Low-Dose Naltrexone (LDN)—Review of Therapeutic Utilization
Abstract
1. Introduction
1.1. Aims and Purpose
1.2. Methods
2. Pharmacological Properties
2.1. Standard Pharmacology of Naltrexone and Naloxone
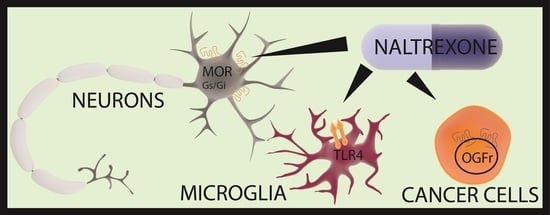
2.2. Mechanism of Action of Low-Dose Naltrexone
2.3. Mechanism of Action of Ultra Low-Dose Naltrexone
2.4. Mechanism of Action of Very Low-Dose Naltrexone
3. Low-Dose Naltrexone in Clinical Medicine
3.1. Multiple Sclerosis
3.2. Complex Regional Pain Syndrome
3.3. Fibromyalgia
3.4. Gastrointestinal Tract Diseases
3.5. Cancer
3.6. Skin Conditions
3.7. Other Diseases or States
4. Very Low-Dose Naltrexone in Clinical Medicine
5. Ultra Low-Dose Naltrexone in Clinical Medicine
6. Safety and Side Effects
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Sudakin, D. Naltrexone: Not Just for Opioids Anymore. J. Med. Toxicol. 2016, 12, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Bihari, B. Low-dose naltrexone for normalizing immune system function. Altern. Ther. Health Med. 2013, 19, 56–65. [Google Scholar] [PubMed]
- Google Search of “Low-Dose Naltrexone” on March 7th 2018. Available online: www.google.com (accessed on 7 March 2018).
- CFS Pharmacy Shop 2018. Available online: https://www.cfspharmacy.pharmacy/human-medicine/naltrexone-low-dose-compounded (accessed on 9 March 2018).
- Raknes, G.; Småbrekke, L. A sudden and unprecedented increase in low dose naltrexone (LDN) prescribing in Norway. Patient and prescriber characteristics, and dispense patterns. A drug utilization cohort study. Pharmacoepidemiol. Drug Saf. 2017, 26, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Basbaum, A.; Ramana, N. Opioid Agonists & Antagonists. In Basic & Clinical Pharmacology & Toxicology, 13th ed.; Katzung, B., Trevor, A., Eds.; Wiley: Hoboken, NJ, USA, 2014; p. 531. [Google Scholar]
- Burns, L.; Wang, H.Y. Ultra-low-dose naloxone or naltrexone to improve opioid analgesia: The history, the mystery and a novel approach. Clin. Med. Insights Ther. 2010, 2, 857–868. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Peng, Y.; Hutchinson, M.R.; Rice, K.C.; Yin, H.; Watkins, L.R. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br. J. Pharmacol. 2016, 173, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Center for Substance Abuse Treatment (CSAT). Chapter 4—Oral Naltrexone. Inc. Alcohol Pharmacother. Into Med. Pract. (Treatment Improv. Protoc. Ser. No. 49); Substance Abuse and Mental Health Services Administration (US): Rockville, MD, USA, 2009; p. 30.
