Polyamine Homeostasis in Snyder-Robinson Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Assay of Polyamine Concentrations and Enzyme Activities
2.3. Protein Isolation and Western Blots
2.4. RNA Isolation and Quantitation of Gene Expression
2.5. Statistical Analyses
3. Results
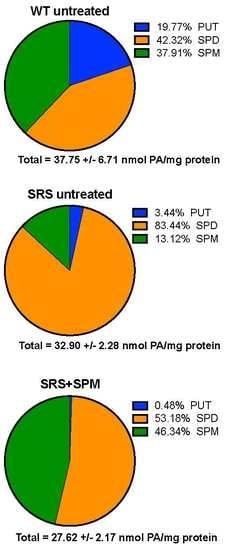
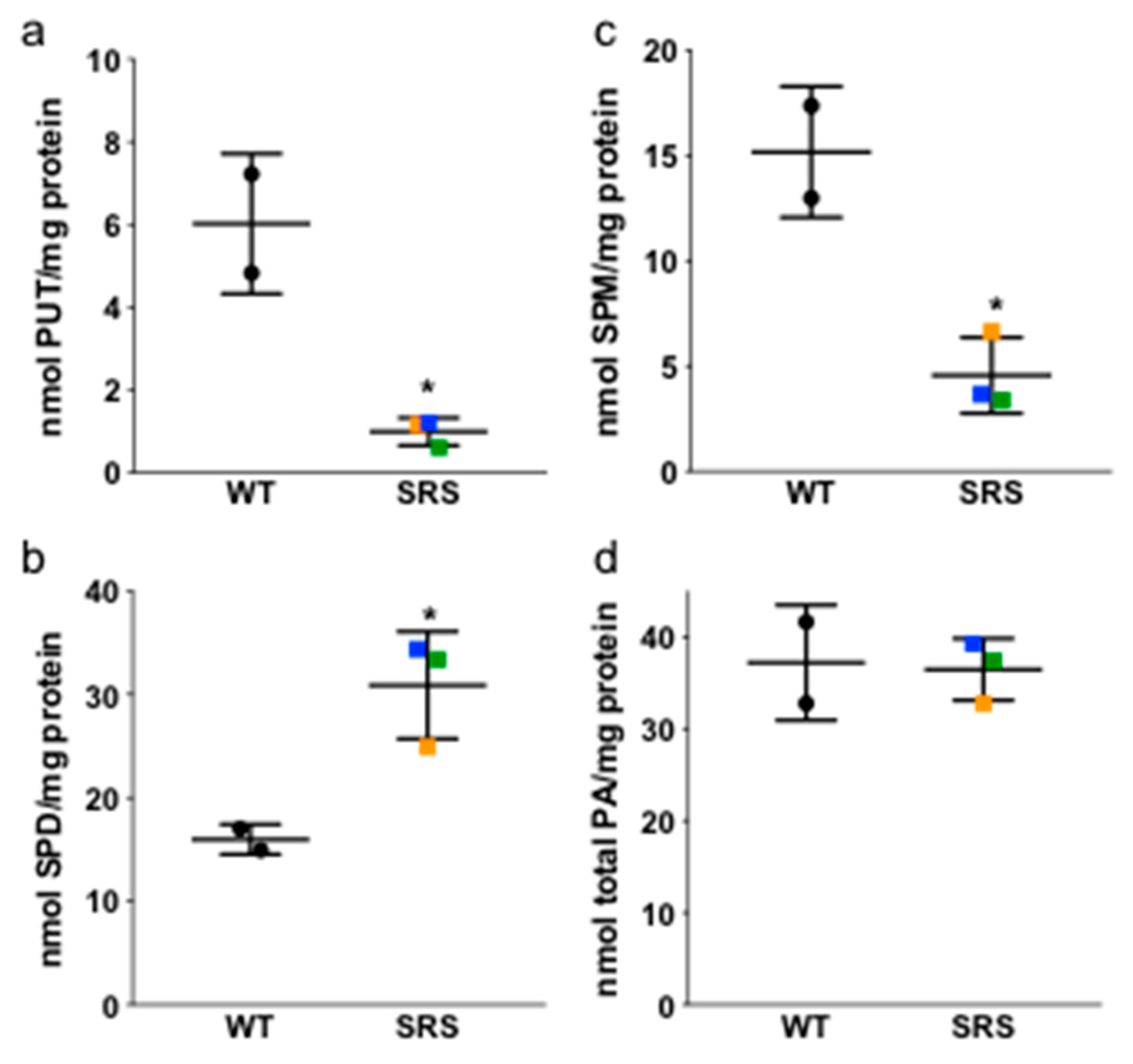
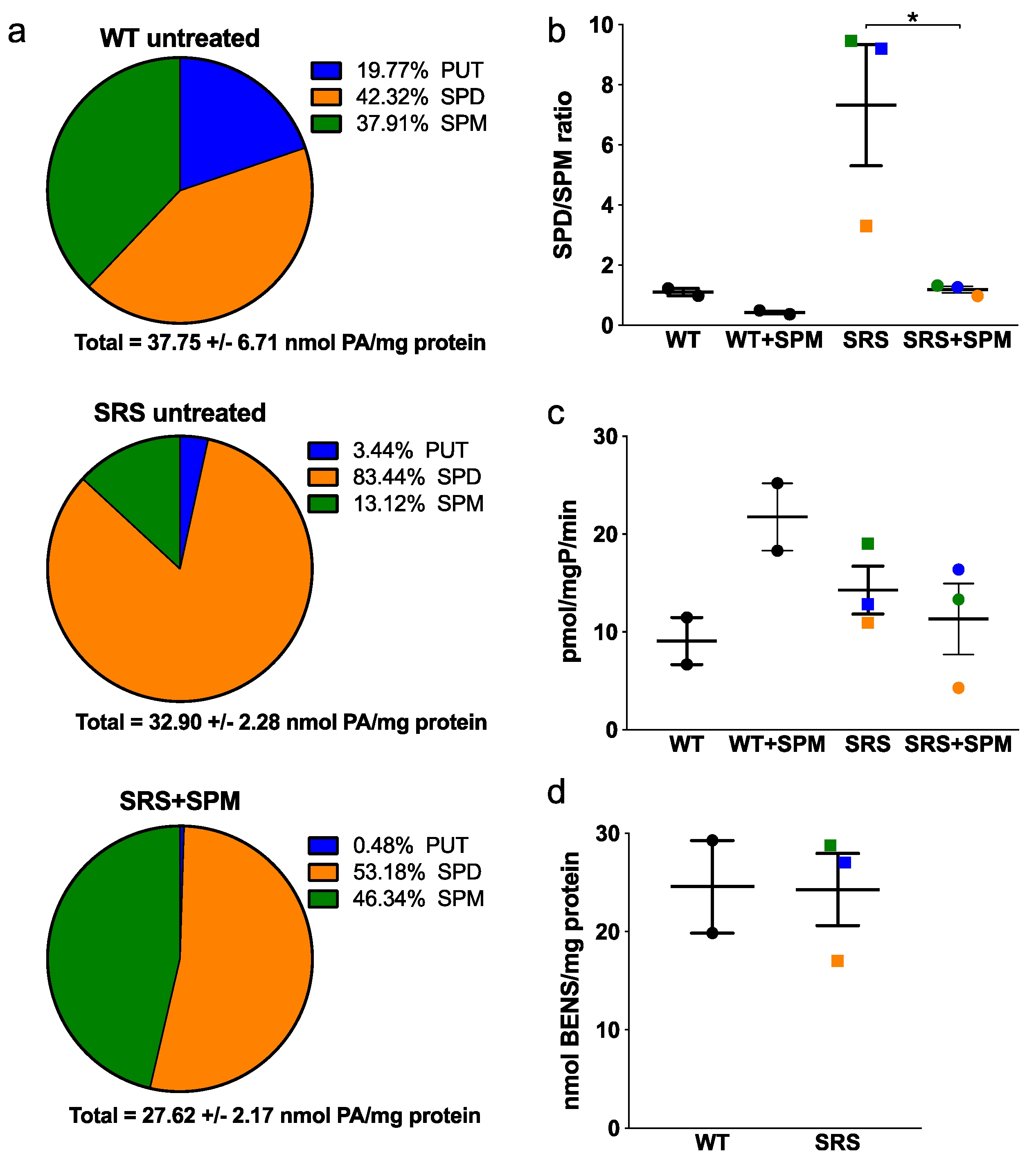
3.1. Alterations in Intracellular Polyamine Distribution
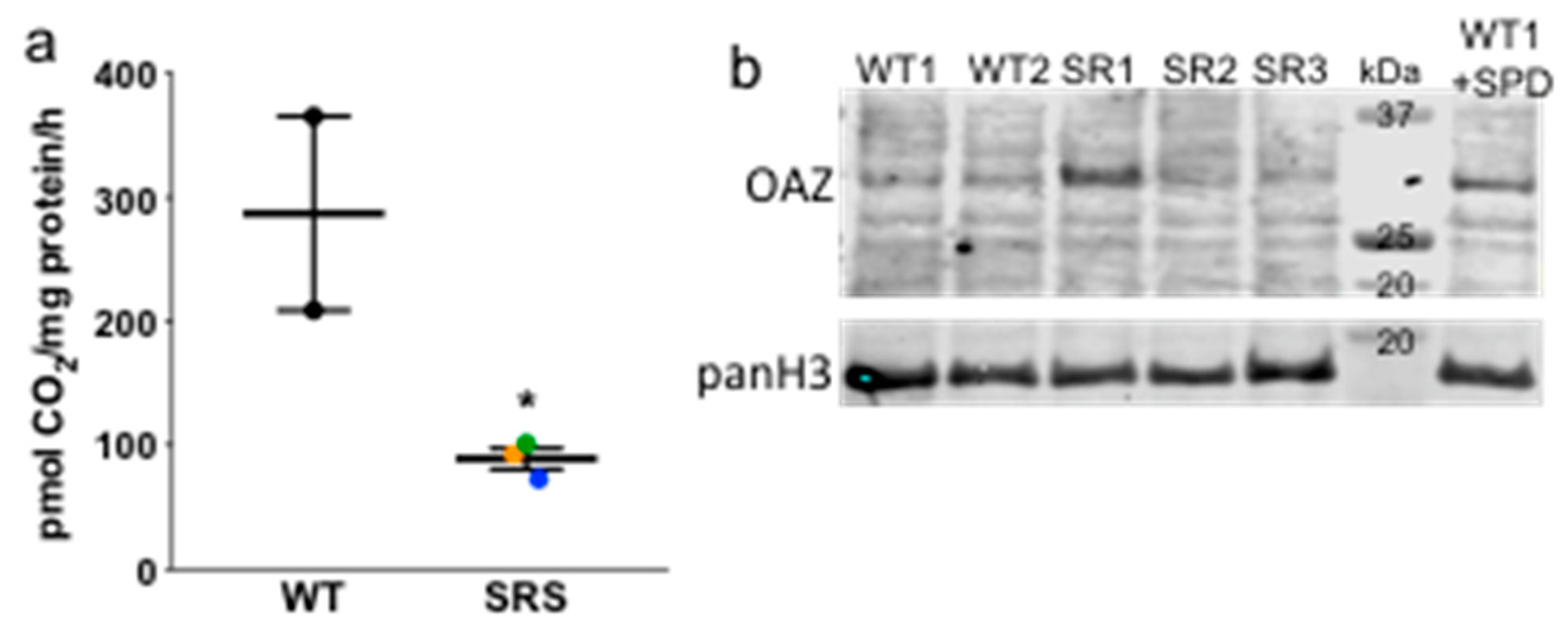
3.2. Ornithine Decarboxylase Activity Is Decreased in Snyder-Robinson Syndrome
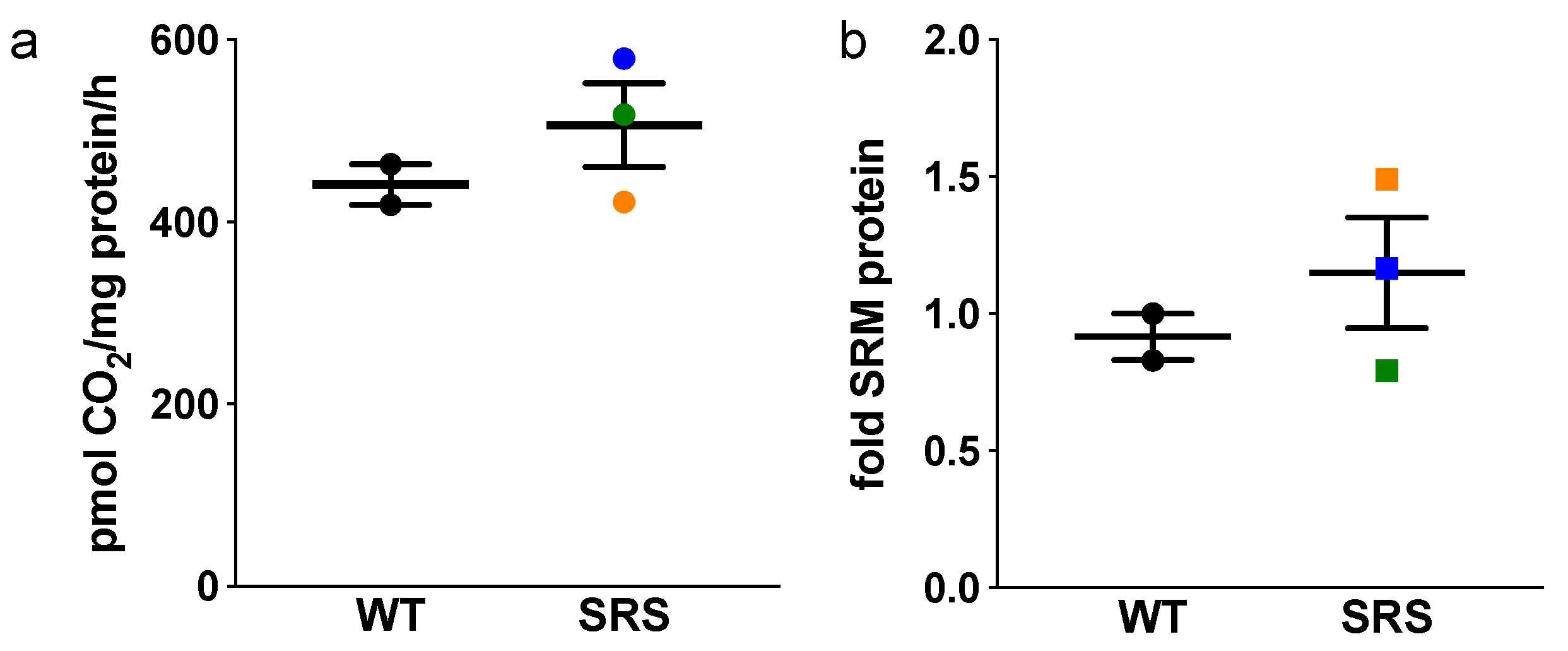
3.3. Effects of Spermine Synthase Mutations on Spermidine Biosynthesis
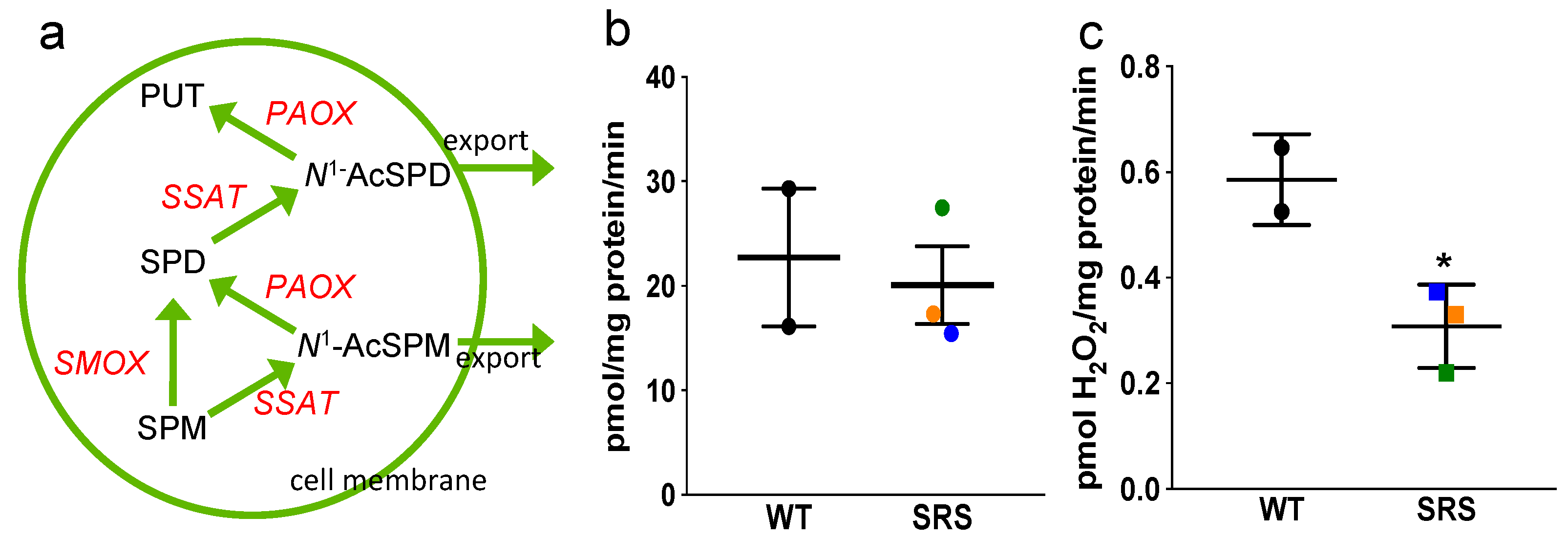
3.4. Effects of Spermine Synthase Mutations on Polyamine Catabolism
3.5. Snyder-Robinson Syndrome Lymphoblasts Maintain Active Polyamine Transport
3.6. N8-Acetylation of Spermidine
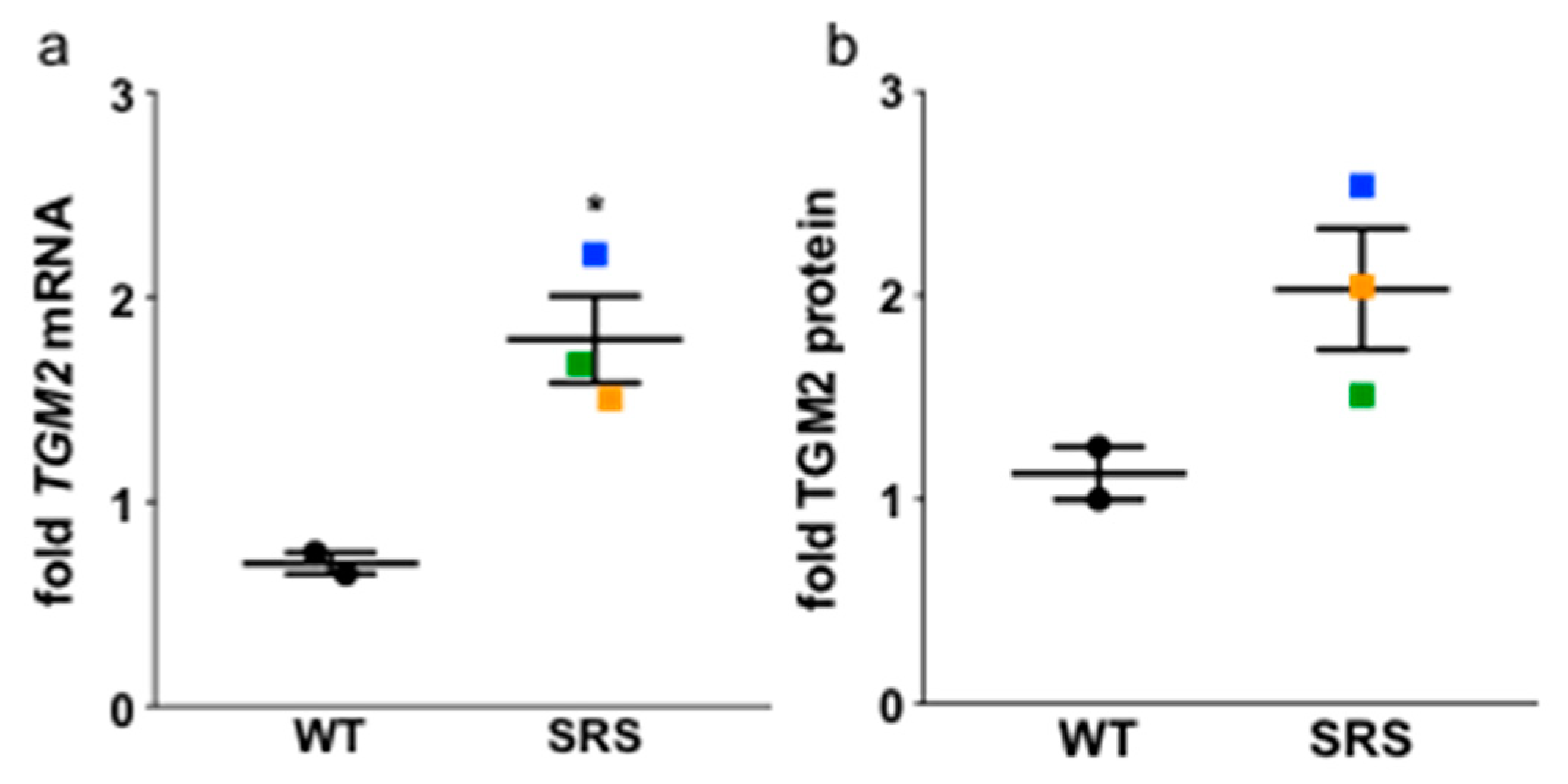
3.7. Transglutaminase 2 Expression Is Upregulated in Snyder-Robinson Syndrome Patient Lymphoblasts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snyder, R.D.; Robinson, A. Recessive sex-linked mental retardation in the absence of other recognizable abnormalities. Report of a family. Clin. Pediatr. 1969, 8, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Arena, J.F.; Schwartz, C.; Ouzts, L.; Stevenson, R.; Miller, M.; Garza, J.; Nance, M.; Lubs, H. X-linked mental retardation with thin habitus, osteoporosis, and kyphoscoliosis: Linkage to Xp21.3-p22.12. Am. J. Med. Genet. 1996, 64, 50–58. [Google Scholar] [CrossRef]
