Cellular and Animal Model Studies on the Growth Inhibitory Effects of Polyamine Analogues on Breast Cancer
Abstract
1. Introduction
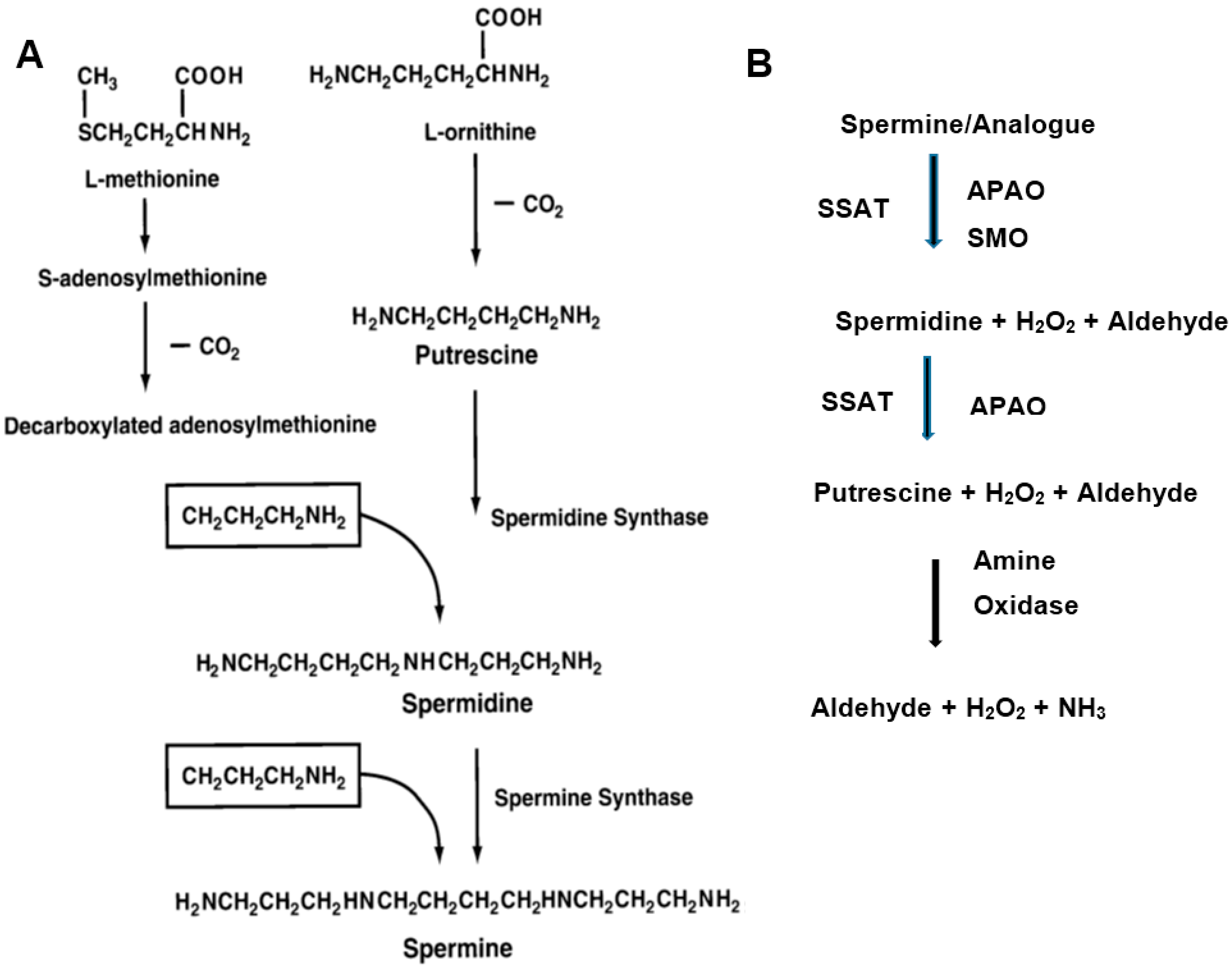
2. Polyamine Metabolism and Breast Cancer
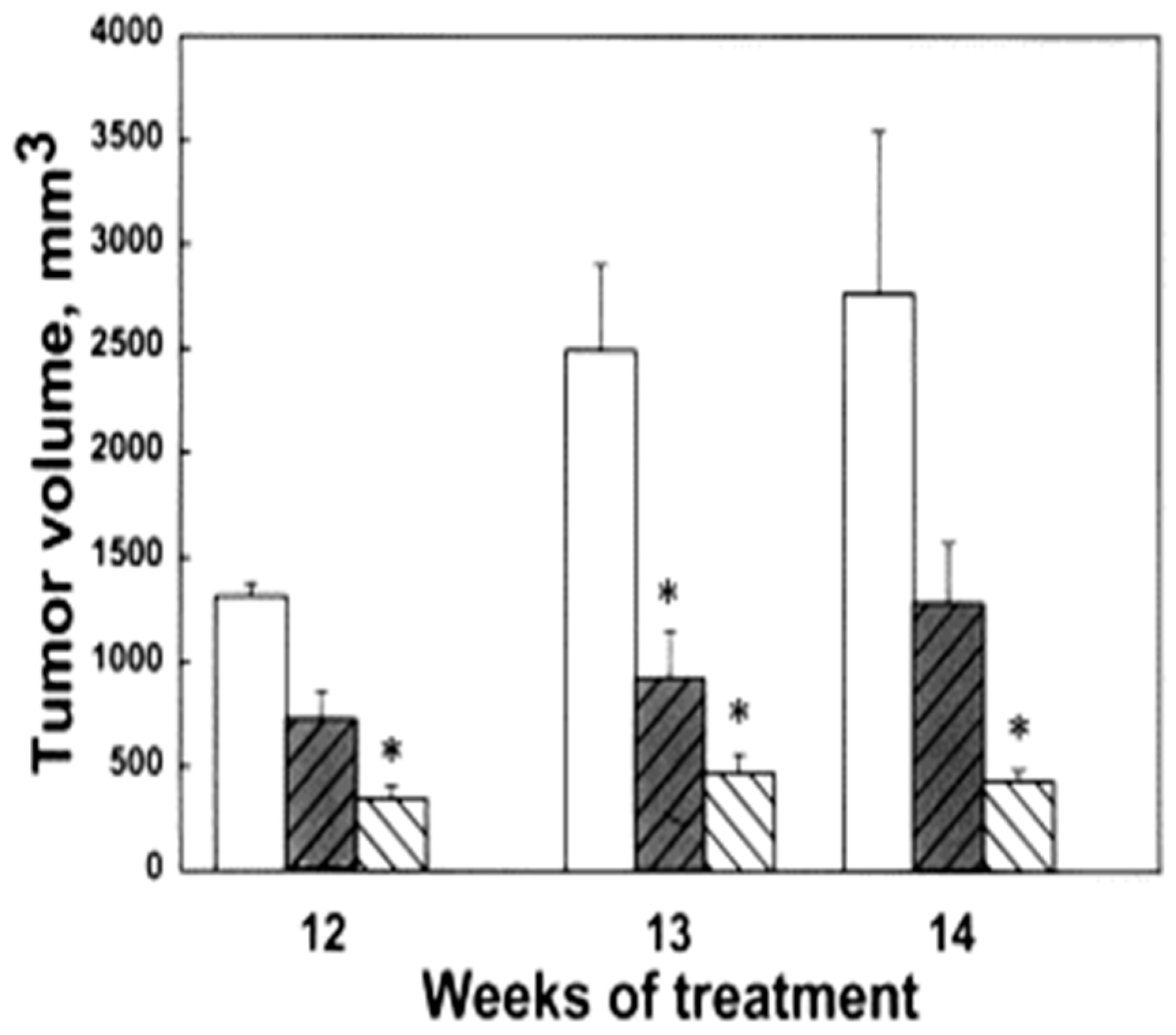
3. Polyamine Analogues and Breast Cancer Therapeutics
4. Conclusions
Author Contributions
Conflicts of Interest
References
- World Cancer Research Fund International, Breast Cancer Statistics. 2017. Available online: http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/breast-cancer-statistics (accessed on 15 January 2018).
