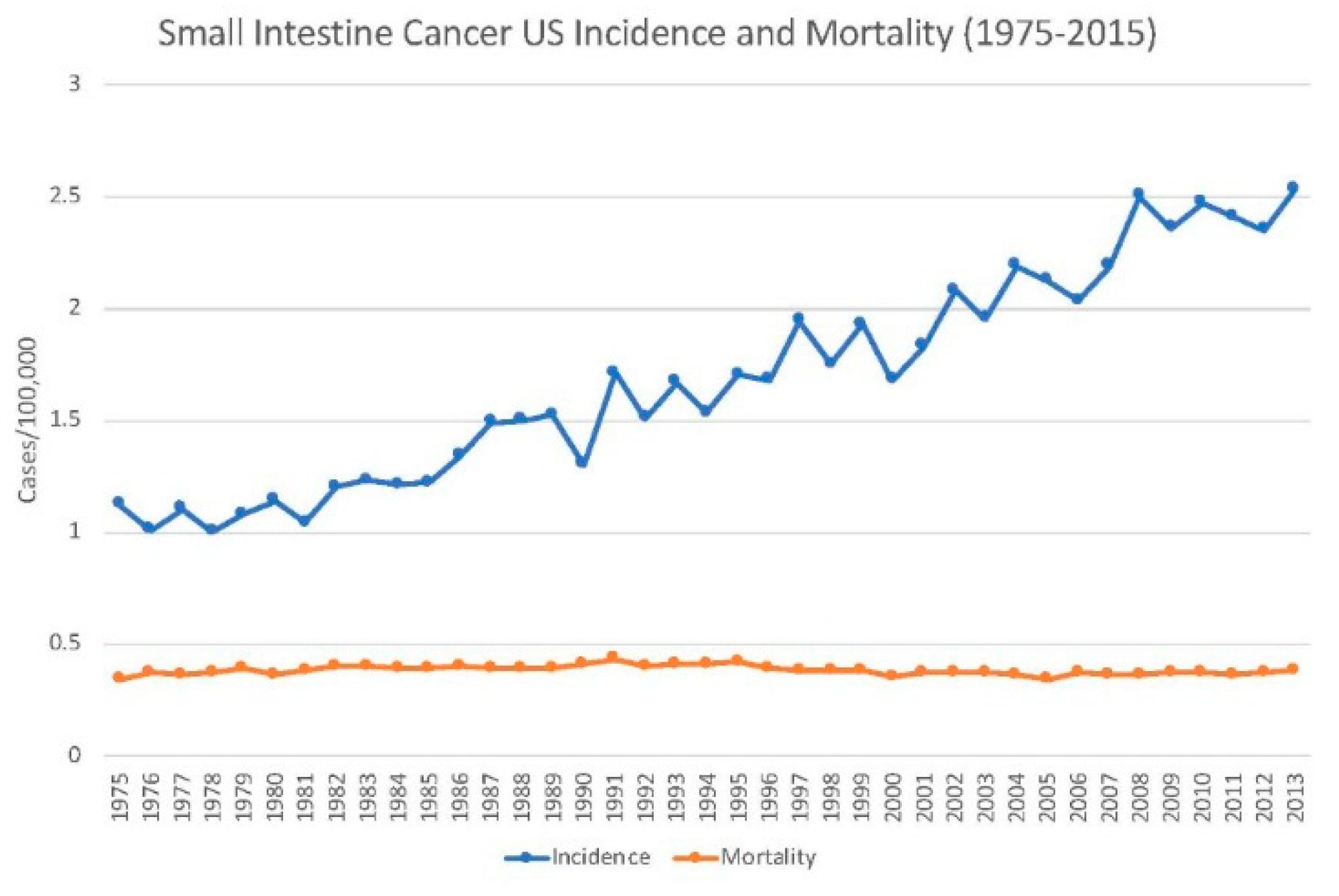
2.1. Incidence
2.2. Mortality
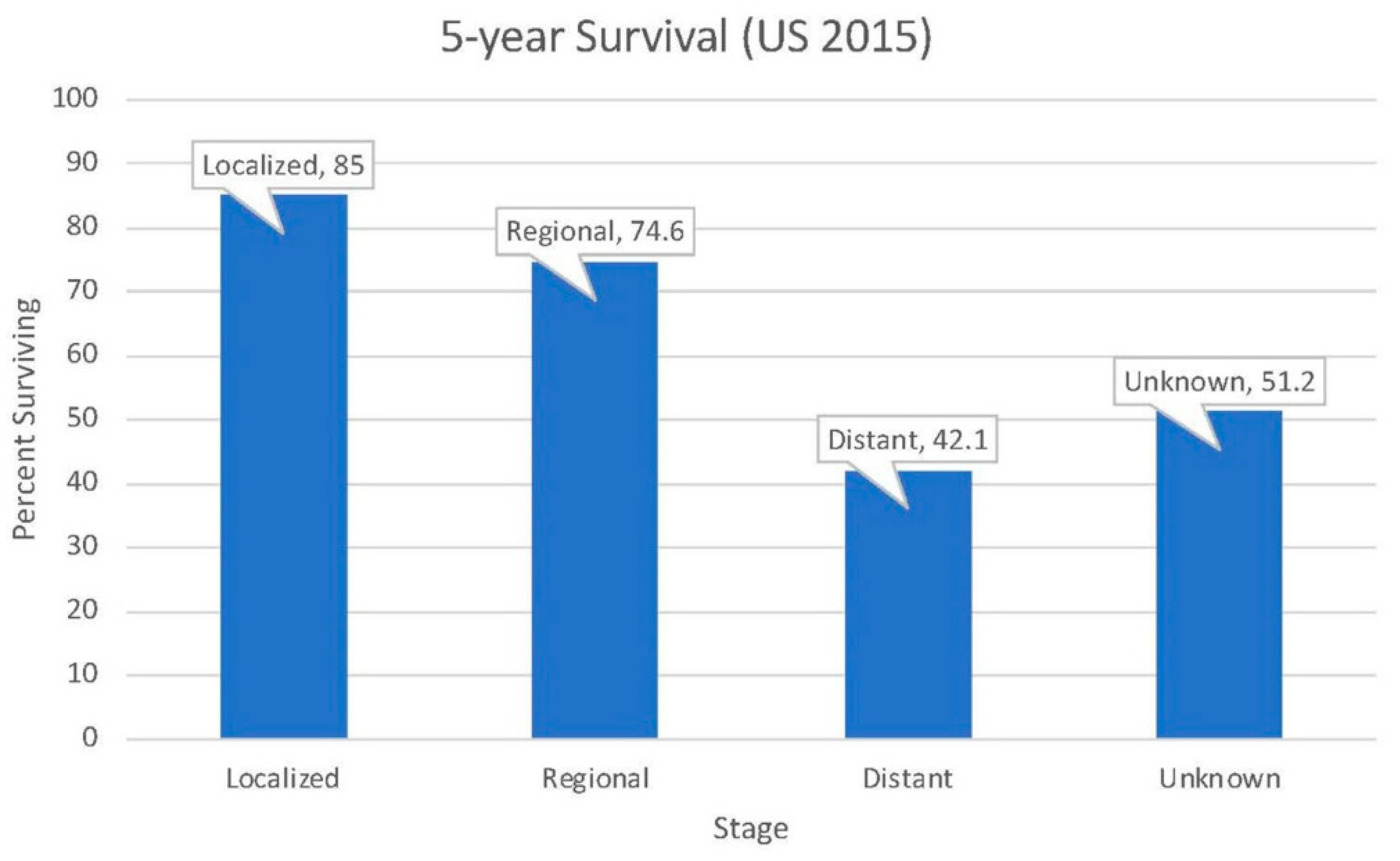
2.3. Survival
2.4. Trends
3. Etiology and Risk Factors
3.1. Etiology
3.2. Non-Modifiable Risk Factors
3.2.1. Race and Ethnicity
3.2.2. Age
3.2.3. Sex
3.2.4. Hereditary Mutations
Lynch Syndrome
Peutz–Jeghers Syndrome
Multiple Endocrine Neoplasia Syndrome Type 1
Neurofibromatosis Type 1
3.2.5. Inflammatory Bowel Disease
3.2.6. Celiac Disease
3.3. Modifiable Risk Factors
3.3.1. Diet
3.3.2. Alcohol
3.3.3. Smoking
3.3.4. Obesity
3.3.5. Biliary Tract Diseases
3.3.6. Occupational Hazards
3.3.7. Vitamins and Medications
4. Prevention
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Neugut, A.I.; Jacobson, J.S.; Suh, S.; Mukherjee, R.; Arber, N. The epidemiology of cancer of the small bowel. Cancer Epidemiol. Biomark. Prev. 1998, 7, 243–251. [Google Scholar]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 18 Regs Research Data+Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973–2013 Varying)—Linked To County Attributes—Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, Released April 2016, Based on the November 2015. Available online: https://seer.cancer.gov/statfacts/html/smint.html (accessed on 15 February 2019).