- Wu, H.; Min, J.; Zeng, H.; McCloskey, D.E.; Ikeguchi, Y.; Loppnau, P.; Michael, A.J.; Pegg, A.E.; Plotnikov, A.N. Crystal structure of human spermine synthase: Implications of substrate binding and catalytic mechanism. J. Biol. Chem. 2008, 283, 16135–16146. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018. [Google Scholar] [CrossRef]
- Baronas, V.A.; Kurata, H.T. Inward rectifiers and their regulation by endogenous polyamines. Front. Physiol. 2014, 5, 325. [Google Scholar] [CrossRef]
- Lightfoot, H.L.; Hall, J. Endogenous polyamine function—The RNA perspective. Nucleic Acids Res. 2014, 42, 11275–11290. [Google Scholar] [CrossRef] [PubMed]
- Pasini, A.; Caldarera, C.M.; Giordano, E. Chromatin remodeling by polyamines and polyamine analogs. Amino Acids 2014, 46, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Folk, J.E.; Park, M.H.; Chung, S.I.; Schrode, J.; Lester, E.P.; Cooper, H.L. Polyamines as physiological substrates for transglutaminases. J. Biol. Chem. 1980, 255, 3695–3700. [Google Scholar] [PubMed]
- Lai, T.S.; Lin, C.J.; Greenberg, C.S. Role of tissue transglutaminase-2 (TG2)-mediated aminylation in biological processes. Amino Acids 2017, 49, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.E.; Wang, X.; Stevenson, R.E.; Pegg, A.E. Spermine synthase deficiency resulting in X-linked intellectual disability (Snyder-Robinson syndrome). Methods Mol. Biol. 2011, 720, 437–445. [Google Scholar] [PubMed]
- Cason, A.L.; Ikeguchi, Y.; Skinner, C.; Wood, T.C.; Holden, K.R.; Lubs, H.A.; Martinez, F.; Simensen, R.J.; Stevenson, R.E.; Pegg, A.E.; et al. X-linked spermine synthase gene (SMS) defect: The first polyamine deficiency syndrome. Eur. J. Hum. Genet. 2003, 11, 937–944. [Google Scholar] [CrossRef]
- de Alencastro, G.; McCloskey, D.E.; Kliemann, S.E.; Maranduba, C.M.; Pegg, A.E.; Wang, X.; Bertola, D.R.; Schwartz, C.E.; Passos-Bueno, M.R.; Sertie, A.L. New SMS mutation leads to a striking reduction in spermine synthase protein function and a severe form of Snyder-Robinson X-linked recessive mental retardation syndrome. J. Med. Genet. 2008, 45, 539–543. [Google Scholar] [CrossRef]
- Becerra-Solano, L.E.; Butler, J.; Castaneda-Cisneros, G.; McCloskey, D.E.; Wang, X.; Pegg, A.E.; Schwartz, C.E.; Sanchez-Corona, J.; Garcia-Ortiz, J.E. A missense mutation, p.V132G, in the X-linked spermine synthase gene (SMS) causes Snyder-Robinson syndrome. Am. J. Med. Genet. A 2009, 149A, 328–335. [Google Scholar] [CrossRef]
- Bergeron, R.J.; Neims, A.H.; McManis, J.S.; Hawthorne, T.R.; Vinson, J.R.; Bortell, R.; Ingeno, M.J. Synthetic polyamine analogues as antineoplastics. J. Med. Chem. 1988, 31, 1183–1190. [Google Scholar] [CrossRef]
