The Role of Adipokines in Intervertebral Disc Degeneration
Abstract
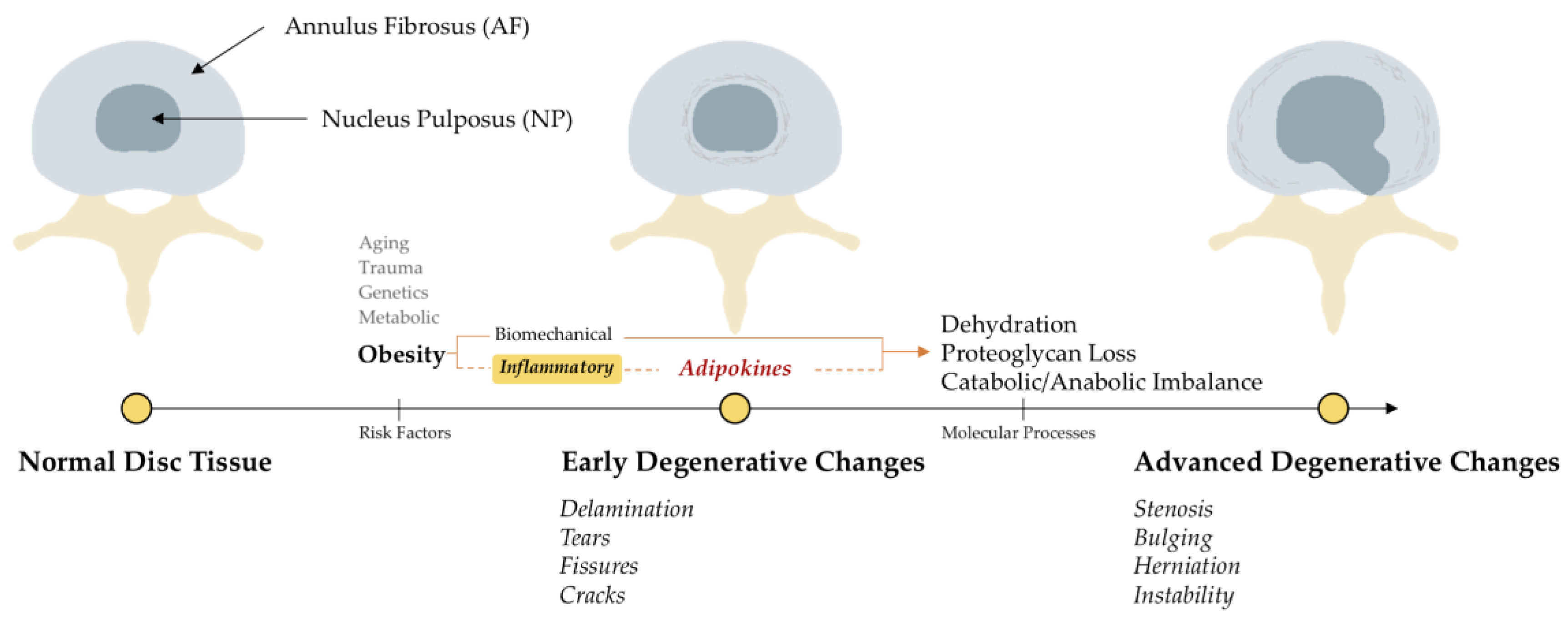
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Criteria
2.3. Study Selection
2.4. Data Collection
3. Results
3.1. Adipokines
3.2. Sample/Tissue
3.3. Species
3.3.1. Human Studies
3.3.2. Animal Studies
4. Discussion
4.1. Presence of Adipokine and Adipokine-Receptors in Intervertebral Discs
4.2. Effects of Adipokine Treatment on Cellular Proteome
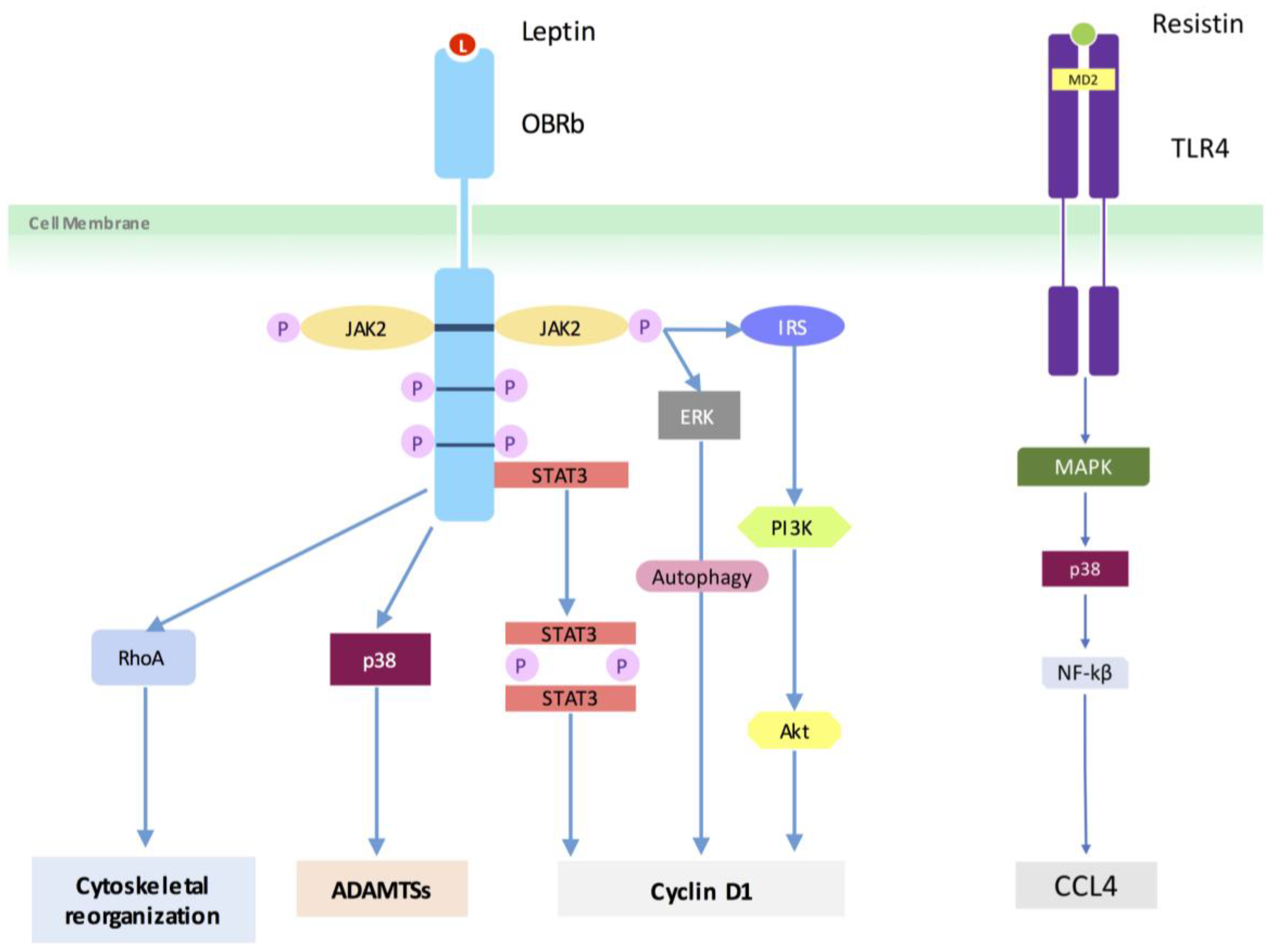
4.3. Adipokine Pathways/Signaling
4.4. Adipokine in Other Fibrocartilaginous Diseases
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hadjipavlou, A.G.; Tzermiadianos, M.N.; Bogduk, N.; Zindrick, M.R. The pathophysiology of disc degeneration: A critical review. J. Bone Joint Surg. Br. 2008, 90, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.P. Intervertebral disc: Anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef] [PubMed]
- Coventry, M.B.; Ghormley, R.K.; Kernohan, J.W. The intervertebral disc: Its microscopic anatomy and pathology. Part I: Anatomy, development, and physiology. J. Bone Joint Surg. Am. 1945, 27, 105–112. [Google Scholar]
- Coventry, M.B.; Ghormley, R.K.; Kernohan, J.W. The intervertebral disc: Its microscopic anatomy and pathology. Part II: Changes in the intervertebral disc concomitant with age. J. Bone Joint Surg. Am. 1945, 27, 233–247. [Google Scholar]
- Coventry, M.B.; Ghormley, R.K.; Kernohan, J.W. The intervertebral disc: Its microscopic anatomy and pathology. Part III: Pathological changes in the intervertebral disc. J. Bone Joint Surg. Am. 1945, 27, 460–474. [Google Scholar]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Luetmer, P.H.; Comstock, B.; Bresnahan, B.W.; Chen, L.E.; Deyo, R.A.; Halabi, S.; Turner, J.A.; Avins, A.L.; James, K.; et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Am. J. Neuroradiol. 2015, 36, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.H.; Yoon, S.T. Update on the pathophysiology of degenerative disc disease and new developments in treatment strategies. Open Access J. Sports Med. 2010, 1, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J. Bone Joint Surg. Am. 2006, 88 (Suppl. 2), 21–24. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Liuke, M.; Solovieva, S.; Lamminen, A.; Luoma, K.; Leino-Arjas, P.; Luukkonen, R.; Riihimaki, H. Disc degeneration of the lumbar spine in relation to overweight. Int. J. Obes. 2005, 29, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Karppinen, J.; Chan, D.; Luk, K.D.; Cheung, K.M. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: A population-based study. Arthritis Rheumtol. 2012, 64, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Riihimaki, H.; Mattsson, T.; Zitting, A.; Wickstrom, G.; Hanninen, K.; Waris, P. Radiographically detectable degenerative changes of the lumbar spine among concrete reinforcement workers and house painters. Spine (Phila Pa 1976) 1990, 15, 114–119. [Google Scholar] [CrossRef]