- Hospira Inc. Naloxone Prescribing Information 2018; Hospira Inc.: Lake Forest, IL, USA, 2018. [Google Scholar]
- Robinson, A.; Wermeling, D.P. Intranasal naloxone administration for treatment of opioid overdose. Am. J. Heal. Pharm. 2014, 71, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Panksepp, J. Low-dose naltrexone for disease prevention and quality of life. Med. Hypotheses 2009, 72, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.; Parkitny, L.; McLain, D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin. Rheumatol. 2014, 33, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Toljan, K.; Qureshi, H.; Vrooman, B. Therapeutic value of naltrexone as a glial modulator. Glia 2017, 65, E103–E578. [Google Scholar] [CrossRef]
- Selfridge, B.R.; Wang, X.; Zhang, Y.; Yin, H.; Grace, P.M.; Watkins, L.R.; Ionescu, D.F.; Alpert, J.E.; Soskin, D.P.; Fava, M. Structure–Activity Relationships of (+)-Naltrexone-Inspired Toll-like Receptor 4 (TLR4) Antagonists. J. Med. Chem. 2015, 58, 5038–5052. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.R.; Zhang, Y.; Brown, K.; Coats, B.D.; Shridhar, M.; Sholar, P.W.; Patel, S.J.; Crysdale, N.Y.; Harrison, J.A.; Maier, S.F.; et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: Involvement of toll-like receptor 4 (TLR4). Eur. J. Neurosci. 2008, 28, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Okun, E.; Griffioen, K.J.; Mattson, M.P. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011, 34, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Wadachi, R.; Hargreaves, K.M. Trigeminal nociceptors express TLR-4 and CD14: A mechanism for pain due to infection. J. Dent. Res. 2006, 85, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Leow-Dyke, S.; Allen, C.; Denes, A.; Nilsson, O.; Maysami, S.; Bowie, A.G.; Rothwell, N.J.; Pinteaux, E. Neuronal toll-like receptor 4 signaling induces brain endothelial activation and neutrophil transmigration in vitro. J. Neuroinflamm. 2012, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S. Innate immunity and neuroinflammation in the CNS: The role of microglia in toll-like receptor-mediated neuronal injury. Glia 2010, 58, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Chopra, P.; Cooper, M.S. Treatment of complex regional pain syndrome (CRPS) using low dose naltrexone (LDN). J. Neuroimmune Pharmacol. 2013, 8, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Janković, B.D.; Radulović, J. Enkephalins, brain and immunity: Modulation of immune responses by methionine-enkephalin injected into the cerebral cavity. Int. J. Neurosci. 1992, 67, 241–270. [Google Scholar] [CrossRef] [PubMed]
- Rahn, K.A.; McLaughlin, P.J.; Zagon, I.S. Prevention and diminished expression of experimental autoimmune encephalomyelitis by low dose naltrexone (LDN) or opioid growth factor (OGF) for an extended period: Therapeutic implications for multiple sclerosis. Brain Res. 2011, 1381, 243–253. [Google Scholar] [CrossRef] [PubMed]
- McCusker, R.H.; Kelley, K.W. Immune-neural connections: How the immune system’s response to infectious agents influences behavior. J. Exp. Biol. 2013, 216, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Yoburn, B.C.; Duttaroy, A.; Shah, S.; Davis, T. Opioid antagonist-induced receptor upregulation: Effects of concurrent agonist administration. Brain Res. Bull. 1994, 33, 237–240. [Google Scholar] [CrossRef]
- Pierzchała-Koziec, K.; Dziedzicka-Wasylewska, M.; Oeltgen, P.; Zubel-Łojek, J.; Latacz, A.; Ocłoń, E. The effect of CRH, dexamethasone and naltrexone on the mu, delta and kappa opioid receptor agonist binding in lamb hypothalamic-pituitary-adrenal axis. Folia Biol. 2015, 63, 187–193. [Google Scholar] [CrossRef]
- Zukin, R.S.; Sugarman, J.R.; Fitz-Syage, M.L.; Gardner, E.L.; Zukin, S.R.; Gintzler, A.R. Naltrexone-induced opiate receptor supersensitivity. Brain Res. 1982, 245, 285–292. [Google Scholar] [CrossRef]
