Eosinophilic Upper Airway Inflammation in a Murine Model Using an Adoptive Transfer System Induces Hyposmia and Epithelial Layer Injury with Convex Lesions
Abstract
:1. Introduction
2. Methods
2.1. Mice
2.2. Preparation of Splenocytes including a High Number of Activated Eosinophils
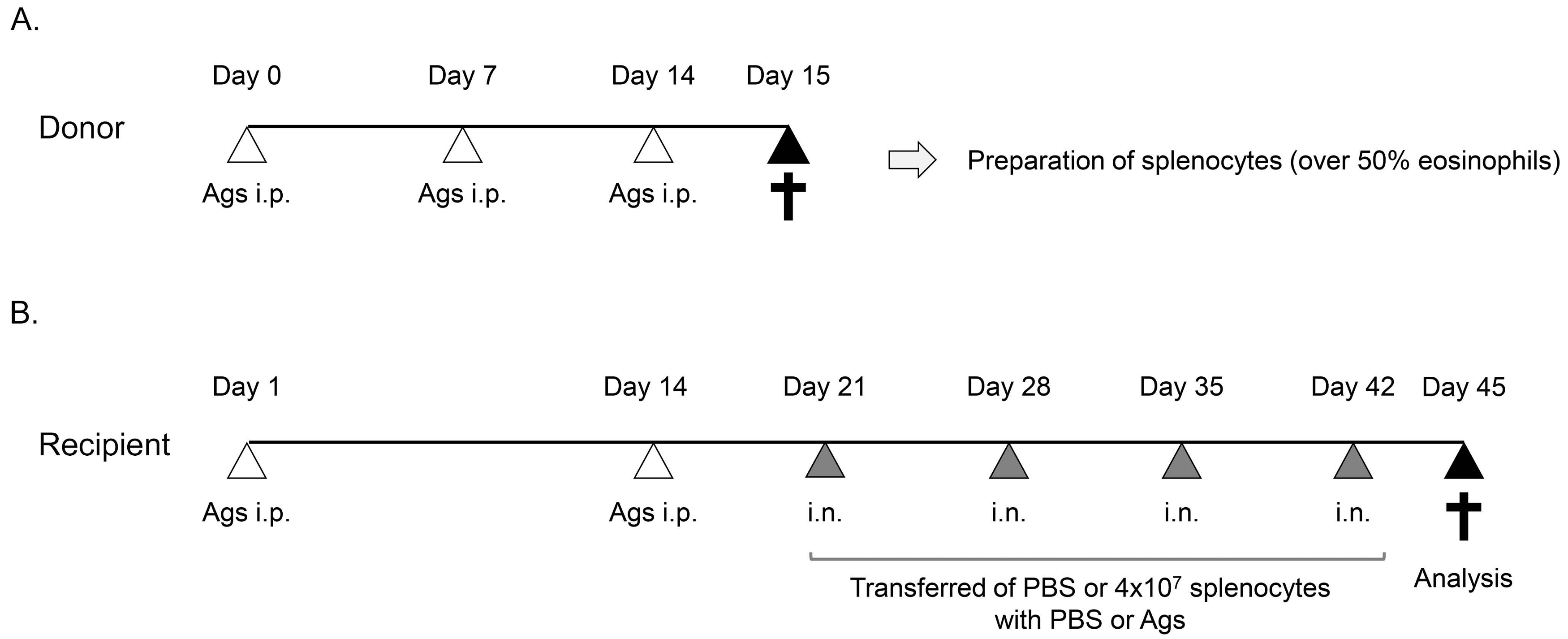
2.3. Adoptive Transfer System
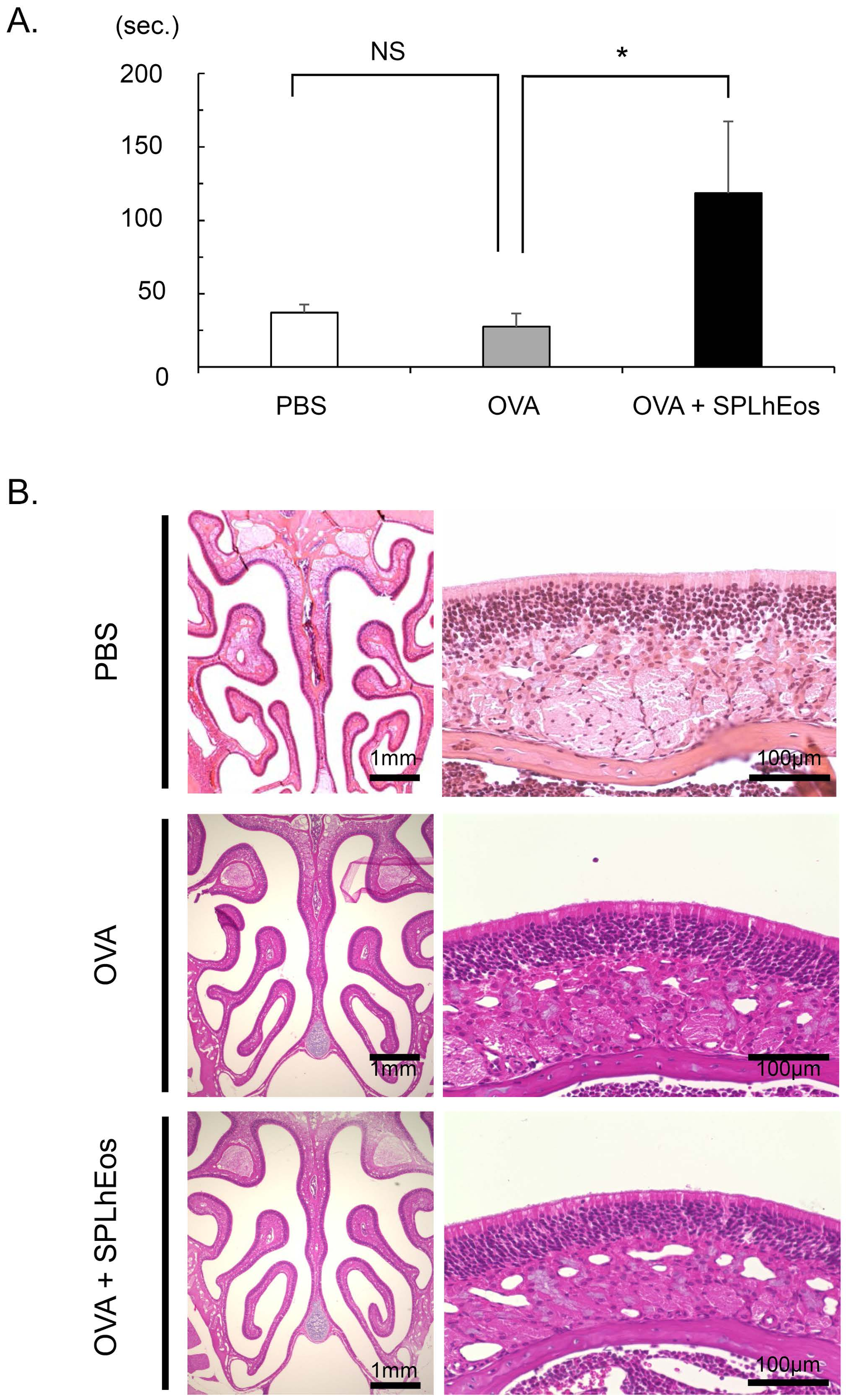
2.4. Buried Food Test
2.5. Histological Analysis
2.6. Computed tomography Analysis
2.7. Statistical Analysis
3. Results
3.1. Hyposmia on Transfer of SPLhEos in Th2-Skewed Response
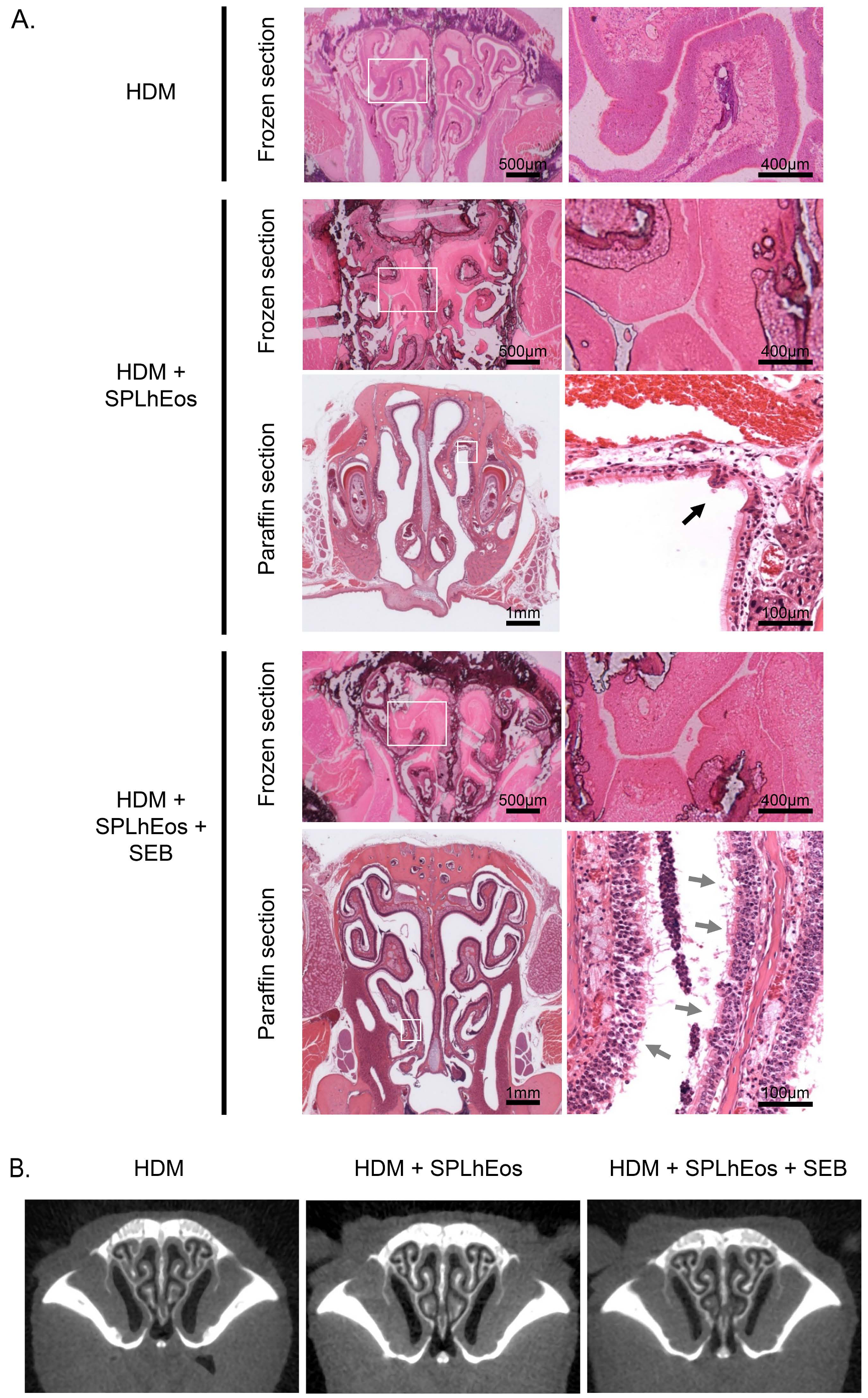
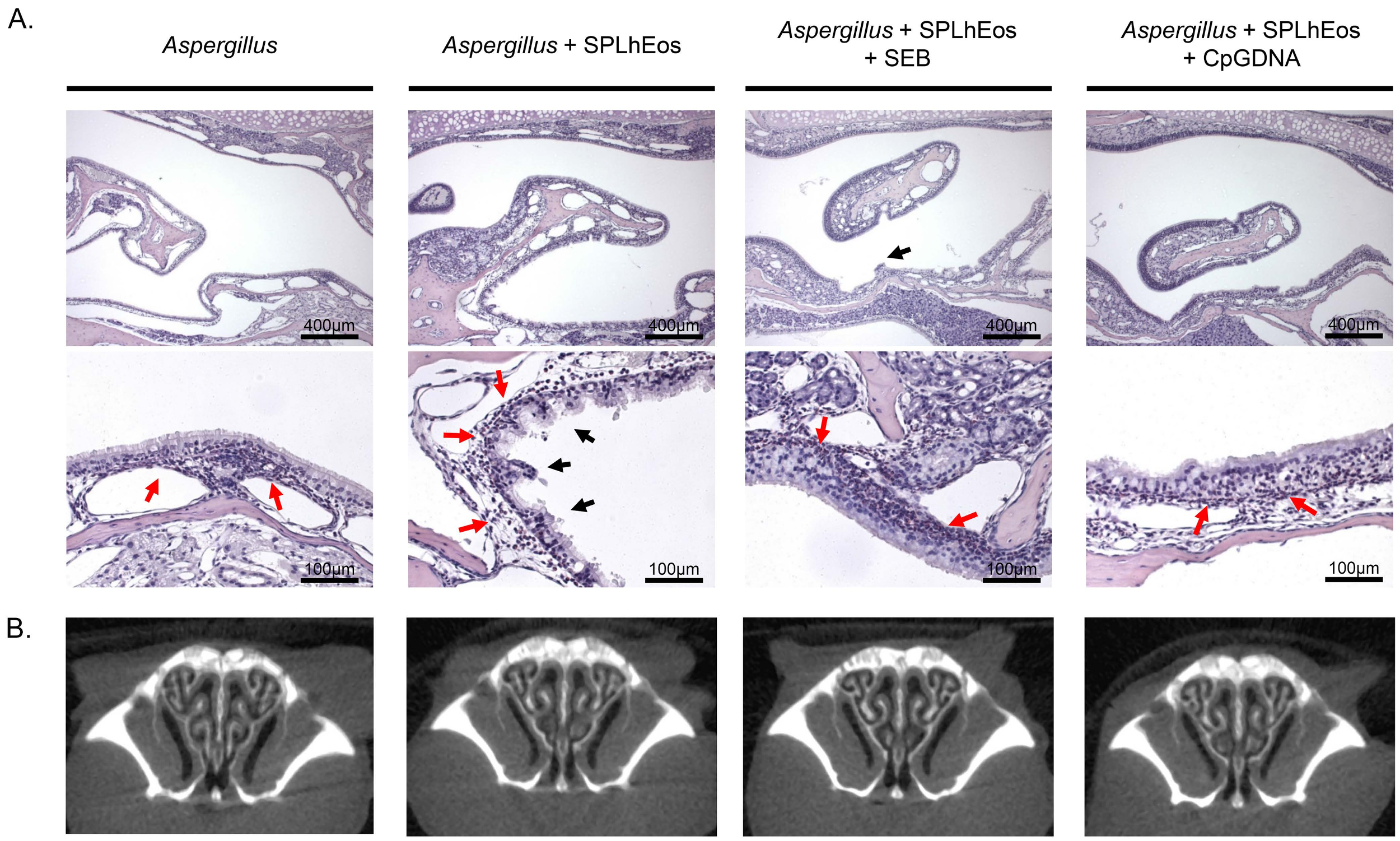
3.2. Epithelia Injury on Transfer of SPLhEos in Adoptive and Innate Responses
3.3. Thin Tissue Component of Turbinate in Murine Paranasal Sinuses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rothenberg, M.E.; Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 2006, 24, 147–174. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.; Rothenberg, M.E. Biology of the eosinophil. Adv. Immunol. 2009, 101, 81–121. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, H.F.; Dyer, K.D.; Foster, P.S. Eosinophils: Changing perspectives in health and disease. Nat. Rev. Immunol. 2013, 13, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.C.; Prefontaine, D.; Hamid, Q. Images in allergy and immunology: Role of eosinophils in airway remodeling. J. Allergy Clin. Immunol. 2007, 119, 1563–1566. [Google Scholar] [CrossRef]
- Schleimer, R.P. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu. Rev. Pathol. 2017, 12, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps. Rhinol. Suppl. 2012, 23, 1–298. [Google Scholar]