- Videman, T.; Gibbons, L.E.; Kaprio, J.; Battie, M.C. Challenging the cumulative injury model: Positive effects of greater body mass on disc degeneration. Spine J. 2010, 10, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Hassett, G.; Hart, D.J.; Manek, N.J.; Doyle, D.V.; Spector, T.D. Risk factors for progression of lumbar spine disc degeneration: The chingford study. Arthritis Rheumtol. 2003, 48, 3112–3117. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Newbold, R.; Schug, T.T. Endocrine disruptors and obesity. Nat. Rev. Endocrinol. 2015, 11, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Bardoloi, A.; Parkash, A.A. Metabolic effects of obesity: A review. World J. Diabetes 2010, 1, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Junker, S.; Schett, G.; Frommer, K.; Muller-Ladner, U. Adipokines in bone disease. Nat. Rev. Rheumatol. 2016, 12, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Takahashi, M.; Smith, H.; Rizek, R.; Mahomed, N.N. The synovial fluid adiponectin-leptin ratio predicts pain with knee osteoarthritis. Clin. Rheumatol. 2010, 29, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Perruccio, A.V.; Rizek, R.; Dessouki, O.; Evans, H.M.; Mahomed, N.N. Obesity-related adipokines predict patient-reported shoulder pain. Obes. Facts 2013, 6, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Kapoor, M.; Mahomed, N.N.; Perruccio, A.V. A comparison of obesity related adipokine concentrations in knee and shoulder osteoarthritis patients. Obes. Res. Clin. Pract. 2015, 9, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Sharma, A.; Kapoor, M.; Sundararajan, K.; Perruccio, A.V. Racial differences in serum adipokine and insulin levels in a matched osteoarthritis sample: A pilot study. J. Obes. 2016, 2016, 8746268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fasshauer, M.; Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Podichetty, V.K. The aging spine: The role of inflammatory mediators in intervertebral disc degeneration. Cell. Mol. Biol. 2007, 53, 4–18. [Google Scholar] [PubMed]
- Wuertz, K.; Haglund, L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Glob. Spine J. 2013, 3, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Molinos, M.; Almeida, C.R.; Caldeira, J.; Cunha, C.; Goncalves, R.M.; Barbosa, M.A. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 2015, 12, 20141191. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhao, C.; Cao, L.; Zhang, K.; Sun, W.; Xie, Y.; Li, H.; Zhao, J. Leptin induces terminal differentiation of rat annulus fibrosus cells via activation of MAPK signaling. Anat. Rec. 2013, 296, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Ingram, J.A.; Hoelscher, G.L.; Hanley, E.N., Jr. Leptin expression by annulus cells in the human intervertebral disc. Spine J. 2007, 7, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Khabour, O.F.; Abu-Rumeh, L.; Al-Jarrah, M.; Jamous, M.; Alhashimi, F. Association