- Tempel, A.; Gardner, E.L.; Zukin, R.S. Neurochemical and functional correlates of naltrexone-induced opiate receptor up-regulation. J. Pharmacol. Exp. Ther. 1985, 232, 439–444. [Google Scholar] [PubMed]
- Zagon, I.S.; Donahue, R.; McLaughlin, P.J. Targeting the opioid growth factor: Opioid growth factor receptor axis for treatment of human ovarian cancer. Exp. Biol. Med. (Maywood) 2013, 238, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Rogosnitzky, M.; Finegold, M.J.; McLaughlin, P.J.; Zagon, I.S. Opioid growth factor (OGF) for hepatoblastoma: A novel non-toxic treatment. Investig. New Drugs 2013, 31, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Donahue, R.N.; McLaughlin, P.J.; Zagon, I.S. Low-dose naltrexone targets the opioid growth factor-opioid growth factor receptor pathway to inhibit cell proliferation: Mechanistic evidence from a tissue culture model. Exp. Biol. Med. 2011, 236, 1036–1050. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.J.; Zagon, I.S. Duration of opioid receptor blockade determines biotherapeutic response. Biochem. Pharmacol. 2015, 97, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Lutz, P.-E.; Kieffer, B.L. Opioid receptors: Distinct roles in mood disorders. Trends Neurosci. 2013, 36, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-F.; Crain, S.M. Dual opioid modulation of the action potential duration of mouse dorsal root ganglion neurons in culture. Brain Res. 1989, 491, 227–242. [Google Scholar] [CrossRef]
- Wang, H.Y.; Burns, L.H. Naloxone’s pentapeptide binding site on filamin A blocks Mu opioid receptor-Gs coupling and CREB activation of acute morphine. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Burns, L.; Wang, H.-Y. PTI-609: A Novel Analgesic that Binds Filamin A to Control Opioid Signaling. Recent Pat. CNS Drug Discov. 2010, 5, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Stock, H.; Bingaman, S.; Mauger, D.; Rogosnitzky, M.; Zagon, I.S. Low-dose naltrexone therapy improves active Crohn’s disease. Am. J. Gastroenterol. 2007, 102, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Berkson, B.M.; Rubin, D.M.; Berkson, A.J. Reversal of signs and symptoms of a B-cell lymphoma in a patient using only low-dose naltrexone. Integr. Cancer Ther. 2007, 6, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Zylicz, Z.; Stork, N.; Krajnik, M. Severe pruritus of cholestasis in disseminated cancer: Developing a rational treatment strategy. A case report. J. Pain Symptom. Manag. 2005, 29, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.; Noor, N.; McCue, R.; MacKey, S. Low-dose naltrexone for the treatment of fibromyalgia: Findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheumatol. 2013, 65, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Sharafaddinzadeh, N.; Moghtaderi, A.; Kashipazha, D.; Majdinasab, N.; Shalbafan, B. The effect of low-dose naltrexone on quality of life of patients with multiple sclerosis: A randomized placebo-controlled trial. Mult. Scler. J. 2010, 16, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; You, Y.; Griffin, N.; Feng, J.; Shan, F. Low-dose naltrexone (LDN): A promising treatment in immune-related diseases and cancer therapy. Int. Immunopharmacol. 2018, 61, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Lie, M.R.K.L.; van der Giessen, J.; Fuhler, G.M.; de Lima, A.; Peppelenbosch, M.P.; van der Ent, C.; van der Woude, C.J. Low dose Naltrexone for induction of remission in inflammatory bowel disease patients. J. Transl. Med. 2018, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.P. Low dose naltrexone therapy in multiple sclerosis. Med. Hypotheses 2005, 64, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Gironi, M.; Martinelli-Boneschi, F.; Sacerdote, P.; Solaro, C.; Zaffaroni, M.; Cavarretta, R.; Moiola, L.; Bucello, S.; Radaelli, M.; Pilato, V.; et al. A pilot trial of low-dose naltrexone in primary progressive multiple sclerosis. Mult. Scler. 