- Bachert, C.; Zhang, N.; Holtappels, G.; De Lobel, L.; van Cauwenberge, P.; Liu, S.; Lin, P.; Bousquet, J.; Van Steen, K. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J. Allergy Clin. Immunol. 2010, 126, 962–968.e6. [Google Scholar] [CrossRef]
- Tomassen, P.; Vandeplas, G.; Van Zele, T.; Cardell, L.O.; Arebro, J.; Olze, H.; Forster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziaber, A.; Holtappels, G.; et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456.e4. [Google Scholar] [CrossRef]
- Sakuma, Y.; Ishitoya, J.; Komatsu, M.; Shiono, O.; Hirama, M.; Yamashita, Y.; Kaneko, T.; Morita, S.; Tsukuda, M. New clinical diagnostic criteria for eosinophilic chronic rhinosinusitis. Auris Nasus Larynx 2011, 38, 583–588. [Google Scholar] [CrossRef]
- Tokunaga, T.; Sakashita, M.; Haruna, T.; Asaka, D.; Takeno, S.; Ikeda, H.; Nakayama, T.; Seki, N.; Ito, S.; Murata, J.; et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: The JESREC Study. Allergy 2015, 70, 995–1003. [Google Scholar] [CrossRef]
- Klimek, L.; Eggers, G. Olfactory dysfunction in allergic rhinitis is related to nasal eosinophilic inflammation. J. Allergy Clin. Immunol. 1997, 100, 158–164. [Google Scholar] [CrossRef]
- Kim, D.W.; Khalmuratova, R.; Hur, D.G.; Jeon, S.Y.; Kim, S.W.; Shin, H.W.; Lee, C.H.; Rhee, C.S. Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am. J. Rhinol. Allergy 2011, 25, e255–e261. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, D.W.; Khalmuratova, R.; Kim, J.H.; Jung, M.H.; Chang, D.Y.; Shin, E.C.; Lee, H.K.; Shin, H.W.; Rhee, C.S.; et al. Resveratrol prevents development of eosinophilic rhinosinusitis with nasal polyps in a mouse model. Allergy 2013, 68, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Lim, J.Y.; Kim, S.; Kim, J.H.; Jang, Y.J. Development of a mouse model of eosinophilic chronic rhinosinusitis with nasal polyp by nasal instillation of an Aspergillus protease and ovalbumin. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 3899–3906. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Driss, V.; Hornez, N.; Abdallah, M.; Roumier, T.; Abboud, G.; Legrand, F.; Staumont-Salle, D.; Queant, S.; Bertout, J.; et al. Eosinophil-derived IFN-gamma induces airway hyperresponsiveness and lung inflammation in the absence of lymphocytes. J. Allergy Clin. Immunol. 2009, 124, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Besse, J.A.; Mingler, M.K.; Fulkerson, P.C.; Rothenberg, M.E. Eosinophil adoptive transfer system to directly evaluate pulmonary eosinophil trafficking in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 6067–6072. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Crawley, J.N. Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. 2009, 8, 8–24. [Google Scholar] [CrossRef]
- Kawamoto, T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch. Histol. Cytol. 2003, 66, 123–143. [Google Scholar] [CrossRef]
- Ryu, J.H.; Yoo, J.Y.; Kim, M.J.; Hwang, S.G.; Ahn, K.C.; Ryu, J.C.; Choi, M.K.; Joo, J.H.; Kim, C.H.; Lee, S.N.; et al. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J. Allergy Clin. Immunol. 2013, 131, 549–561. [Google Scholar] [CrossRef]
- Liu, C.F.; Drocourt, D.; Puzo, G.; Wang, J.Y.; Riviere, M. Innate immune response of alveolar macrophage to house dust mite allergen is mediated through TLR2/-4 co-activation. PLoS ONE 2013, 8, e75983. [Google Scholar] [CrossRef]
