Transglutaminase 2 Up-Regulation Is Associated with Inflammatory Response in PBMC from Healthy Subjects with Hypovitaminosis D
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Assessment of Vitamin D Serum Levels
2.3. Isolation of Peripheral Blood Mononuclear Cells
2.4. Quantitative Real-Time PCR Analyses
2.5. Western Blotting
2.6. Electrophoretic Mobility Shift Assay
2.7. Statistical Analysis
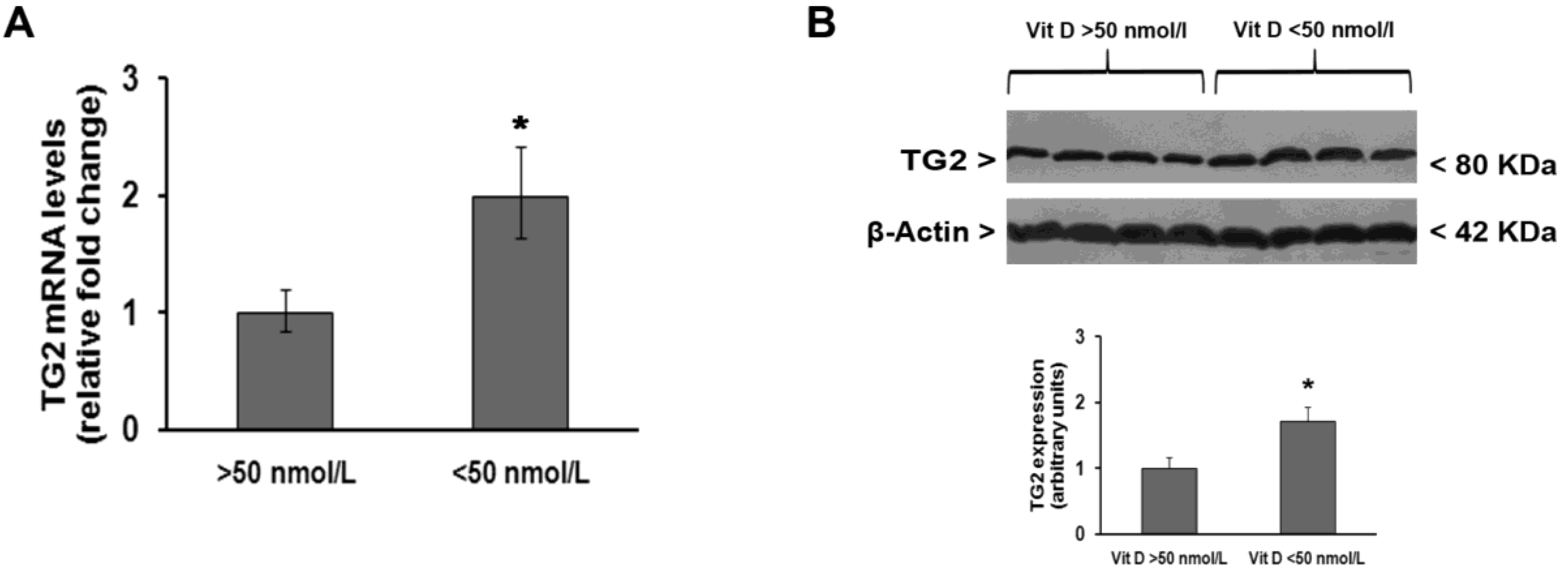
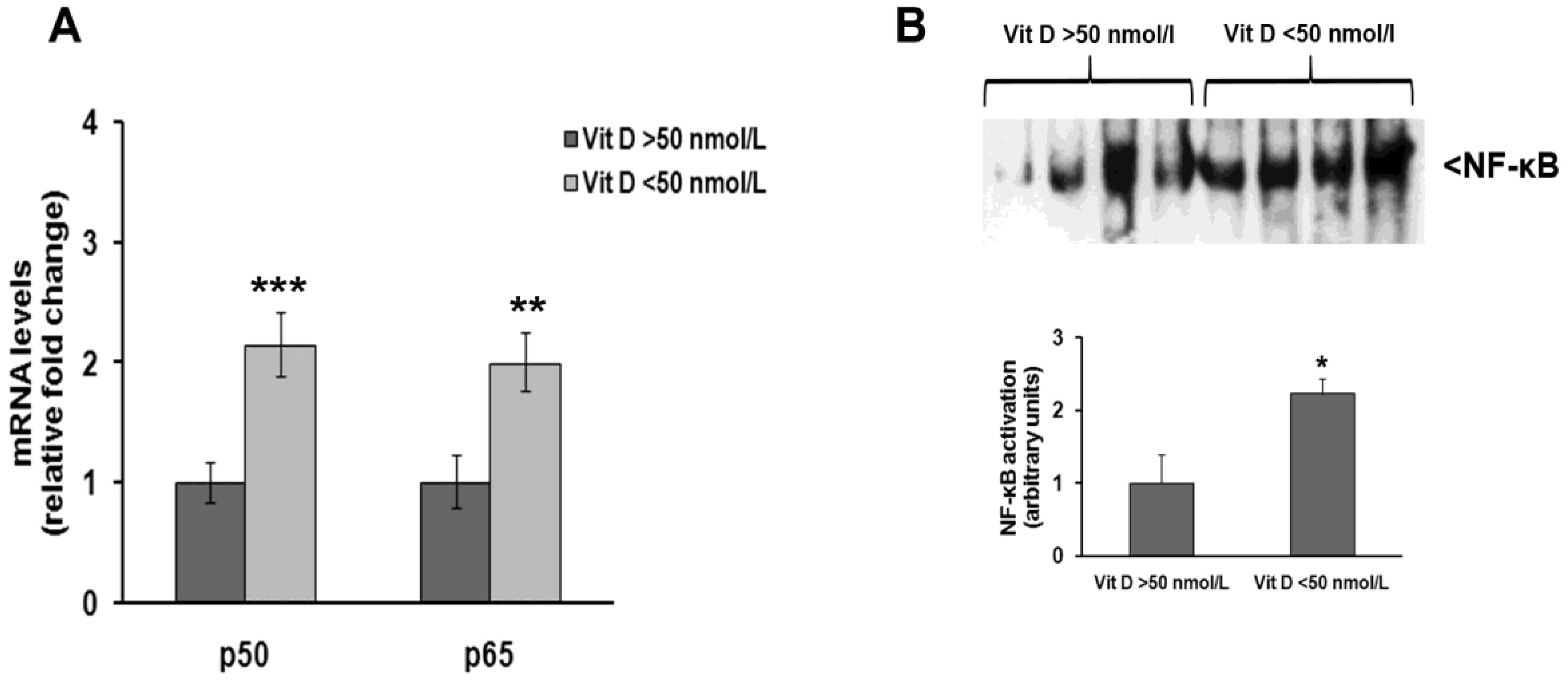
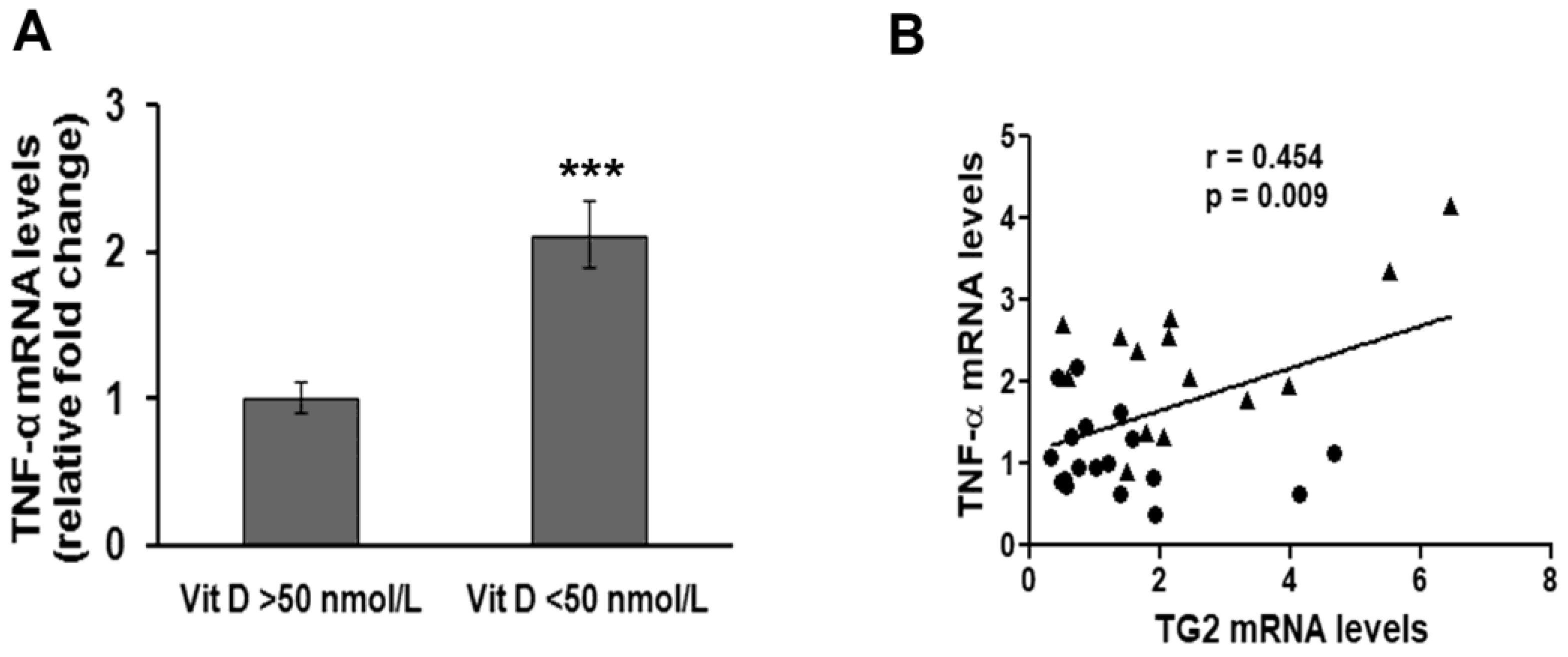
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fesus, L.; Piacentini, M. Transglutaminase 2: An enigmatic enzyme with diverse functions. Trends Biochem. Sci. 2002, 27, 534–539. [Google Scholar] [CrossRef]
- Park, D.; Choi, S.S.; Ha, K.-S. Transglutaminase 2: A multi-functional protein in multiple subcellular compartments. Amino Acids 2010, 39, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.W.; Mehta, K. Transglutaminase regulation of cell function. Physiol. Rev. 2014, 94, 383–417. [Google Scholar] [CrossRef] [PubMed]
- Tatsukawa, H.; Furutani, Y.; Hitomi, K.; Kojima, S. Transglutaminase 2 has opposing roles in the regulation of cellular functions as well as cell growth and death. Cell Death Dis. 2016, 7, e2244. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, K.; Fuxreiter, M.; Fésüs, L. Physiological, pathological, and structural implications of non-enzymatic protein-protein interactions of the multifunctional human transglutaminase 2. Cell. Mol. Life Sci. 2015, 72, 3009–3035. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Jeong, E.M.; Jeong, Y.-J.; Lee, W.J.; Kang, J.S.; Kim, I.-G.; Hwang, Y. Transglutaminase 2 on the surface of dendritic cells is proposed to be involved in dendritic cell-T cell interaction. Cell. Immunol. 2014, 289, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef]