2008, 14, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Turel, A.P.; Oh, K.H.; Zagon, I.S.; McLaughlin, P.J. Low Dose Naltrexone for Treatment of Multiple Sclerosis: A Retrospective Chart Review of Safety and Tolerability. J. Clin. Psychopharmacol. 2015, 35, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Cree, B.A.C.; Kornyeyeva, E.; Goodin, D.S. Pilot trial of low-dose naltrexone and quality of life in multiple sclerosis. Ann. Neurol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.D.; Turel, A.P.; Zagon, I.S.; McLaughlin, P.J. Long-term treatment with low dose naltrexone maintains stable health in patients with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Raknes, G.; Småbrekke, L. Low dose naltrexone in multiple sclerosis: Effects on medication use. A quasi-experimental study. PLoS ONE 2017, 12, e0187423. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.D.; Zagon, I.S.; McLaughlin, P.J. Featured Article: Serum [Met5]-enkephalin levels are reduced in multiple sclerosis and restored by low-dose naltrexone. Exp. Biol. Med. 2017, 242, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Donahue, R.N.; Bonneau, R.H.; McLaughlin, P.J. T lymphocyte proliferation is suppressed by the opioid growth factor ([Met5]-enkephalin)-opioid growth factor receptor axis: Implication for the treatment of autoimmune diseases. Immunobiology 2011, 216, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Donahue, R.N.; Bonneau, R.H.; McLaughlin, P.J. B lymphocyte proliferation is suppressed by the opioid growth factor-opioid growth factor receptor axis: Implication for the treatment of autoimmune diseases. Immunobiology 2011, 216, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.D.; Zagon, I.S.; McLaughlin, P.J. Featured Article: Modulation of the OGF–OGFr pathway alters cytokine profiles in experimental autoimmune encephalomyelitis and multiple sclerosis. Exp. Biol. Med. 2018, 243, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, L.B.; Myers, T.L.; Walters, A.S.; Schwartz, O.A.; Younger, J.W.; Chopra, P.J.; Guarino, A.H. Identification and Treatment of New Inflammatory Triggers for Complex Regional Pain Syndrome: Small Intestinal Bacterial Overgrowth and Obstructive Sleep Apnea. A A Case Rep. 2016, 6, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.; Mackey, S. Fibromyalgia Symptoms Are Reduced by Low-Dose Naltrexone: A Pilot Study. Pain Med. 2009, 10, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Parkitny, L.; Younger, J. Reduced Pro-Inflammatory Cytokines after Eight Weeks of Low-Dose Naltrexone for Fibromyalgia. Biomedicines 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Panksepp, J.; Johnson, B. Is fibromyalgia an endocrine/endorphin deficit disorder? Is low dose naltrexone a new treatment option? Psychosomatics 2012, 53, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Kariv, R.; Tiomny, E.; Grenshpon, R.; Dekel, R.; Waisman, G.; Ringel, Y.; Halpern, Z. Low-dose naltrexone for the treatment of irritable bowel syndrome: A pilot study. Dig. Dis. Sci. 2006, 51, 2128–2133. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.; Alkhouri, N.; Mayacy, S.; Kaplan, B.; Mahajan, L. Low-dose naltrexone for treatment of duodenal Crohn’s disease in a pediatric patient. Inflamm. Bowel Dis. 2010, 16, 1457. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.E.; Nguyen, T.M.; Segal, D.; MacDonald, J.K.; Chande, N. Low dose naltrexone for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 4, CD010410. [Google Scholar] [CrossRef] [PubMed]
- Raknes, G.; Simonsen, P.; Småbrekke, L. The effect of Low Dose Naltrexone on Medication in Inflammatory Bowel Disease: A Quasi Experimental before-and-after Prescription Database Study. J. Crohns Colitis 2018. [Google Scholar] [CrossRef] [PubMed]
- Segal, D.; MacDonald, J.K.; Chande, N.; MacDonald John, K.; Chande, N. Low dose naltrexone for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2014, 10–13. [Google Scholar] [CrossRef]