- Schubert, M.S. Allergic fungal sinusitis: Pathophysiology, diagnosis and management. Med. Mycol. 2009, 47, S324–S330. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Kaur, H. Allergic Aspergillus Rhinosinusitis. J. Fungi 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.B.; Barnes, C.S.; Portnoy, J.M. Innate and Adaptive Immune Response to Fungal Products and Allergens. J. Allergy Clin. Immunol. Pract. 2016, 4, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.W.; Schleimer, R.P.; Kern, R.C. Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2016, 4, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Gaines, A.D. Anosmia and hyposmia. Allergy Asthma Proc. 2010, 31, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Syed, I.; Philpott, C. Hyposmia. Br. J. Hosp. Med. 2015, 76, C41–C42. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. Office procedures for quantitative assessment of olfactory function. Am. J. Rhinol. 2007, 21, 460–473. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Su, S.B.; Zhang, P.; Kurosaka, K.; Caspi, R.R.; Michalek, S.M.; Rosenberg, H.F.; Zhang, N.; Oppenheim, J.J. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J. Exp. Med. 2008, 205, 79–90. [Google Scholar] [CrossRef]
- Li, X.; Hawkins, G.A.; Ampleford, E.J.; Moore, W.C.; Li, H.; Hastie, A.T.; Howard, T.D.; Boushey, H.A.; Busse, W.W.; Calhoun, W.J.; et al. Genome-wide association study identifies TH1 pathway genes associated with lung function in asthmatic patients. J. Allergy Clin. Immunol. 2013, 132, 313–320. [Google Scholar] [CrossRef]
- Cao, P.P.; Wang, Z.C.; Schleimer, R.P.; Liu, Z. Pathophysiologic mechanisms of chronic rhinosinusitis and their roles in emerging disease endotypes. Ann. Allergy Asthma Immunol. 2018. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.S.; Han, D.M.; Sy, C.; Huang, Q.; Sun, Y.; Fan, E.Z.; Li, Y.; Zhou, B. Differential expression of Toll-like receptor pathway genes in chronic rhinosinusitis with or without nasal polyps. Acta Oto-Laryngol. 