of adiponectin protein and ADIPOQ gene variants with lumbar disc degeneration. Exp. Ther. Med. 2014, 8, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Koerner, J.D.; Markova, D.Z.; Yadla, S.; Mendelis, J.; Hilibrand, A.; Vaccaro, A.R.; Risbud, M.V.; Albert, T.J.; Anderson, D.G.; Kepler, C.K. Differential gene expression in anterior and posterior annulus fibrosus. Spine (Phila Pa 1976) 2014, 39, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liang, J.; Wu, W.K.; Yu, X.; Yu, J.; Weng, X.; Shen, J. Leptin activates rhoa/rock pathway to induce cytoskeleton remodeling in nucleus pulposus cells. Int. J. Mol. Sci. 2014, 15, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shen, J.; Wu, W.K.; Yu, X.; Liang, J.; Qiu, G.; Liu, J. Leptin induces cyclin D1 expression and proliferation of human nucleus pulposus cells VIA JAK/STAT, PI3K/Akt and MEK/ERK pathways. PLoS ONE 2012, 7, e53176. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shen, J.; Wu, W.K.; Yu, X.; Liang, J.; Qiu, G.; Liu, J. The role of leptin on the organization and expression of cytoskeleton elements in nucleus pulposus cells. J. Orthop. Res. 2013, 31, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, X.; Liang, J.; Wu, W.K.; Yu, J.; Shen, J. Leptin downregulates aggrecan through the p38-ADAMST pathway in human nucleus pulposus cells. PLoS ONE 2014, 9, e109595. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Zhang, L. Leptin modulates the expression of catabolic genes in rat nucleus pulposus cells through the mitogen-activated protein kinase and Janus kinase 2/signal transducer and activator of transcription 3 pathways. Mol. Med. Rep. 2015, 12, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Liu, D.; Li, H.; Jiang, L.S.; Dai, L.Y. Expression of leptin and its functional receptor on disc cells: Contribution to cell proliferation. Spine (Phila Pa 1976) 2008, 33, E858–E864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.L.; Zhang, Q. Leptin inhibits apoptosis of nucleus pulposus cells via promoting autophagy. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 786–795. [Google Scholar] [PubMed]
- Yuan, B.; Huang, L.; Yan, M.; Zhang, S.; Zhang, Y.; Jin, B.; Ma, Y.; Luo, Z. Adiponectin downregulates TNF-alpha expression in degenerated intervertebral discs. Spine (Phila Pa 1976) 2018, 43, E381–E389. [Google Scholar] [CrossRef] [PubMed]
- Terashima, Y.; Kakutani, K.; Yurube, T.; Takada, T.; Maeno, K.; Hirata, H.; Miyazaki, S.; Ito, M.; Kakiuchi, Y.; Takeoka, Y.; et al. Expression of adiponectin receptors in human and rat intervertebral disc cells and changes in receptor expression during disc degeneration using a rat tail temporary static compression model. J. Orthop. Surg. Res. 2016, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, H.; Gao, F.; Li, X.; An, Y.; Wang, J.; Jin, A. Resistin promotes intervertebral disc degeneration by upregulation of adamts-5 through p38 MAPK signaling pathway. Spine (Phila Pa 1976) 2016, 41, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, X.; Pan, H.; Yang, H.; Li, X.; Zhang, K.; Wang, H.; Zheng, Z.; Liu, H.; Wang, J. Resistin promotes CCL4 expression through toll-like receptor-4 and activation of the p38-MAPK and NF-κβ signaling pathways: Implications for intervertebral disc degeneration. Osteoarthr. Cartil. 