- Tawfik, D.I.; Osman, A.S.; Tolba, H.M.; Khattab, A.; Abdel-Salam, L.O.; Kamel, M.M. Evaluation of therapeutic effect of low dose naltrexone in experimentally-induced Crohn’s disease in rats. Neuropeptides 2016, 59, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Matters, G.L.; Harms, J.F.; McGovern, C.; Fitzpatrick, L.; Parikh, A.; Nilo, N.; Smith, J.P. The opioid antagonist naltrexone improves murine inflammatory bowel disease. J. Immunotoxicol. 2008, 5, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; McLaughlin, P.J. Naltrexone Modulates Tumor Response in Mice with Neuroblastoma. Science 1983, 221, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Berkson, B.M.; Rubin, D.M.; Berkson, A.J. The long-term survival of a patient with pancreatic cancer with metastases to the liver after treatment with the intravenous α-lipoic acid/low-dose naltrexone protocol. Integr. Cancer Ther. 2006, 5, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Berkson, B.M.; Rubin, D.M.; Berkson, A.J. Revisiting the ALA/N (α-Lipoic Acid/Low-Dose Naltrexone) protocol for people with metastatic and nonmetastatic pancreatic cancer: A report of 3 new cases. Integr. Cancer Ther. 2009, 8, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; Buhler, L.; Icard, P.; Lincet, H.; Steyaert, J.-M. Metabolic treatment of cancer: Intermediate results of a prospective case series. Anticancer Res. 2014, 34, 973–980. [Google Scholar] [PubMed]
- Ding, E.L.; Song, Y.; Manson, J.E.; Hunter, D.J.; Lee, C.C.; Rifai, N.; Buring, J.E.; Gaziano, J.M.; Liu, S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 2009, 361, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Campbell, V.; McGrath, C.; Corry, A. Low-dose naltrexone: A novel treatment for Hailey-Hailey disease. Br. J. Dermatol. 2018, 12, 3218–3221. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.; Hogan, S.R.; Vij, A.; Fernandez, A.P. Low-dose naltrexone treatment of familial benign pemphigus (Hailey-Hailey disease). JAMA Dermatol. 2017, 153, 1015–1017. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, M.P.; Leboyer, M.; Launay, J.M.; Recasens, C.; Plumet, M.H.; Waller-Perotte, D.; Tabuteau, F.; Bondoux, D.; Dugas, M.; Lensing, P.; et al. Low-dose naltrexone effects on plasma chemistries and clinical symptoms in autism: A double-blind, placebo-controlled study. Psychiatry Res. 1995, 58, 191–201. [Google Scholar] [CrossRef]
- Mischoulon, D.; Hylek, L.; Yeung, A.S.; Clain, A.J.; Baer, L.; Cusin, C.; Ionescu, D.F.; Alpert, J.E.; Soskin, D.P.; Fava, M. Randomized, proof-of-concept trial of low dose naltrexone for patients with breakthrough symptoms of major depressive disorder on antidepressants. J. Affect. Disord. 2017, 208, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Frech, T.; Novak, K.; Revelo, M.P.; Murtaugh, M.; Markewitz, B.; Hatton, N.; Scholand, M.B.; Frech, E.; Markewitz, D.; Sawitzke, A.D. Low-dose naltrexone for pruritus in systemic sclerosis. Int. J. Rheumatol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Hota, D.; Srinivasan, A.; Dutta, P.; Bhansali, A.; Chakrabarti, A. Off-Label, Low-Dose Naltrexone for Refractory Painful Diabetic Neuropathy. Pain Med. 2016, 17, 790–791. [Google Scholar] [CrossRef] [PubMed]
- Roginsky, G.; Alexoff, A.; Ehrenpreis, E.D. Initial Findings of an Open-Label Trial of Low-Dose Naltrexone for Symptomatic Mesenteric Panniculitis. J. Clin. Gastroenterol. 2015, 49, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, L.B.; Brook, J.B.; Myers, T.L.; Goodman, B. Successful treatment of postural orthostatic tachycardia and mast cell activation syndromes using naltrexone, immunoglobulin and antibiotic treatment. BMJ Case Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Attarian, S.; Vallat, J.-M.; Magy, L.; Funalot, B.; Gonnaud, P.-M.; Lacour, A.; Péréon, Y.; Dubourg, O.; Pouget, J.; Micallef, J.; et al. An exploratory randomised double-blind and placebo-controlled phase 2 study of a combination of baclofen, naltrexone and sorbitol (PXT3003) in patients with Charcot-Marie-Tooth disease type 1A. Orphanet J. Rare Dis. 2014, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J.M.K.; Kopsky, D.J. Enhancing acupuncture by low dose naltrexone. Acupunct. Med. 2011, 29, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, P.; Patkar, A.A.; Peindl, K.; Gottheil, E.; Wu, L.