2013, 133, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Jin, S.Y.; Yeon, S.H.; Lee, S.B.; Xu, J.; Yoon, Y.H.; Rha, K.S.; Kim, Y.M. Role of Toll-like receptor 9 signaling on activation of nasal polyp-derived fibroblasts and its association with nasal polypogenesis. Int. Forum Allergy Rhinol. 2018, 8, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Mandron, M.; Aries, M.F.; Brehm, R.D.; Tranter, H.S.; Acharya, K.R.; Charveron, M.; Davrinche, C. Human dendritic cells conditioned with Staphylococcus aureus enterotoxin B promote TH2 cell polarization. J. Allergy Clin. Immunol. 2006, 117, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Blom, E.; Camberis, M.; Prout, M.; Tang, S.C.; Le Gros, G. Staphylococcal-derived superantigen enhances peanut induced Th2 responses in the skin. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2012, 42, 305–314. [Google Scholar] [CrossRef]
- Okano, M.; Fujiwara, T.; Haruna, T.; Kariya, S.; Makihara, S.; Higaki, T.; Nishizaki, K. Role of fungal antigens in eosinophilia-associated cellular responses in nasal polyps: A comparison with enterotoxin. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2011, 41, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.E.; Asaduzzaman, M.; Arizmendi, N.G.; Polley, D.; Wu, Y.; Gordon, J.R.; Hollenberg, M.D.; Cameron, L.; Vliagoftis, H. Proteinase-activated receptor-2 activation participates in allergic sensitization to house dust mite allergens in a murine model. Clin. Exp. Allergy 2013, 43, 1274–1285. [Google Scholar] [CrossRef]

| Maxillary Sinus | Turbinate | |||||
|---|---|---|---|---|---|---|
| Mucosal Layer | Interstitium Tissue | Gland | Mucosal Layer | Interstitium Tissue | Gland | |