- Kabra, P.M.; Lee, H.K.; Lubich, W.P.; Marton, L.J. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: Improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J. Chromatogr. 1986, 380, 19–32. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Celano, P.; Ervin, S.J.; Porter, C.W.; Bergeron, R.J.; Libby, P.R. Differential induction of spermidine/spermine N1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res. 1989, 49, 3829–3833. [Google Scholar]
- Seely, J.E.; Pegg, A.E. Ornithine decarboxylase (mouse kidney). Methods Enzymol. 1983, 94, 158–161. [Google Scholar]
- Pegg, A.E. The role of polyamine depletion and accumulation of decarboxylated S-adenosylmethionine in the inhibition of growth of SV-3T3 cells treated with alpha-difluoromethylornithine. Biochem. J. 1984, 224, 29–38. [Google Scholar] [CrossRef]
- Goodwin, A.C.; Murray-Stewart, T.R.; Casero, R.A., Jr. A simple assay for mammalian spermine oxidase: A polyamine catabolic enzyme implicated in drug response and disease. Methods Mol. Biol. 2011, 720, 173–181. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mitchell, J.L.; Judd, G.G.; Bareyal-Leyser, A.; Ling, S.Y. Feedback repression of polyamine transport is mediated by antizyme in mammalian tissue-culture cells. Biochem. J. 1994, 299, 19–22. [Google Scholar] [CrossRef]