-T.; Gorelick, D.A. Early Outcomes Following Low Dose Naltrexone Enhancement of Opioid Detoxification. Am. J. Addict. 2009, 18, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, P.; Patkar, A.A.; Peindl, K.; Gorelick, D.A.; Wu, L.-T.; Gottheil, E. Very low dose naltrexone addition in opioid detoxification: A randomized, controlled trial. Addict. Biol. 2009, 14, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, P.; Peindl, K.; Wu, L.T.; Patkar, A.A.; Gorelick, D.A. The combination very low-dose naltrexone-clonidine in the management of opioid withdrawal. Am. J. Drug Alcohol. Abuse 2012, 38, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, P.; Wu, L.T.; Peindl, K.S.; Gorelick, D.A. Smoking and opioid detoxification: Behavioral changes and response to treatment. Nicotine Tob. Res. 2013, 15, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, P.; Wu, L.-T.; Peindl, K.S.; Swartz, M.S.; Woody, G.E. Extended release naltrexone injection is performed in the majority of opioid dependent patients receiving outpatient induction: A very low dose naltrexone and buprenorphine open label trial. Drug Alcohol. Depend. 2014, 138, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.R.; Butera, P.G.; Moran, L.V.; Wu, N.; Burns, L.H.; Friedmann, N. Oxytrex Minimizes Physical Dependence While Providing Effective Analgesia: A Randomized Controlled Trial in Low Back Pain. J. Pain 2006, 7, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.R. Oxytrex: An oxycodone and ultra-low-dose naltrexone formulation. Expert Opin. Investig. Drugs 2007, 16, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Chindalore, V.L.; Craven, R.A.; Yu, K.P.; Butera, P.G.; Burns, L.H.; Friedmann, N. Adding ultralow-dose naltrexone to oxycodone enhances and prolongs analgesia: A randomized, controlled trial of oxytrex. J. Pain 2005, 6, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Krebs, E.E.; Gravely, A.; Nugent, S.; Jensen, A.C.; DeRonne, B.; Goldsmith, E.S.; Kroenke, K.; Bair, M.J.; Noorbaloochi, S. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain. JAMA 2018, 319, 872. [Google Scholar] [CrossRef] [PubMed]
- Firouzian, A.; Gholipour Baradari, A.; Alipour, A.; Emami Zeydi, A.; Zamani Kiasari, A.; Emadi, S.A.; Kheradmand, B.; Hadadi, K. Ultra–low-dose Naloxone as an Adjuvant to Patient Controlled Analgesia (PCA) With Morphine for Postoperative Pain Relief Following Lumber Discectomy. J. Neurosurg. Anesthesiol. 2016, 30, 1. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, L.; Zhou, Q.; Xiong, W.; Duan, X.; Huang, X. A randomized clinical trial of the effects of ultra-low-dose naloxone infusion on postoperative opioid requirements and recovery. Acta Anaesthesiol. Scand. 2015, 59, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Movafegh, A.; Nouralishahi, B.; Sadeghi, M.; Nabavian, O. An ultra-low dose of naloxone added to lidocaine or lidocaine-fentanyl mixture prolongs axillary brachial plexus blockade. Anesth. Analg. 2009, 109, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.L.; La Vincente, S.F.; Somogyi, A.A.; Chapleo, C.B.; White, J.M. Potentiation of buprenorphine antinociception with ultra-low dose naltrexone in healthy subjects. Eur. J. Pain 2011, 15, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Werner, M.U. Combining an oral opioid-receptor agonist and the antagonist naloxone: A smart drug design that removes some but not all adverse effects of the opioid analgesic. Scand. J. Pain 2014, 5, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Torkildsen, O.; Myhr, K.-M.; Wergeland, S. Treatment-resistant immune thrombocytopenic purpura associated with LDN use in a patient with MS. Neurol Neuroimmunol. Neuroinflamm. 2014, 1, e25. [Google Scholar] [CrossRef] [PubMed]