- American Cancer Society. Cancer Facts and Figures 2017; American Cancer Society: Atlanta, GA, USA, 2017. [Google Scholar]
- Polyak, K. Heterogeneity in breast cancer. J. Clin. Investig. 2011, 121, 3786–3788. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Tarulli, G.; Portman, N.; Hickey, T.E.; Tilley, W.D.; Palmieri, C. Pushing estrogen receptor around in breast cancer. Endocr. Relat. Cancer 2016, 23, T227–T241. [Google Scholar] [CrossRef] [PubMed]
- Nagini, S. Breast cancer: Current molecular therapeutic targets and new players. Anticancer Agents Med. Chem. 2017, 17, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Gallo, M.A.; Thomas, T.J. Estrogen receptors as targets for drug development for breast cancer, osteoporosis and cardiovascular diseases. Curr. Cancer Drug Targets 2004, 4, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.T.; Jordan, V.C. The biological role of estrogen receptors alpha and beta in cancer. Crit. Rev. Oncol. Hematol. 2004, 50, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Maximov, P.Y.; Lee, T.M.; Jordan, V.C. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr. Clin. Pharmacol. 2013, 8, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, D.; Kumari, K.M.; Avin, S.; Babu, V.A.M. The evolutionary tale and future directions of aromatase inhibitors in breast carcinoma. Anticancer Agents Med. Chem. 2017, 17, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Coleman, R.E.; Cortés, J.; Janni, W. Advances in the management of HER2-positive early breast cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; He, G.; Yan, S.; Chen, C.; Song, L.; Rosol, T.J.; Deng, X. Triple-negative breast cancer: Is there a treatment on the horizon? Oncotarget 2017, 8, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.R.; Massarweh, S.A. Endocrine therapy and strategies to overcome therapeutic resistance in breast cancer. Curr. Probl. Cancer 2016, 40, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Balabhadrapathruni, S.; Gallo, M.A.; Thomas, T.J. Development of polyamine analogs as cancer therapeutic agents. Oncol. Res. 2002, 13, 123–135. [Google Scholar] [PubMed]
- Cervelli, M.; Pietropaoli, S.; Signore, F.; Amendola, R.; Mariottini, P. Polyamines metabolism and breast cancer: State of the art and perspectives. Breast Cancer Res. Treat. 2014, 148, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Vijayanathan, V.; Agostinelli, E.; Thomas, T.; Thomas, T.J. Innovative approaches to the use of polyamines for DNA nanoparticle preparation for gene therapy. Amino Acids 2014, 46, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.E.; Hahm, H.A.; McCloskey, D.E.; Woster, P.M.; Casero, R.A., Jr. Clinical aspects of cell death in breast cancer: The polyamine pathway as a new target for treatment. Endocr. Relat. Cancer 1999, 6, 69–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murray-Stewart, T.R.; Woster, P.M.; Casero, R.A., Jr. Targeting polyamine metabolism for cancer therapy and prevention. Biochem. J. 2016, 473, 2937–2953. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Thomas, T.J. Polyamines in cell growth and cell death: Molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. 2001, 58, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining mysteries of molecular biology: The role of polyamines in the cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Thomas, T.J. Polyamine metabolism and cancer. J. Cell. Mol. Med. 2003, 7, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Nowotarski, S.L.; Woster, P.M.; Casero, R.A., Jr. Polyamines and cancer: Implications for chemotherapy and chemoprevention. Expert Rev. Mol. Med. 2013, 15, e3. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Tajmir-Riahi, H.A.; Thomas, T. Polyamine-DNA interactions and development of gene delivery vehicles. Amino Acids 2016, 48, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Thomas, T. Collapse of DNA in packaging and cellular transport. Int. J. Biol. Macromol. 