- Visvikis-Siest, S.; Marteau, J.-B.; Samara, A.; Berrahmoune, H.; Marie, B.; Pfister, M. Peripheral blood mononuclear cells (PBMCs): A possible model for studying cardiovascular biology systems. Clin. Chem. Lab. Med. 2007, 45, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Kleiveland, C.R. Peripheral Blood Mononuclear Cells. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer Nature: Basel, Switzerland, 2015; ISBN 978-3-319-15791-7. [Google Scholar]
- Lemire, J.M. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J. Cell. Biochem. 1992, 49, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mahon, B.D.; Froicu, M.; Cantorna, M.T. Calcium and 1α,25-dihydroxyvitamin D3 target the TNF-α pathway to suppress experimental inflammatory bowel disease. Eur. J. Immunol. 2005, 35, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Wöbke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; West, A.P.; Ghosh, S. NF-κβ and the immune response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.-H.; Kim, M.-S.; Le, M.-Q.; Song, Y.-S.; Bak, Y.; Ryu, H.-W.; Oh, S.-R.; Yoon, D.-Y. Fargesin exerts anti-inflammatory effects in THP-1 monocytes by suppressing PKC-dependent AP-1 and NF-ĸB signaling. Phytomedicine 2017, 24, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Parnsamut, C.; Brimson, S. Effects of silver nanoparticles and gold nanoparticles on IL-2, IL-6, and TNF-α production via MAPK pathway in leukemic cell lines. Genet. Mol. Res. 2015, 14, 3650–3668. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-S.; Kim, J.-M.; Kim, D.-S.; Kim, I.-H.; Kim, S.-Y. Transglutaminase 2 mediates polymer formation of I-κβα through C-terminal glutamine cluster. J. Biol. Chem. 2006, 281, 34965–34972. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, D.; Campisi, A.; Currò, M.; Aguennouz, M.; Li Volti, G.; Avola, R.; Ientile, R. Nuclear factor-kappab activation is associated with glutamate-evoked tissue transglutaminase up-regulation in primary astrocyte cultures. J. Neurosci. Res. 2005, 82, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Amendola, A.; Rodolfo, C.; Di Caro, A.; Ciccosanti, F.; Falasca, L.; Piacentini, M. “Tissue” transglutaminase expression in HIV-infected cells: An enzyme with an antiviral effect? Ann. N. Y. Acad. Sci. 2001, 946, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Chrobok, N.L.; Sestito, C.; Wilhelmus, M.M.M.; Drukarch, B.; van Dam, A.-M. Is monocyte- and macrophage-derived tissue transglutaminase involved in inflammatory processes? Amino Acids 2017, 49, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Kumar, A.; Kim, H.I. Transglutaminase 2: A multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem. Pharmacol. 2010, 80, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Currò, M.; Isola, G.; Caccamo, D.; Vecchio, M.; Giunta, M.L.; Ramaglia, L.; Cordasco, G.; Williams, R.C.; Ientile, R. Transglutaminase 2 up-regulation is associated with RANKL/OPG pathway in cultured HPDL cells and THP-1-differentiated macrophages. Amino Acids 2015, 47, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Willis, W.L.; Wang, L.; Wada, T.T.; Gardner, M.; Abdouni, O.; Hampton, J.; Valiente, G.; Young, N.; Ardoin, S.; Agarwal, S.; et al. The proinflammatory protein HMGB1 is a substrate of transglutaminase-2 and forms high-molecular weight complexes with autoantigens. J. Biol. Chem. 2018, 293, 8394–8409. [Google Scholar] [CrossRef] [PubMed]
- Post, L.; Ilich, Z. Controversies in Vitamin D Recommendations and Its Possible Roles in Non skeletal Health Issues. J. Nutr. Food Sci. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Jones, G. Interpreting vitamin D assay results: Proceed with caution. Clin. J. Am. Soc. Nephrol. 2015, 10, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, I.; Pawliczak, R. The Active Metabolite of Vitamin D3 as a Potential Immunomodulator. Scand. J. Immunol. 2016, 83, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Overbergh, L.; Decallonne, B.; Valckx, D.; Verstuyf, A.; Depovere, J.; Laureys, J.; Rutgeerts, O.; Saint-Arnaud, R.; Bouillon, R.; Mathieu, C. Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin. Exp. Immunol. 2000, 120, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zügel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef] [PubMed]