2017, 25, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Balague, F.; Mannion, A.F.; Pellise, F.; Cedraschi, C. Non-specific low back pain. Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef]
- Gore, M.; Sadosky, A.; Stacey, B.R.; Tai, K.S.; Leslie, D. The burden of chronic low back pain: Clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Phila Pa 1976) 2012, 37, E668–E677. [Google Scholar] [CrossRef] [PubMed]
- Maniadakis, N.; Gray, A. The economic burden of back pain in the UK. Pain 2000, 84, 95–103. [Google Scholar] [CrossRef]
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef]
- De Schepper, E.I.; Damen, J.; van Meurs, J.B.; Ginai, A.Z.; Popham, M.; Hofman, A.; Koes, B.W.; Bierma-Zeinstra, S.M. The association between lumbar disc degeneration and low back pain: The influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976) 2010, 35, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Shao, Z.W.; Xiong, L.M. Cell death in intervertebral disc degeneration. Apoptosis 2013, 18, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Ahima, R.S. Leptin signaling. F1000Prime Rep. 2014, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeck, G. Intracellular signalling pathways activated by leptin. Biochem. J. 2006, 393, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Poonpet, T.; Honsawek, S. Adipokines: Biomarkers for osteoarthritis? World J. Orthop. 2014, 5, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Abella, V.; Scotece, M.; Conde, J.; Lopez, V.; Lazzaro, V.; Pino, J.; Gomez-Reino, J.J.; Gualillo, O. Adipokines, metabolic syndrome and rheumatic diseases. J. Immunol. Res. 2014, 2014, 343746. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Scotece, M.; Lopez, V.; Gomez, R.; Lago, F.; Pino, J.; Gomez-Reino, J.J.; Gualillo, O. Adipokines: Novel players in rheumatic diseases. Discov. Med. 2013, 15, 73–83. [Google Scholar] [PubMed]
- Hu, P.F.; Bao, J.P.; Wu, L.D. The emerging role of adipokines in osteoarthritis: A narrative review. Mol. Biol. Rep. 2011, 38, 873–878. [Google Scholar] [CrossRef] [PubMed]

| Authors | Year | Study Design | Key Findings | Species | Sample |
|---|---|---|---|---|---|
| Ding et al. [28] | 2013 | MAPK pathway analyzed using PCR, Western blot and IHC. | p38 and ERK1/2 signalling plays a distinct role in leptin-induced AF cells terminal differentiation. | Rat | AF |
| Gruber et al. [29] | 2007 | Disc tissue from n = 7 young (normal); n = 29 adults (degenerative) examined. | Leptin and its receptor are present in human annulus cells. | Human | AF |