- Albert, J.S.; Bhattacharyya, N.; Wolfe, L.A.; Bone, W.P.; Maduro, V.; Accardi, J.; Adams, D.R.; Schwartz, C.E.; Norris, J.; Wood, T.; et al. Impaired osteoblast and osteoclast function characterize the osteoporosis of Snyder—Robinson syndrome. Orphanet. J. Rare Dis. 2015, 10, 27. [Google Scholar] [CrossRef]
- Peron, A.; Spaccini, L.; Norris, J.; Bova, S.M.; Selicorni, A.; Weber, G.; Wood, T.; Schwartz, C.E.; Mastrangelo, M. Snyder-Robinson syndrome: A novel nonsense mutation in spermine synthase and expansion of the phenotype. Am. J. Med. Genet. A 2013, 161A, 2316–2320. [Google Scholar] [CrossRef]
- Korhonen, V.P.; Niiranen, K.; Halmekyto, M.; Pietila, M.; Diegelman, P.; Parkkinen, J.J.; Eloranta, T.; Porter, C.W.; Alhonen, L.; Janne, J. Spermine deficiency resulting from targeted disruption of the spermine synthase gene in embryonic stem cells leads to enhanced sensitivity to antiproliferative drugs. Mol. Pharmacol. 2001, 59, 231–238. [Google Scholar] [CrossRef]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Pegg, A.E. Spermidine/spermine N1-acetyltransferase—The turning point in polyamine metabolism. FASEB J. 1993, 7, 653–661. [Google Scholar] [CrossRef]
- Pegg, A.E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem. J. 1986, 234, 249–262. [Google Scholar] [CrossRef]
- Abela, L.; Simmons, L.; Steindl, K.; Schmitt, B.; Mastrangelo, M.; Joset, P.; Papuc, M.; Sticht, H.; Baumer, A.; Crowther, L.M.; et al. N(8)-acetylspermidine as a potential plasma biomarker for Snyder-Robinson syndrome identified by clinical metabolomics. J. Inherit. Metab. Dis. 2016, 39, 131–137. [Google Scholar] [CrossRef]