- Collins, J.T.; Badireddy, M. Anatomy, Abdomen and Pelvis, Small Intestine. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2018. [Google Scholar]
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.; Bennett, C.L.; Talamonti, M.S. Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann. Surg. 2009, 249, 63–71. [Google Scholar] [CrossRef]
- Lepage, C.; Bouvier, A.M.; Manfredi, S.; Dancourt, V.; Faivre, J. Incidence and management of primary malignant small bowel cancers: A well-defined French population study. Am. J. Gastroenterol. 2006, 101, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Dabaja, B.S.; Suki, D.; Pro, B.; Bonnen, M.; Ajani, J. Adenocarcinoma of the small bowel: Presentation, prognostic factors, and outcome of 217 patients. Cancer 2004, 101, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.W.; Rha, S.Y.; Shin, S.J.; Chang, H.; Shim, H.S.; Roh, J.K. Adenocarcinoma of the small bowel at a single Korean institute: Management and prognosticators. J. Cancer Res. Clin. Oncol. 2010, 136, 387–394. [Google Scholar] [CrossRef]
- Schottenfeld, D.; Beebe-Dimmer, J.L.; Vigneau, F.D. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann. Epidemiol. 2009, 19, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.M.D. An insight into the genetic pathway of adenocarcinoma of the small intestine. Gut 2002, 50, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Shimizu, S.; Imai, Y.; Nakatsuru, Y.; Oda, H.; Oohara, T.; Ishikawa, T. Mutations of the Ki-ras, p53 and APC genes in adenocarcinomas of the human small intestine. Int. J. Cancer 1997, 70, 390–395. [Google Scholar] [CrossRef]
- Breuhahn, K.; Singh, S.; Schirmacher, P.; Blaker, H. Large-scale N-terminal deletions but not point mutations stabilize beta-catenin in small bowel carcinomas, suggesting divergent molecular pathways of small and large intestinal carcinogenesis. J. Pathol. 2008, 215, 300–307. [Google Scholar] [CrossRef]
- Offerhaus, G.J.; Giardiello, F.M.; Krush, A.J.; Booker, S.V.; Tersmette, A.C.; Kelley, N.C.; Hamilton, S.R. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology 1992, 102, 1980–1982. [Google Scholar] [CrossRef]
- Watson, P.; Lynch, H.T. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer 1993, 71, 677–685. [Google Scholar] [CrossRef]
- Marini, F.; Falchetti, A.; Luzi, E.; Tonelli, F.; Luisa, B.M. Multiple endocrine neoplasia type 1 (MEN1) syndrome. In Cancer Syndromes [Internet]; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2008. [Google Scholar]
- Talamonti, M.S.; Goetz, L.H.; Rao, S.; Joehl, R.J. Primary cancers of the small bowel: Analysis of prognostic factors and results of surgical management. Arch. Surg. 2002, 137, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Cancer Registration Statistics, England Statistical Bulletins. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/previousReleases (accessed on 16 February 2019).