| Khabour et al. [30] | 2014 | n = 168 LDD patients and n = 122 healthy controls were genotyped for rs266729 and rs2241766 SNPs and measured for plasma levels of adiponectin. | Adiponectin was elevated in patients with LDD. However, SNPs in the ADIPOQ gene were not associated with LDD. | Human | Plasma |
| Koerner et al. [31] | 2014 | Degenerative (n = 7) and normal disc tissue (n = 2) was separated into anterior and posterior AF. Expression levels of 42 cytokines were determined and compared between anterior vs. posterior AF. | The posterior AF expressed increased levels of IL-4, IL-5, IL-6, M-CSF, TNF-β, EGF, IGF 1, angiogenin and leptin compared with the anterior AF in patients with degenerative discs. | Human | AF |
| Li et al. [32] | 2014 | NP cells isolated from n = 7 patients, treated with leptin. RhoA signalling in NP cells was determined. Protein expression of LIMK1 and cofilin-2 was analyzed. F-actin cytoskeletal reorganization was assessed. | Leptin activated the RhoA/ROCK/LIMK/cofilin-2 cascade to induce cytoskeleton reorganization in NP cells. | Human | NP |
| Li et al. [33] | 2012 | Effects of leptin on the proliferation of primary cultured human NP cells (n = 8) and the underlying mechanism. | Leptin induced human NP cell proliferation and cyclin D1 expression via activation of JAK/STAT3, PI3K/Akt or MEK/ERK signaling. | Human | NP |
| Li et al. [34] | 2013 | Do NP tissues and cells express leptin receptors (OBRa and OBRb) and whether leptin affects the organization and expression of major cytoskeletal elements in NP cells (n = 45). | mRNA and proteins of OBRa and OBRb were expressed in all NP tissues and cells, and OBRb expression was correlated with body weight. Increased expression of beta-actin, vimentin and reorganization of F-actin were evident in leptin-stimulated NP cells. | Human | NP |
| Li et al. [35] | 2014 | Effects of leptin on the expression of aggrecan and ADAMTSs in primary human NP cells (n = 4). | Leptin induced p38 to upregulate ADAMTSs and thereby promoting aggrecan degradation in human NP cells. | Human | NP |
| Li et al. [42] | 2017 | Resistin and CCL4 expression measured in degenerated human NP tissue. TLR-4, p38-MAPK, and NF-kappaβ signaling pathways studied. | Expression of resistin and CCL4 was elevated in degenerated NP tissue. Resistin via TLR4 receptor increased the expression of CCL4 through p38-MAPK and NF-kappaβ signaling pathways. | Human | NP |
| Liu et al. [41] | 2016 | Transcriptional activity, gene expression, and protein levels of ADAMTS-5 were measured in resistin-exposed NP cells along with detection of activation of p38 MAPK. | p38-MAPK signaling pathway was activated after exposure to resistin. p38 inhibitor decreased the upregulation of ADAMTS-5 by resistin. | Rat | NP |