- Libby, P.R. Rat liver nuclear N-acetyltransferases: Separation of two enzymes with both histone and spermidine acetyltransferase activity. Arch. Biochem. Biophys. 1980, 203, 384–389. [Google Scholar] [CrossRef]
- Burgio, G.; Corona, D.F.; Nicotra, C.M.; Carruba, G.; Taibi, G. P/CAF-mediated spermidine acetylation regulates histone acetyltransferase activity. J. Enzyme Inhib. Med. Chem. 2016, 31, 75–82. [Google Scholar] [CrossRef]
- Hai, Y.; Shinsky, S.A.; Porter, N.J.; Christianson, D.W. Histone deacetylase 10 structure and molecular function as a polyamine deacetylase. Nat. Commun. 2017, 8, 15368. [Google Scholar] [CrossRef]
- Mackintosh, C.A.; Pegg, A.E. Effect of spermine synthase deficiency on polyamine biosynthesis and content in mice and embryonic fibroblasts, and the sensitivity of fibroblasts to 1,3-bis-(2-chloroethyl)-N-nitrosourea. Biochem. J. 2000, 351, 439–447. [Google Scholar] [CrossRef]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef]
- Park, M.H.; Cooper, H.L.; Folk, J.E. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc. Natl. Acad. Sci. USA 1981, 78, 2869–2873. [Google Scholar] [CrossRef]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.; Mehta, K. Transglutaminase regulation of cell function. Physiol. Rev. 2014, 94, 383–417. [Google Scholar] [CrossRef]