- Curado, M.P.; Edwards, B.; Shin, H.R.; Storm, H.; Ferlay, J.; Heanue, M.; Boyle, P. Cancer Incidence in Five Continents; IARC Scientific Publications: Lyon, France, 2008; Volume IX, pp. 1–837. [Google Scholar]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE-5—A population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef]
- Howe, J.R.; Cardona, K.; Fraker, D.L.; Kebebew, E.; Untch, B.R.; Wang, Y.Z.; Law, C.H.; Liu, E.H.; Kim, M.K.; Menda, Y.; et al. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas 2017, 46, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Stryker, S.J.; Wolff, B.G.; Culp, C.E.; Libbe, S.D.; Ilstrup, D.M.; MacCarty, R.L. Natural history of untreated colonic polyps. Gastroenterology 1987, 93, 1009–1013. [Google Scholar] [CrossRef]
- Stewart, S.L.; Wike, J.M.; Kato, I.; Lewis, D.R.; Michaud, F. A population-based study of colorectal cancer histology in the United States, 1998–2001. Cancer 2006, 107, 1128–1141. [Google Scholar] [CrossRef]
- Delaunoit, T.; Neczyporenko, F.; Limburg, P.J.; Erlichman, C. Pathogenesis and risk factors of small bowel adenocarcinoma: A colorectal cancer sibling? Am. J. Gastroenterol. 2005, 100, 703–710. [Google Scholar] [CrossRef]
- Weiss, N.S.; Yang, C.P. Incidence of histologic types of cancer of the small intestine. J. Natl. Cancer Inst. 1987, 78, 653–656. [Google Scholar]
- KlÖPpel, G.; Perren, A.; Heitz, P.U. The Gastroenteropancreatic Neuroendocrine Cell System and Its Tumors: The WHO Classification. Ann. N. Y. Acad. Sci. 2004, 1014, 13–27. [Google Scholar] [CrossRef]
- Moertel, C.G.; Sauer, W.G.; Dockerty, M.B.; Baggenstoss, A.H. Life history of the carcinoid tumor of the small intestine. Cancer 1961, 14, 901–912. [Google Scholar] [CrossRef]
- Banck, M.S.; Kanwar, R.; Kulkarni, A.A.; Boora, G.K.; Metge, F.; Kipp, B.R.; Zhang, L.; Thorland, E.C.; Minn, K.T.; Tentu, R.; et al. The genomic landscape of small intestine neuroendocrine tumors. J. Clin. Investing. 2013, 123, 2502–2508. [Google Scholar] [CrossRef]
- Li, S.C.; Essaghir, A.; Martijn, C.; Lloyd, R.V.; Demoulin, J.B.; Oberg, K.; Giandomenico, V. Global microRNA profiling of well-differentiated small intestinal neuroendocrine tumors. Mod. Pathol. 2013, 26, 685–696. [Google Scholar] [CrossRef]
- De Rosa, M.; Pace, U.; Rega, D.; Costabile, V.; Duraturo, F.; Izzo, P.; Delrio, P. Genetics, diagnosis and management of colorectal cancer (Review). Oncol. Rep. 2015, 34, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Jagelman, D.G.; DeCosse, J.J.; Bussey, H.J. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet 1988, 1, 1149–1151. [Google Scholar] [CrossRef]
- Shenoy, S. Genetic risks and familial associations of small bowel carcinoma. World J. Gastrointest. Oncol. 2016, 8, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Vasen, H.F.; Bulow, S.; Myrhoj, T.; Mathus-Vliegen, L.; Griffioen, G.; Buskens, E.; Taal, B.G.; Nagengast, F.; Slors, J.F.; de Ruiter, P. Decision analysis in the management of duodenal adenomatosis in familial adenomatous polyposis. Gut 1997, 40, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Bulow, S.; Alm, T.; Fausa, O.; Hultcrantz, R.; Jarvinen, H.; Vasen, H. Duodenal adenomatosis in familial adenomatous polyposis. Int. J. Colorectal Dis. 1995, 10, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Bonadona, V.; Bonaiti, B.; Olschwang, S.; Grandjouan, S.; Huiart, L.; Longy, M.; Guimbaud, R.; Buecher, B.; Bignon, Y.J.; Caron, O.; et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011, 305, 2304–2310. [Google Scholar] [CrossRef]