2018, 109, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Chanphai, P.; Thomas, T.J.; Tajmir-Riahi, H.A. Conjugation of biogenic and synthetic polyamines with serum proteins: A comprehensive review. Int. J. Biol. Macromol. 2016, 92, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Bignon, E.; Chan, C.H.; Morell, C.; Monari, A.; Ravanat, J.L.; Dumont, E. Molecular dynamics insights into polyamine-DNA binding modes: Implications for cross-link selectivity. Chemistry 2017, 23, 12845–12852. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Thomas, T.J.; Lewis, J.S.; Klinge, C.M.; Shirahata, A.; Gelinas, C.; Thomas, T. Regulation of estrogenic and nuclear factor κB functions by polyamines and their role in polyamine analog-induced apoptosis of breast cancer cells. Oncogene 2001, 20, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Thomas, T.; Shirahata, A.; Sigal, L.H.; Thomas, T.J. Activation of nuclear factor κB by polyamines in breast cancer cells. Biochemistry 1999, 38, 14763–14774. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Jin, L.; Casero, R.A.; Davidson, N.E.; Huang, Y. Role of ornithine decarboxylase in regulation of estrogen receptor alpha expression and growth in human breast cancer cells. Breast Cancer Res. Treat. 2012, 136, 57–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vijayanathan, V.; Thomas, T.J.; Nair, S.K.; Shirahata, A.; Gallo, M.A.; Thomas, T. Bending of the estrogen response element by polyamines and estrogen receptors α and β: A fluorescence resonance energy transfer study. Int. J. Biochem. Cell Biol. 2006, 38, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Pegg, A.E. Polyamine catabolism and disease. Biochem. J. 2009, 421, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, E.; Vianello, F.; Magliulo, G.; Thomas, T.; Thomas, T.J. Nanoparticle strategies for cancer therapeutics: Nucleic acids, polyamines, bovine serum amine oxidase and iron oxide nanoparticles. Int. J. Oncol. 2015, 46, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Chaturvedi, R.; Piazuelo, M.J.; Coburn, L.A.; Williams, C.S.; Delgado, A.G.; Casero, R.A.; Pegg, A.G.; Schwartz, D.A.; Wilson, K.T. Increased expression and cellular localization of spermine oxidase in ulcerative colitis and relationship to disease activity. Inflamm. Bowel Dis. 2010, 16, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, E.; Arancia, G.; Vedova, L.D.; Belli, F.; Marra, M.; Salvi, M.; Toninello, A. The biological functions of polyamine oxidation products by amine oxidases: Perspectives of clinical applications. Amino Acids 2004, 27, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.J.; Wallace, H.M. The polyamine transport system as a target for anticancer drug development. Amino Acids 2010, 38, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Kahana, C. Antizyme and antizyme inhibitor, a regulatory tango. Cell. Mol. Life Sci. 2009, 66, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.; Casero, R.A. Regulation of polyamine metabolism by curcumin for cancer prevention and therapy. Med. Sci. (Basel) 2017, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Thomas, T.; John, S.; Hsu, H.C.; Yang, P.; Keinänen, T.A.; Hyvönen, M.T. Tamoxifen metabolite endoxifen interferes with the polyamine pathway in breast cancer. Amino Acids 2016, 48, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Thomas, T.J. Estradiol control of ornithine decarboxylase mRNA, enzyme activity, and polyamine levels in MCF-7 breast cancer cells: Therapeutic implications. Breast Cancer Res. Treat. 1994, 29, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Thomas, T.J. Regulation of cyclin B1 by estradiol and polyamines in MCF-7 breast cancer cells. Cancer Res. 1994, 54, 1077–1084. [Google Scholar] [PubMed]