| Miao et al. [36] | 2015 | Effects of leptin on the expression of degeneration-associated genes in n = 30 rat NP cells, and its possible mechanism. | Leptin promoted catabolic metabolism in the rat NP cells via the MAPK and JAK2/STAT3 pathways. | Rat | NP |
| Terashima et al. [40] | 2016 | Adiponectin and adiponectin receptors AdipoR1 and AdipoR2 were detected in disc tissue (n = 4, Humans) and (n = 21, Rats). IL-1beta and/or adiponectin-treated rat NP and AF tissues were evaluated for mRNA expression of TNF-alpha and IL-6. | AdipoR1 and AdipoR2 were widely expressed in both human and rat IVD tissues, were inversely related to disease severity. TNF-alpha expression in the IL-1beta + Ad group was significantly lower than that in the IL-1beta group in both NP and AF cells. | Human, Rat | NP, AF |
| Yuan et al. [39] | 2018 | Examined the expression levels of and effect of adiponectin on TNF-alpha in IVD tissues and isolated NP cells. | Adiponectin levels were downregulated, while AdipoR1 and AdipoR2 expression was upregulated in degenerated IVD tissues and NP cells compared to healthy controls. TNF-alpha production by degenerated NP cells was downregulated by adiponectin administration. | Human | NP |
| Zhang et al. [38] | 2018 | Human degenerative NP cells were extracted and cultured, then treated with leptin, leptin inhibitor and leptin neutralizing antibody and expressions of LC3 II/I, Beclin-1 were studied and change of apoptosis rate was detected. Leptin/bafilomycin A and (PI3K)/(MEK) inhibitor-treated cells were used to detect the expressions of LC3II/I, cleaved caspase 3, apoptosis rate, Akt and Erk1/2 signal pathway. | Leptin-treated cells showed increased expressions of LC3II/I and Beclin-1 and decreased apoptosis rate. Leptin inhibitor or neutralizing antibody showed the opposite results. Bafilomycin A increased the expression of LC3II/I and apoptosis rate. Inhibition of Akt phosphorylation was partially offset by leptin while inhibition of Erk1/2 phosphorylation was not. | Human | NP |
| Zhao et al. [37] | 2008 | Determined the expression of leptin and its functional receptor in human herniated disc tissues (n = 45), and to elucidate whether leptin can stimulate rat NP cells to proliferate in vitro. | Disc cells express leptin and its functional receptor. Leptin stimulated proliferation of disc cells in vitro. | Human, Rat | NP |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A. The Role of Adipokines in Intervertebral Disc Degeneration. Med. Sci. 2018, 6, 34. https://doi.org/10.3390/medsci6020034
Sharma A. The Role of Adipokines in Intervertebral Disc Degeneration. Medical Sciences. 2018; 6(2):34. https://doi.org/10.3390/medsci6020034
Chicago/Turabian StyleSharma, Anirudh. 2018. "The Role of Adipokines in Intervertebral Disc Degeneration" Medical Sciences 6, no. 2: 34. https://doi.org/10.3390/medsci6020034
APA StyleSharma, A. (2018). The Role of Adipokines in Intervertebral Disc Degeneration. Medical Sciences, 6(2), 34. https://doi.org/10.3390/medsci6020034