- Vasen, H.F.; Wijnen, J.T.; Menko, F.H.; Kleibeuker, J.H.; Taal, B.G.; Griffioen, G.; Nagengast, F.M.; Meijers-Heijboer, E.H.; Bertario, L.; Varesco, L.; et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology 1996, 110, 1020–1027. [Google Scholar] [CrossRef]
- Jun, S.Y.; Lee, E.J.; Kim, M.J.; Chun, S.M.; Bae, Y.K.; Hong, S.U.; Choi, J.; Kim, J.M.; Jang, K.T.; Kim, J.Y.; et al. Lynch syndrome-related small intestinal adenocarcinomas. Oncotarget 2017, 8, 21483–21500. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Singhi, A.D.; Dudley, B.; Brand, R.; Nikiforova, M.; Pai, R.K. Small Bowel Adenocarcinoma Frequently Exhibits Lynch Syndrome-associated Mismatch Repair Protein Deficiency But Does Not Harbor Sporadic MLH1 Deficiency. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Babba, T.; Schischmanoff, O.; Lagorce, C.; Wind, P.; Des Guetz, G.; Aparicio, T.; Benamouzig, R. Small bowel carcinoma revealing HNPCC syndrome. Gastroenterol. Clin. Biol. 2010, 34, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz-Correa, M.; Offerhaus, J.A. Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterology 2000, 119, 1447–1453. [Google Scholar] [CrossRef]
- Benafif, S.; Eeles, R. Diagnosis and Management of Hereditary Carcinoids. Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les Recherches sur le Cancer 2016, 205, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Lodish, M.B.; Stratakis, C.A. Endocrine tumours in neurofibromatosis type 1, tuberous sclerosis and related syndromes. Best practice & research. Clin. Endocrinol. Metab. 2010, 24, 439–449. [Google Scholar] [CrossRef]
- Bojesen, R.D.; Riis, L.B.; Hogdall, E.; Nielsen, O.H.; Jess, T. Inflammatory Bowel Disease and Small Bowel Cancer Risk, Clinical Characteristics, and Histopathology: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2017, 15, 1900–1907. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Kliewer, E.; Wajda, A. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer 2001, 91, 854–862. [Google Scholar] [CrossRef]
- Palascak-Juif, V.; Bouvier, A.M.; Cosnes, J.; Flourié, B.; Bouché, O.; Cadiot, G.; Lémann, M.; Bonaz, B.; Denet, C.; Marteau, P.; et al. Small Bowel Adenocarcinoma in Patients with Crohn’s Disease Compared with Small Bowel Adenocarcinoma De Novo. Inflamm. Bowel Dis. 2005, 11, 828–832. [Google Scholar] [CrossRef]
- Shaukat, A.; Virnig, D.J.; Howard, D.; Sitaraman, S.V.; Liff, J.M.; Lederle, F.A. Crohn’s disease and small bowel adenocarcinoma: A population-based case-control study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1120–1123. [Google Scholar] [CrossRef]
- Swinson, C.; Coles, E.C.; Slavin, G.; Booth, C.C. Coeliac Disease and Malignancy. Lancet 1983, 321, 111–115. [Google Scholar] [CrossRef]
- Howdle, P.D.; Jalal, P.K.; Holmes, G.K.; Houlston, R.S. Primary small-bowel malignancy in the UK and its association with coeliac disease. QJM 2003, 96, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Askling, J.; Linet, M.; Gridley, G.; Halstensen, T.S.; Ekstrom, K.; Ekbom, A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology 2002, 123, 1428–1435. [Google Scholar] [CrossRef]
- Aparicio, T.; Manfredi, S.; Tougeron, D.; Henriques, J.; Bouche, O.; Pezet, D.; Piessen, G.; Coriat, R.; Zaanan, A.; Legoux, J.L.; et al. 772PARCAD-NADEGE cohort: Result of a small bowel adenocarcinomas prospective cohort. Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Aparicio, T.; Zaanan, A.; Svrcek, M.; Laurent-Puig, P.; Carrere, N.; Manfredi, S.; Locher, C.; Afchain, P. Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis and treatment. Dig. Liver Dis. 2014, 46, 97–104. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Sonni, A. Distribution of small bowel tumors. Cancer Lett. 1977, 3, 83–86. [Google Scholar] [CrossRef]