- Manni, A.; Badger, B.; Glikman, P.; Bartholomew, M.; Santner, S.; Demers, L. Individual and combined effects of α-difluoromethylornithine and ovariectomy on the growth and polyamine milieu of experimental breast cancer in rats. Cancer Res. 1989, 49, 3529–3534. [Google Scholar] [PubMed]
- Alexiou, G.A.; Lianos, G.D.; Ragos, V.; Galani, V.; Kyritsis, A.P. Difluoromethylornithine in cancer: New advances. Future Oncol. 2017, 13, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Faaland, C.A.; Adhikarakunnathu, S.; Thomas, T.J. Structure-activity relations of S-adenosylmethionine decarboxylase inhibitors on the growth of MCF-7 breast cancer cells. Breast Cancer Res. Treat. 1996, 39, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Bellavia, G.; Fratini, E.; Amendola, R.; Polticelli, F.; Barba, M.; Federico, R.; Signore, F.; Gucciardo, G.; Grillo, R.; et al. Spermine oxidase (SMO) activity in breast tumor tissues and biochemical analysis of the anticancer spermine analogues BENSpm and CPENSpm. BMC Cancer 2010, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.M. The physiological role of the polyamines. Eur. J. Clin. Investig. 2000, 30, 1–3. [Google Scholar] [CrossRef]
- Hu, X.; Washington, S.; Verderame, M.F.; Demers, L.M.; Mauger, D.; Manni, A. Biological activity of the S-adenosylmethionine decarboxylase inhibitor SAM486A in human breast cancer cells in vitro and in vivo. Int. J. Oncol. 2004, 25, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Richert, M.M.; Phadke, P.A.; Matters, G.; DiGirolamo, D.J.; Washington, S.; Demers, L.M.; Bond, J.S.; Manni, A.; Welch, D.R. Metastasis of hormone-independent breast cancer to lung and bone is decreased by α-difluoromethylornithine treatment. Breast Cancer Res. 2005, 7, R819. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J.; Kimler, B.F.; Brady, D.A.; Mayo, M.S.; Chang, C.H.; Ferraro, J.A.; Zalles, C.M.; Stanton, A.L.; Masood, S.; Grizzle, W.E.; et al. A phase II breast cancer chemoprevention trial of oral α-difluoromethylornithine: Breast tissue, imaging, and serum and urine biomarkers. Clin. Cancer Res. 2002, 8, 3105–3117. [Google Scholar] [PubMed]
- Lao, C.D.; Backoff, P.; Shotland, L.I.; McCarty, D.; Eaton, T.; Ondrey, F.G.; Viner, J.L.; Spechler, S.J.; Hawk, E.T.; Brenner, D.E. Irreversible ototoxicity associated with difluoromethylornithine. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 1250–1252. [Google Scholar] [PubMed]
- Porter, C.W.; Bergeron, R.J. Spermidine requirement for cell proliferation in eukaryotic cells: Structural specificity and quantitation. Science 1983, 219, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Israel, M.; Zol, E.C.; Muhammad, N.; Modest, E.J. Synthesis and antitumor evaluation of the presumed cytotoxic metabolites of spermine and N,N′-bis(3-aminopropyl)nonane-1,9-diamine. J. Med. Chem. 1973, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, T.A.; Hyvönen, M.T.; Alhonen, L.; Vepsäläinen, J.; Khomutov, A.R. Selective regulation of polyamine metabolism with methylated polyamine analogues. Amino Acids 2014, 46, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.B.; Thomas, T.J.; Thomas, T.; Singh, M.; Mann, R.A. Differential effects of polyamine homologues on the prevention of DL-α-difluoromethylornithine-mediated inhibition of malignant cell growth and normal immune response. Cancer Res. 1992, 52, 1840–1847. [Google Scholar] [PubMed]
- Wang, Y.; Murray-Stewart, T.; Devereux, W.; Hacker, A.; Frydman, B.; Woster, P.M.; Casero, R.A., Jr. Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem. Biophys. Res. Commun. 2003, 304, 605–611. [Google Scholar] [CrossRef]
- Sjögren, T.; Wassvik, C.M.; Snijder, A.; Aagaard, A.; Kumanomidou, T.; Barlind, L.; Kaminski, T.P.; Kashima, A.; Yokota, T.; Fjellström, O. The structure of murine N1-acetylspermine oxidase reveals molecular details of vertebrate polyamine catabolism. Biochemistry 2017, 56, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.W.; Ganis, B.; Vinson, T.; Marton, L.J.; Kramer, D.L.; Bergeron, R.J. Comparison and characterization of growth inhibition in L1210 cells by α-difluoromethylornithine, an inhibitor of ornithine decarboxylase, and N1,N8-bis(ethyl)spermidine, an apparent regulator of the enzyme. Cancer Res. 1986, 46, 6279–6285. [Google Scholar] [PubMed]