| Human | Thin | Poor | Poor | Thick | Rich | Rich |
| Mouse | Thin–moderate | Poor | Rich | Thin | Poor | Poor |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, A.; Kondo, K.; Hosaka, N.; Kobayashi, Y.; Bui, D.V.; Yun, Y.; Suzuki, K.; Sawada, S.; Asako, M.; Nakamura, A.; et al. Eosinophilic Upper Airway Inflammation in a Murine Model Using an Adoptive Transfer System Induces Hyposmia and Epithelial Layer Injury with Convex Lesions. Med. Sci. 2019, 7, 22. https://doi.org/10.3390/medsci7020022
Kanda A, Kondo K, Hosaka N, Kobayashi Y, Bui DV, Yun Y, Suzuki K, Sawada S, Asako M, Nakamura A, et al. Eosinophilic Upper Airway Inflammation in a Murine Model Using an Adoptive Transfer System Induces Hyposmia and Epithelial Layer Injury with Convex Lesions. Medical Sciences. 2019; 7(2):22. https://doi.org/10.3390/medsci7020022
Chicago/Turabian StyleKanda, Akira, Kenji Kondo, Naoki Hosaka, Yoshiki Kobayashi, Dan Van Bui, Yasutaka Yun, Kensuke Suzuki, Shunsuke Sawada, Mikiya Asako, Akihiko Nakamura, and et al. 2019. "Eosinophilic Upper Airway Inflammation in a Murine Model Using an Adoptive Transfer System Induces Hyposmia and Epithelial Layer Injury with Convex Lesions" Medical Sciences 7, no. 2: 22. https://doi.org/10.3390/medsci7020022
APA StyleKanda, A., Kondo, K., Hosaka, N., Kobayashi, Y., Bui, D. V., Yun, Y., Suzuki, K., Sawada, S., Asako, M., Nakamura, A., Tomoda, K., Sakata, Y., Tsuta, K., Dombrowicz, D., Kawauchi, H., Fujieda, S., & Iwai, H. (2019). Eosinophilic Upper Airway Inflammation in a Murine Model Using an Adoptive Transfer System Induces Hyposmia and Epithelial Layer Injury with Convex Lesions. Medical Sciences, 7(2), 22. https://doi.org/10.3390/medsci7020022