- Chow, W.H.; Linet, M.S.; McLaughlin, J.K.; Hsing, A.W.; Chien, H.T.; Blot, W.J. Risk factors for small intestine cancer. Cancer Causes Control 1993, 4, 163–169. [Google Scholar] [CrossRef]
- Wu, A.H.; Yu, M.C.; Mack, T.M. Smoking, alcohol use, dietary factors and risk of small intestinal adenocarcinoma. Int. J. Cancer 1997, 70, 512–517. [Google Scholar] [CrossRef]
- Cross, A.J.; Leitzmann, M.F.; Subar, A.F.; Thompson, F.E.; Hollenbeck, A.R.; Schatzkin, A. A prospective study of meat and fat intake in relation to small intestinal cancer. Cancer Res. 2008, 68, 9274–9279. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, Q.; Yin, Z.; Shuang, J.; Bai, B.; Yu, P.; Guo, M.; Zhao, Q. Red and processed meat consumption and colorectal cancer risk: A systematic review and meta-analysis. Oncotarget 2017, 8, 83306–83314. [Google Scholar] [CrossRef] [PubMed]
- Schatzkin, A.; Park, Y.; Leitzmann, M.F.; Hollenbeck, A.R.; Cross, A.J. Prospective study of dietary fiber, whole grain foods, and small intestinal cancer. Gastroenterology 2008, 135, 1163–1167. [Google Scholar] [CrossRef]
- Negri, E.; Bosetti, C.; La Vecchia, C.; Fioretti, F.; Conti, E.; Franceschi, S. Risk factors for adenocarcinoma of the small intestine. Int. J. Cancer 1999, 82, 171–174. [Google Scholar] [CrossRef]
- Hassan, M.M.; Phan, A.; Li, D.; Dagohoy, C.G.; Leary, C.; Yao, J.C. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int. J. Cancer 2008, 123, 867–873. [Google Scholar] [CrossRef]
- Kaerlev, L.; Teglbjaerg, P.S.; Sabroe, S.; Kolstad, H.A.; Ahrens, W.; Eriksson, M.; Guénel, P.; Hardell, L.; Launoy, G.; Merler, E.; et al. Is there an association between alcohol intake or smoking and small bowel adenocarcinoma? Results from a European multi-center case–control study. Cancer Causes Control 2000, 11, 791–797. [Google Scholar] [CrossRef]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Grant, E.J.; Ozasa, K.; Tsuji, I.; Kakizaki, M.; Nagai, M.; Nishino, Y.; You, S.L.; Yoo, K.Y.; Yuan, J.M.; et al. Body mass, tobacco smoking, alcohol drinking and risk of cancer of the small intestine—A pooled analysis of over 500 000 subjects in the Asia Cohort Consortium. Ann. Oncol. 2011, 23, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Kaerlev, L.; Teglbjaerg, P.S.; Sabroe, S.; Kolstad, H.A.; Ahrens, W.; Eriksson, M.; Guenel, P.; Hardell, L.; Cyr, D.; Ballard, T.; et al. Occupational risk factors for small bowel carcinoid tumor: A European population-based case-control study. J. Occup. Environ. Med. 2002, 44, 516–522. [Google Scholar] [CrossRef]
- Chen, C.C.; Neugut, A.I.; Rotterdam, H. Risk factors for adenocarcinomas and malignant carcinoids of the small intestine: Preliminary findings. Cancer Epidemiol. Biomark. Prev. 1994, 3, 205–207. [Google Scholar]
- Botteri, E.; Iodice, S.; Bagnardi, V.; Raimondi, S.; Lowenfels, A.B.; Maisonneuve, P. Smoking and colorectal cancer: A meta-analysis. JAMA 2008, 300, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Wolk, A.; Gridley, G.; Svensson, M.; Nyrén, O.; McLaughlin, J.K.; Fraumeni, J.F.; Adami, H.-O. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 2001, 12, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Samanic, C.; Chow, W.H.; Gridley, G.; Jarvholm, B.; Fraumeni, J.F., Jr. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control 2006, 17, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Bjorge, T.; Tretli, S.; Engeland, A. Height and body mass index in relation to cancer of the small intestine in two million Norwegian men and women. Br. J. Cancer 2005, 93, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Samanic, C.; Gridley, G.; Chow, W.H.; Lubin, J.; Hoover, R.N.; Fraumeni, J.F., Jr. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control 2004, 15, 35–43. [Google Scholar] [CrossRef]