- Porter, CW.; Bergeron, R.J. Regulation of polyamine biosynthetic activity by spermidine and spermine analogs—A novel antiproliferative strategy. Adv. Exp. Med. Biol. 1988, 250, 677–690. [Google Scholar] [PubMed]
- Bernacki, R.J.; Bergeron, R.J.; Porter, C.W. Antitumor activity of N,N′-bis(ethyl)spermine homologues against human MALME-3 melanoma xenografts. Cancer Res. 1992, 52, 2424–2430. [Google Scholar] [PubMed]
- Bergeron, R.J.; Neims, A.H.; McManis, J.S.; Hawthorne, T.R.; Vinson, J.R.; Bortell, R.; Ingeno, M.J. Synthetic polyamine analogues as antineoplastics. J. Med. Chem. 1988, 31, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.V.; Goodwin, A.C.; Hacker-Prietz, A.; Sugar, E.; Woster, P.M.; Casero, R.A., Jr. Knockdown of ornithine decarboxylase antizyme 1 causes loss of uptake regulation leading to increased N1,N11-bis(ethyl)norspermine (BENSpm) accumulation and toxicity in NCI H157 lung cancer cells. Amino Acids 2012, 42, 529–538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.; Pledgie, A.; Casero, R.A., Jr.; Davidson, N.E. Molecular mechanisms of polyamine analogs in cancer cells. Anticancer Drugs 2005, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Seiler, N. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Part 2. Structural analogues and derivatives. Curr. Drug Targets 2003, 4, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.E.; Mank, A.R.; Prestigiacomo, L.J.; Bergeron, R.J.; Casero, R.A., Jr. Growth inhibition of hormone-responsive and -resistant human breast cancer cells in culture by N1,N12-bis(ethyl)spermine. Cancer Res. 1993, 53, 2071–2075. [Google Scholar] [PubMed]
- Faaland, C.A.; Thomas, T.J.; Balabhadrapathruni, S.; Langer, T.; Mian, S.; Shirahata, A.; Gallo, M.A.; Thomas, T. Molecular correlates of the action of bis(ethyl)polyamines in breast cancer cell growth inhibition and apoptosis. Biochem. Cell Biol. 2000, 78, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Manni, A.; Wecher, R.; Verderame, M.F.; Mauger, D. Cooperativity between the polyamine pathway and HER2neu in transformation of human mammary epithelial cells in culture: Role of the MAPK pathway. Int. J. Cancer 1998, 76, 563–570. [Google Scholar] [CrossRef]
- Guy, C.T.; Webster, M.A.; Schaller, M.; Parsons, T.J.; Cardiff, R.D.; Muller, W.J. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. USA 1992, 89, 10578–10582. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Antony, T.; Haddad, S.; Amenta, P.; Shirahata, A.; Thomas, T.J.; Thomas, T. Antitumor effects of bis(ethyl)polyamine analogs on mammary tumor development in FVB/NTgN (MMTVneu) transgenic mice. Cancer Lett. 1999, 146, 15–23. [Google Scholar] [CrossRef]
- Wolff, A.C.; Armstrong, D.K.; Fetting, J.H.; Carducci, M.K.; Riley, C.D.; Bender, J.F.; Casero, R.A., Jr.; Davidson, N.E. A Phase II study of the polyamine analog N1,N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin. Cancer Res. 2003, 9, 5922–5928. [Google Scholar] [PubMed]
- Balabhadrapathruni, S.; Santhakumaran, L.M.; Thomas, T.J.; Shirahata, A.; Gallo, M.A.; Thomas, T. Bis(ethyl)norspermine potentiates the apoptotic activity of the pure antiestrogen ICI 182780 in breast cancer cells. Oncol. Rep. 2005, 13, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Holst, C.M.; Staaf, J.; Jönsson, G.; Hegardt, C.; Oredsson, S.M. Molecular mechanisms underlying N1,N11-diethylnorspermine-induced apoptosis in a human breast cancer cell line. Anticancer Drugs 2008, 19, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Vijayanathan, V.; Venkiteswaran, S.; Nair, S.K.; Verma, A.; Thomas, T.J.; Zhu, B.T.; Thomas, T. Physiologic levels of 2-methoxyestradiol interfere with nongenomic signaling of 17β-estradiol in human breast cancer cells. Clin. Cancer Res. 2006, 12, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Pledgie-Tracy, A.; Billam, M.; Hacker, A.; Sobolewski, M.D.; Woster, P.M.; Zhang, Z.; Casero, R.A.; Davidson, N.E. The role of the polyamine catabolic enzymes SSAT and SMO in the synergistic effects of standard chemotherapeutic agents with a polyamine analogue in human breast cancer cell lines. Cancer Chemother. Pharmacol. 