- Wang, J.Y.; Viar, M.J.; Johnson, L.R. Regulation of transglutaminase activity by polyamines in the gastrointestinal mucosa of rats. Proc. Soc. Exp. Biol. Med. 1994, 205, 20–28. [Google Scholar] [CrossRef]
- Bupp, C.P.; Schultz, C.R.; Uhl, K.L.; Rajasekaran, S.; Bachmann, A.S. Novel de novo pathogenic variant in the ODC1 gene in a girl with developmental delay, alopecia, and dysmorphic features. Am. J. Med. Genet. A 2018. [Google Scholar] [CrossRef]
- Rodan, L.H.; Anyane-Yeboa, K.; Chong, K.; Klein Wassink-Ruiter, J.S.; Wilson, A.; Smith, L.; Kothare, S.V.; Rajabi, F.; Blaser, S.; Ni, M.; et al. Gain-of-function variants in the ODC1 gene cause a syndromic neurodevelopmental disorder associated with macrocephaly, alopecia, dysmorphic features, and neuroimaging abnormalities. Am. J. Med. Genet. A 2018. [Google Scholar] [CrossRef]
- Megosh, L.; Gilmour, S.K.; Rosson, D.; Soler, A.P.; Blessing, M.; Sawicki, J.A.; O’Brien, T.G. Increased frequency of spontaneous skin tumors in transgenic mice which overexpress ornithine decarboxylase. Cancer Res. 1995, 55, 4205–4209. [Google Scholar]
- McKenna, J.; Kapfhamer, D.; Kinchen, J.M.; Wasek, B.; Dunworth, M.; Murray-Stewart, T.; Bottiglieri, T.; Casero, R.A.; Gambello, M.J. Metabolomic studies identify changes in transmethylation and polyamine metabolism in a brain-specific mouse model of tuberous sclerosis complex. Hum. Mol. Genet. 2018, 27, 2113–2124. [Google Scholar] [CrossRef]