- Johansen, C.; Chow, W.H.; Jorgensen, T.; Mellemkjaer, L.; Engholm, G.; Olsen, J.H. Risk of colorectal cancer and other cancers in patients with gall stones. Gut 1996, 39, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.R.; Abdallah, S.M.; Law, M.; Kaldor, J. Cancer Incidence among Australian Nuclear Industry Workers. J. Occup. Health 2006, 48, 358–365. [Google Scholar] [CrossRef]
- Boscoe, F.P.; Schymura, M.J. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer 2006, 6, 264. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D.; et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J. Natl. Cancer Inst. 2019, 111, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Wilson, M.; Elwin, C.E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef]
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Aoki, Y.; Ogden, C.L. Differences in Obesity Prevalence by Demographic Characteristics and Urbanization Level among Adults in the United States, 2013–2016. JAMA 2018, 319, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.W.; Clarke, T.C.; Nugent, C.N.; Schiller, J.S. Early release of selected estimates based on data from the 2015 National Health Interview Survey (National Center for Health Statistics). 2016. Available online: https://www.cdc.gov/nchs/data/nhis/earlyrelease/earlyrelease201605.pdf (accessed on 15 February 2019).
- Jamal, A.; Phillips, E.; Gentzke, A.S.; Homa, D.M.; Babb, S.D.; King, B.A.; Neff, L.J. Current Cigarette Smoking Among Adults—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 53–59. [Google Scholar] [CrossRef] [PubMed]
| Etiology | Adenocarcinoma | Neuroendocrine (i.e., Carcinoid) | Lymphoma | Sarcoma (i.e., Stromal) |
|---|---|---|---|---|
| Proportion | 30–40% | 35–42% | ~17% | ~8% |
| U.S. incidence (per 100,000) | 0.7 | 1.0 | 0.4 | 0.2 |
| Most probable location | Duodenum | Ileum | Throughout GI tract | Throughout GI tract |
| Predisposing Condition for SBA | Genetic Mutation | Increased Risk | Predisposing Condition for NET | Genetic Mutation | Increased Risk |
|---|---|---|---|---|---|
| Familial adenomatous polyposis (FAP) | APC deletion/inactivation | ~330× | Multiple endocrine neoplasia type I | Defective MEN1 | Accounts for 5–10% of NET in GI tract |
| Lynch syndrome (HNPCC [Hereditary nonpolyposis colorectal cancer]) | Mismatch repair-deficient | 25–291× | Neurofibromatosis 1 (also predisposes to sarcoma) | Defective NF1 | 2–4× increased risk of all tumors |
| Peutz–Jeghers syndrome (PJS) | STK11 suppressor defect | 520× (both NET and SBA) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barsouk, A.; Rawla, P.; Barsouk, A.; Thandra, K.C. Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention. Med. Sci. 2019, 7, 46. https://doi.org/10.3390/medsci7030046
Barsouk A, Rawla P, Barsouk A, Thandra KC. Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention. Medical Sciences. 2019; 7(3):46. https://doi.org/10.3390/medsci7030046
Chicago/Turabian StyleBarsouk, Adam, Prashanth Rawla, Alexander Barsouk, and Krishna Chaitanya Thandra. 2019. "Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention" Medical Sciences 7, no. 3: 46. https://doi.org/10.3390/medsci7030046
APA StyleBarsouk, A., Rawla, P., Barsouk, A., & Thandra, K. C. (2019). Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention. Medical Sciences, 7(3), 46. https://doi.org/10.3390/medsci7030046