- Patten, D.K.; Schultz, B.G.; Berlau, D.J. The Safety and Efficacy of Low-Dose Naltrexone in the Management of Chronic Pain and Inflammation in Multiple Sclerosis, Fibromyalgia, Crohn’s Disease, and Other Chronic Pain Disorders. Pharmacotherapy 2018, 12, 3218–3221. [Google Scholar] [CrossRef] [PubMed]
| Dose Range | Dose Specific Mechanism of Action | Clinical Use |
|---|---|---|
| Standard (50–100 mg) | Opioid receptor antagonism | Alcohol and opiate abuse |
| Low-dose (1–5 mg) | Toll-like receptor 4 antagonism, opioid growth factor antagonism | Fibromyalgia, multiple sclerosis, Crohn’s disease, cancer, Hailey-Hailey disease, complex-regional pain syndrome |
| Very low-dose (0.001–1 mg) | Possibly same as low-dose | Add-on to methadone detoxification taper |
| Ultra low-dose (<0.001 mg) | Binding to high affinity filamin-A (FLNA) site and reducing μ-opioid receptor associated Gs-coupling | Potentiating opioid analgesia |
| Disease Classification | Type of Study (Number of Subjects) | Notable Outcomes | Reference |
|---|---|---|---|
| Primary progressive multiple sclerosis | Open-label uncontrolled phase II (40) |
| Gironi et al. [46] |
| Multiple sclerosis | Randomized placebo-controlled trial (60) |
| Cree et al. [48] |
| Relapsing-remitting and secondary progressive multiple sclerosis | Randomized placebo-controlled trial (96) |
| Sharafaddinzadeh et al. [42] |
| Relapsing-remitting and secondary progressive multiple sclerosis | Retrospective cohort (215) |
| Turel et al. [47] |
| Relapsing-remitting multiple sclerosis | Retrospective cohort (54) |
| Ludwig et al. [49] |
| Multiple sclerosis | Quasi-experimental pharmacoepidemiological cohort (341) |
| Raknes and Småbrekke [50] |
| Type of Study (Number of Subjects) | Treatment Duration | Notable Outcomes | Reference |
|---|---|---|---|
| Open label prospective (17 adult patients affected by Crohn’s disease) | 12 weeks + 4 weeks follow-up |
| Smith et al. [38] |
| Pediatric case report on Crohn’s disease (1) | 4 weeks + 3 months follow-up |
| Shannon et al. [60] |
| Cochrane review of placebo-controlled trials (34 adult and 12 pediatric patients affected by Crohn’s disease) | 12 weeks (adults) and 8 weeks (children) |
| Parker et al. [61] |
| Open label prospective (19 adult patients affected by Crohn’s disease and 28 by ulcerative colitis) | 12 weeks |
| Lie et al. [44] |
| Quasi-experimental pharmacoepidemiological cohort of patients affected by inflammatory bowel disease (582) | 4 years |
| Raknes et al. [62] |
| Syndrome/Model | Type of Study (Number of Subjects) | Notable Outcomes | Reference |
|---|---|---|---|
| Cholestasis pruritus | Case report (1) |
| Zylicz et al. [40] |
| Osteoarthritis | Phase II randomized controlled trial (362) |
| Chindalore et al. [88] |
| Low back pain | Phase III randomized controlled trial (719) |
| Webster et al. [86] |
| Axillary brachial plexus blockade | Randomized controlled trial (112) |
| Movafegh et al. [92] |
| Buprenorphine antinociception in healthy subjects | Double-blind crossover trial (10) |
| Hay et al. [93] |
| Postoperative pain control following colorectal surgery | Randomized controlled trial (72) |
| Xiao et al. [91] |
| Postoperative pain control following lumbar discectomy | Randomized controlled trial (80) |
| Firouzian et al. [90] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toljan, K.; Vrooman, B. Low-Dose Naltrexone (LDN)—Review of Therapeutic Utilization. Med. Sci. 2018, 6, 82. https://doi.org/10.3390/medsci6040082
Toljan K, Vrooman B. Low-Dose Naltrexone (LDN)—Review of Therapeutic Utilization. Medical Sciences. 2018; 6(4):82. https://doi.org/10.3390/medsci6040082
Chicago/Turabian StyleToljan, Karlo, and Bruce Vrooman. 2018. "Low-Dose Naltrexone (LDN)—Review of Therapeutic Utilization" Medical Sciences 6, no. 4: 82. https://doi.org/10.3390/medsci6040082
APA StyleToljan, K., & Vrooman, B. (2018). Low-Dose Naltrexone (LDN)—Review of Therapeutic Utilization. Medical Sciences, 6(4), 82. https://doi.org/10.3390/medsci6040082