| Cell Line | Mutation | Protein | SMS Activity | SPD/SPM |
|---|---|---|---|---|
| WT1 | none | wildtype | yes | 1.17 (0.04) |
| WT2 | none | wildtype | yes | 0.83 (0.07) |
| SRS1 | c.329+5 G>A aberrant splice site | truncated; some functional SMS from read-through | reduced | 3.76 (0.25) |
| SRS2 | V132G | decreased dimerization | ND | 9.56 (0.73) |
| SRS3 | G56S | no dimerization | ND | 9.85 (0.36) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murray-Stewart, T.; Dunworth, M.; Foley, J.R.; Schwartz, C.E.; Casero, R.A., Jr. Polyamine Homeostasis in Snyder-Robinson Syndrome. Med. Sci. 2018, 6, 112. https://doi.org/10.3390/medsci6040112
Murray-Stewart T, Dunworth M, Foley JR, Schwartz CE, Casero RA Jr. Polyamine Homeostasis in Snyder-Robinson Syndrome. Medical Sciences. 2018; 6(4):112. https://doi.org/10.3390/medsci6040112
Chicago/Turabian StyleMurray-Stewart, Tracy, Matthew Dunworth, Jackson R. Foley, Charles E. Schwartz, and Robert A. Casero, Jr. 2018. "Polyamine Homeostasis in Snyder-Robinson Syndrome" Medical Sciences 6, no. 4: 112. https://doi.org/10.3390/medsci6040112
APA StyleMurray-Stewart, T., Dunworth, M., Foley, J. R., Schwartz, C. E., & Casero, R. A., Jr. (2018). Polyamine Homeostasis in Snyder-Robinson Syndrome. Medical Sciences, 6(4), 112. https://doi.org/10.3390/medsci6040112