2010, 65, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, M.A.; Bakhshinejad, B.; Sadeghizadeh, M.; Babashah, S. Combination treatment with dendrosomal nanocurcumin and doxorubicin improves anticancer effects on breast cancer cells through modulating CXCR4/NF-κB/Smo regulatory network. Mol. Biol. Rep. 2017, 44, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Bunjobpol, W.; Dulloo, I.; Igarashi, K.; Concin, N.; Matsuo, K.; Sabapathy, K. Suppression of acetylpolyamine oxidase by selected AP-1 members regulates DNp73 abundance: Mechanistic insights for overcoming DNp73-mediated resistance to chemotherapeutic drugs. Cell Death Differ. 2014, 21, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Obakan, P.; Arısan, E.D.; Ozfiliz, P.; Çoker-Gurkan, A. Palavan-Unsal, N. Purvalanol A is a strong apoptotic inducer via activating polyamine catabolic pathway in MCF-7 estrogen receptor positive breast cancer cells. Mol. Biol. Rep. 2014, 41, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Pledgie, A.; Huang, Y.; Hacker, A.; Zhang, Z.; Woster, P.M.; Davidson, N.E.; Casero, R.A., Jr. Spermine oxidase SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J. Biol. Chem. 2005, 280, 39843–39851. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.K.; Verma, A.; Thomas, T.J.; Chou, T.C.; Gallo, M.A.; Shirahata, A.; Thomas, T. Synergistic apoptosis of MCF-7 breast cancer cells by 2-methoxyestradiol and bis(ethyl)norspermine. Cancer Lett. 2007, 250, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.; Fiuza, SM.; Marques, M.P.; Persson, L.; Oredsson, S. Increased breast cancer cell toxicity by palladination of the polyamine analogue N1N11-bis(ethyl)norspermine. Amino Acids 2014, 46, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Shah, N.; Faaland, C.A.; Gallo, M.A.; Yurkow, E.; Satyaswaroop, P.G.; Thomas, T. Effects of a bis(benzyl)spermine analog on MCF-7 breast cancer cells in culture and nude mice xenografts. Oncol. Rep. 1977, 4, 5–13. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Woster, P.M. Recent advances in the development of polyamine analogues as antitumor agents. J. Med. Chem. 2009, 52, 4551–4573. [Google Scholar] [CrossRef] [PubMed]
- Jagu, E.; Pomel, S.; Pethe, S.; Loiseau, P.M.; Labruère, R. Polyamine-based analogs and conjugates as antikinetoplastid agents. Eur. J. Med. Chem. 2017, 139, 982–1015. [Google Scholar] [CrossRef] [PubMed]
- Valasinas, A.; Reddy, V.K.; Blokhin, A.V.; Basu, H.S.; Bhattacharya, S.; Sarkar, A.; Marton, L.J.; Frydman, B. Long-chain polyamines (oligoamines) exhibit strong cytotoxicities against human prostate cancer cells. Bioorg. Med. Chem. 2003, 11, 4121–4131. [Google Scholar] [CrossRef]
- Huang, Y.; Keen, J.C.; Hager, E.; Smith, R.; Hacker, A.; Frydman, B.; Valasinas, A.L.; Reddy, V.K.; Marton, L.J.; Casero, R.A., Jr.; et al. Regulation of polyamine analogue cytotoxicity by c-Jun in human MDA-MB-435 cancer cells. Mol. Cancer Res. 2004, 2, 81–88. [Google Scholar] [PubMed]