- McGarry, T.; Biniecka, M.; Gao, W.; Cluxton, D.; Canavan, M.; Wade, S.; Wade, S.; Gallagher, L.; Orr, C.; Veale, D.J.; et al. Resolution of TLR2-induced inflammation through manipulation of metabolic pathways in Rheumatoid Arthritis. Sci. Rep. 2017, 7, 43165. [Google Scholar] [CrossRef] [PubMed]
- Calton, E.K.; Keane, K.N.; Soares, M.J.; Rowlands, J.; Newsholme, P. Prevailing vitamin D status influences mitochondrial and glycolytic bioenergetics in peripheral blood mononuclear cells obtained from adults. Redox Biol. 2016, 10, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Reddy, G.S.; Kobayashi, T.; Okano, T.; Park, J.; Sharma, S. Nuclear factor of activated T cells (NFAT) as a molecular target for 1alpha,25-dihydroxyvitamin D3-mediated effects. J. Immunol. 1998, 160, 209–218. [Google Scholar] [PubMed]
- Brtko, J.; Rock, E.; Nezbedova, P.; Krizanova, O.; Dvorcakova, M.; Minet-Quinard, R.; Farges, M.-C.; Ribalta, J.; Winklhofer-Roob, B.M.; Vasson, M.-P.; et al. Age-related change in the retinoid X receptor beta gene expression in peripheral blood mononuclear cells of healthy volunteers: Effect of 13-cis retinoic acid supplementation. Mech. Ageing Dev. 2007, 128, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Dong, X.; Kumar, R. Vitamin D receptor-mediated suppression of RelB in antigen presenting cells: A paradigm for ligand-augmented negative transcriptional regulation. Arch. Biochem. Biophys. 2007, 460, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Yu, Z.; Fu, L.; Wang, H.; Chen, X.; Zhang, C.; Lv, Z.-M.; Xu, D.-X. Vitamin D3 inhibits lipopolysaccharide-induced placental inflammation through reinforcing interaction between vitamin D receptor and nuclear factor kappa B p65 subunit. Sci. Rep. 2015, 5, 10871. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Kim, D.-S.; Ko, C.; Lee, S.-J.; Oh, S.H.; Kim, S.-Y. TNF-alpha mediated NF-kappaB activation is constantly extended by transglutaminase 2. Front. Biosci. 2011, 3, 341–354. [Google Scholar]
| Gene | Primer | SequenzaPrimer 5′→3′ |
|---|---|---|
| ACT-β | forward | TGGTTACAGGAAGTCCCTTGCC |
| ACT-β | reverse | ATGCTATCACCTCCCCTGTGTG |
| NF-κB p50 | forward | ACACTGGAAGCACGAATGACAGA |
| NF-κB p50 | reverse | CCTCCACCTTCTGCTTGCAA |
| NF-κB p65 | forward | CAGGCGAGAGGAGCACAGATAC |
| NF-κB p65 | reverse | TCCTTTCCTACAAGCTCGTGGG |
| TNF-α | forward | GTGAGGAGGACGAACATC |
| TNF-α | reverse | GAGCCAGAAGAGGTTGAG |
| TG2 | forward | CCTTACGGAGTCCAACCTCA |
| TG2 | reverse | CCGTCTTCTGCTCCTCAGTC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caccamo, D.; Ferlazzo, N.; Currò, M.; Ricca, S.; Ientile, R. Transglutaminase 2 Up-Regulation Is Associated with Inflammatory Response in PBMC from Healthy Subjects with Hypovitaminosis D. Med. Sci. 2018, 6, 103. https://doi.org/10.3390/medsci6040103
Caccamo D, Ferlazzo N, Currò M, Ricca S, Ientile R. Transglutaminase 2 Up-Regulation Is Associated with Inflammatory Response in PBMC from Healthy Subjects with Hypovitaminosis D. Medical Sciences. 2018; 6(4):103. https://doi.org/10.3390/medsci6040103
Chicago/Turabian StyleCaccamo, Daniela, Nadia Ferlazzo, Monica Currò, Sergio Ricca, and Riccardo Ientile. 2018. "Transglutaminase 2 Up-Regulation Is Associated with Inflammatory Response in PBMC from Healthy Subjects with Hypovitaminosis D" Medical Sciences 6, no. 4: 103. https://doi.org/10.3390/medsci6040103
APA StyleCaccamo, D., Ferlazzo, N., Currò, M., Ricca, S., & Ientile, R. (2018). Transglutaminase 2 Up-Regulation Is Associated with Inflammatory Response in PBMC from Healthy Subjects with Hypovitaminosis D. Medical Sciences, 6(4), 103. https://doi.org/10.3390/medsci6040103