- Zhu, Q.; Huang, Y.; Marton, L.J.; Woster, P.M.; Davidson, N.E.; Casero, R.A., Jr. Polyamine analogs modulate gene expression by inhibiting lysine-specific demethylase 1 (LSD1) and altering chromatin structure in human breast cancer cells. Amino Acids 2012, 42, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Pledgie, A.; Rubin, E.; Marton, L.J.; Woster, P.M.; Sukumar, S.; Casero, R.A., Jr.; Davidson, N.E. Role of p53/p21(Waf1/Cip1) in the regulation of polyamine analogue-induced growth inhibition and cell death in human breast cancer cells. Cancer Biol. Ther. 2005, 4, 1006–1013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vujcic, S.; Halmekyto, M.; Diegelman, P.; Gan, G.; Kramer, D.L.; Janne, J.; Porter, C.W. Effects of conditional overexpression of spermidine/spermine N1-acetyltransferase on polyamine pool dynamics, cell growth, and sensitivity to polyamine analogs. J. Biol. Chem. 2000, 275, 38319–38328. [Google Scholar] [CrossRef] [PubMed]
- Akyol, Z.; Çoker-Gürkan, A.; Arisan, E.D.; Obakan-Yerlikaya, P.; Palavan-Ünsal, N. DENSpm overcame Bcl-2 mediated resistance against Paclitaxel treatment in MCF-7 breast cancer cells via activating polyamine catabolic machinery. Biomed. Pharmacother. 2016, 84, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.C.; Woster, P.M.; Yager, J.D.; Casero, R.A., Jr. The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc. Natl. Acad. Sci. USA 1997, 94, 11557–11562. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feith, D.J.; Welsh, P.; Coleman, C.S.; Lopez, C.; Woster, P.M.; O’Brien, T.G.; Pegg, A.E. Studies of the mechanism by which increased spermidine/spermine N1-acetyltransferase activity increases susceptibility to skin carcinogenesis. Carcinogenesis 2007, 28, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.C.; Jadallah, S.; Toubaji, A.; Lecksell, K.; Hicks, J.L.; Kowalski, J.; Bova, G.S.; De Marzo, A.M.; Netto, G.J.; Casero, R.A., Jr. Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate 2008, 68, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Greene, E.; Murray Stewart, T.; Goodwin, A.C.; Baylin, S.B.; Woster, P.M.; Casero, R.A., Jr. Inhibition of lysine-specific demethylase 1 by polyamine analogues results in reexpression of aberrantly silenced genes. Proc. Natl. Acad. Sci. USA 2007, 104, 8023–8028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Casero, R.A., Jr. Mammalian polyamine catabolism: A therapeutic target, a pathological problem, or both? J. Biochem. 2006, 139, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.M.; Thomas, T.; Wada, M.; Sigal, L.H.; Shirahata, A.; Thomas, T.J. Facilitation of the cellular uptake of a triplex-forming oligonucleotide by novel polyamine analogues: Structure-activity relationships. Biochemistry 1999, 38, 13328–13337. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Murray-Stewart, T.; Wang, Y.; Yu, F.; Li, J.; Marton, L.J.; Casero, R.A., Jr.; Oupický, D. Self-immolative nanoparticles for simultaneous delivery of microRNA and targeting of polyamine metabolism in combination cancer therapy. J. Control. Release 2017, 246, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Ucal, S.; Häkkinen, M.R.; Alanne, A.L.; Alhonen, L.; Vepsäläinen, J.; Keinänen, T.A.; Hyvönen, M.T. Controlling of N-Alkylpolyamine Analogue Metabolism by Selective Deuteration. Biochem. J. 2018, 475, 663–676. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, T.J.; Thomas, T. Cellular and Animal Model Studies on the Growth Inhibitory Effects of Polyamine Analogues on Breast Cancer. Med. Sci. 2018, 6, 24. https://doi.org/10.3390/medsci6010024
Thomas TJ, Thomas T. Cellular and Animal Model Studies on the Growth Inhibitory Effects of Polyamine Analogues on Breast Cancer. Medical Sciences. 2018; 6(1):24. https://doi.org/10.3390/medsci6010024
Chicago/Turabian StyleThomas, T. J., and Thresia Thomas. 2018. "Cellular and Animal Model Studies on the Growth Inhibitory Effects of Polyamine Analogues on Breast Cancer" Medical Sciences 6, no. 1: 24. https://doi.org/10.3390/medsci6010024
APA StyleThomas, T. J., & Thomas, T. (2018). Cellular and Animal Model Studies on the Growth Inhibitory Effects of Polyamine Analogues on Breast Cancer. Medical Sciences, 6(1), 24. https://doi.org/10.3390/medsci6010024




