Chemo-Immunotherapy Using Lentinan for the Treatment of Gastric Cancer with Liver Metastases
Abstract
:1. Introduction
2. Patients and Methods
3. Results
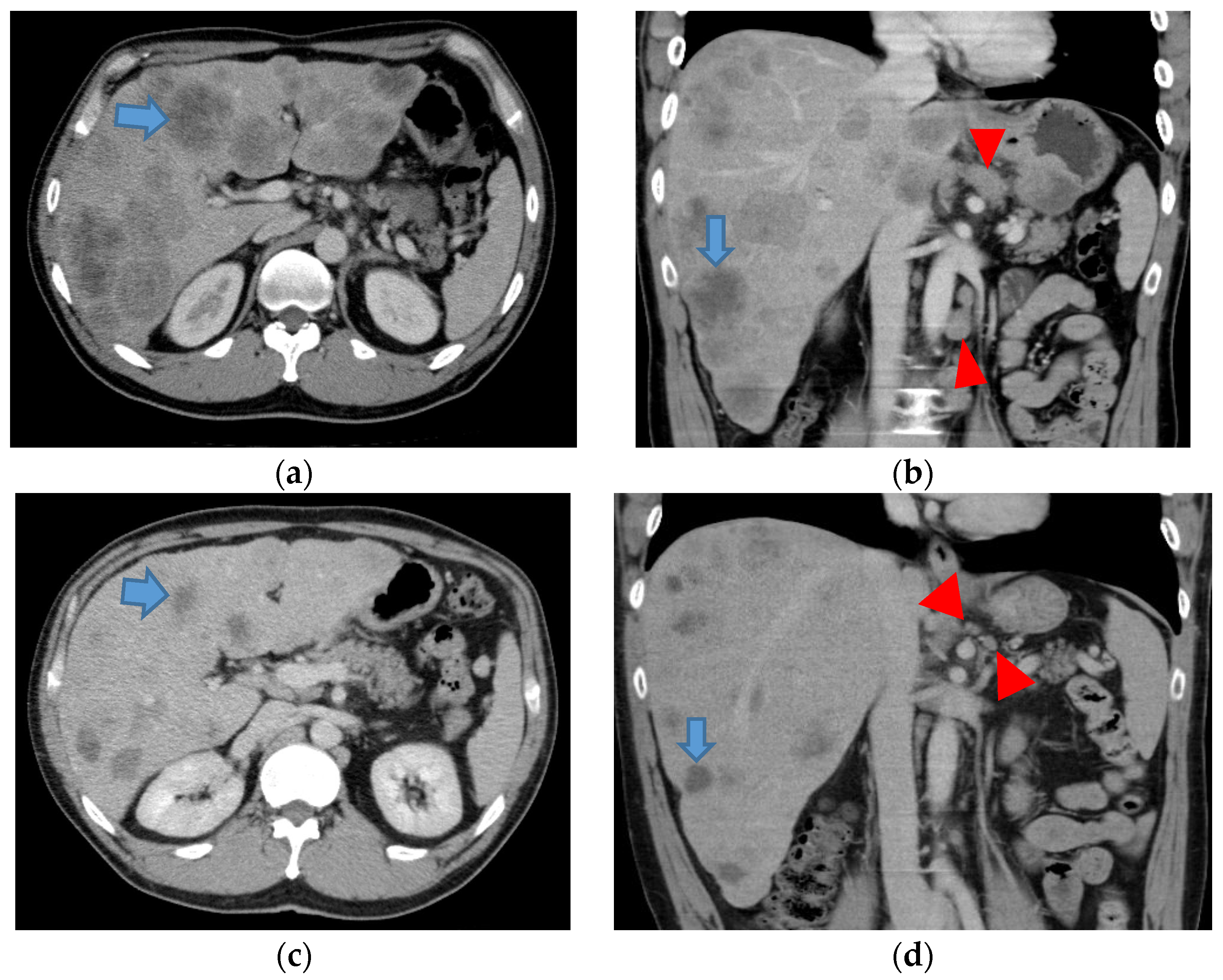
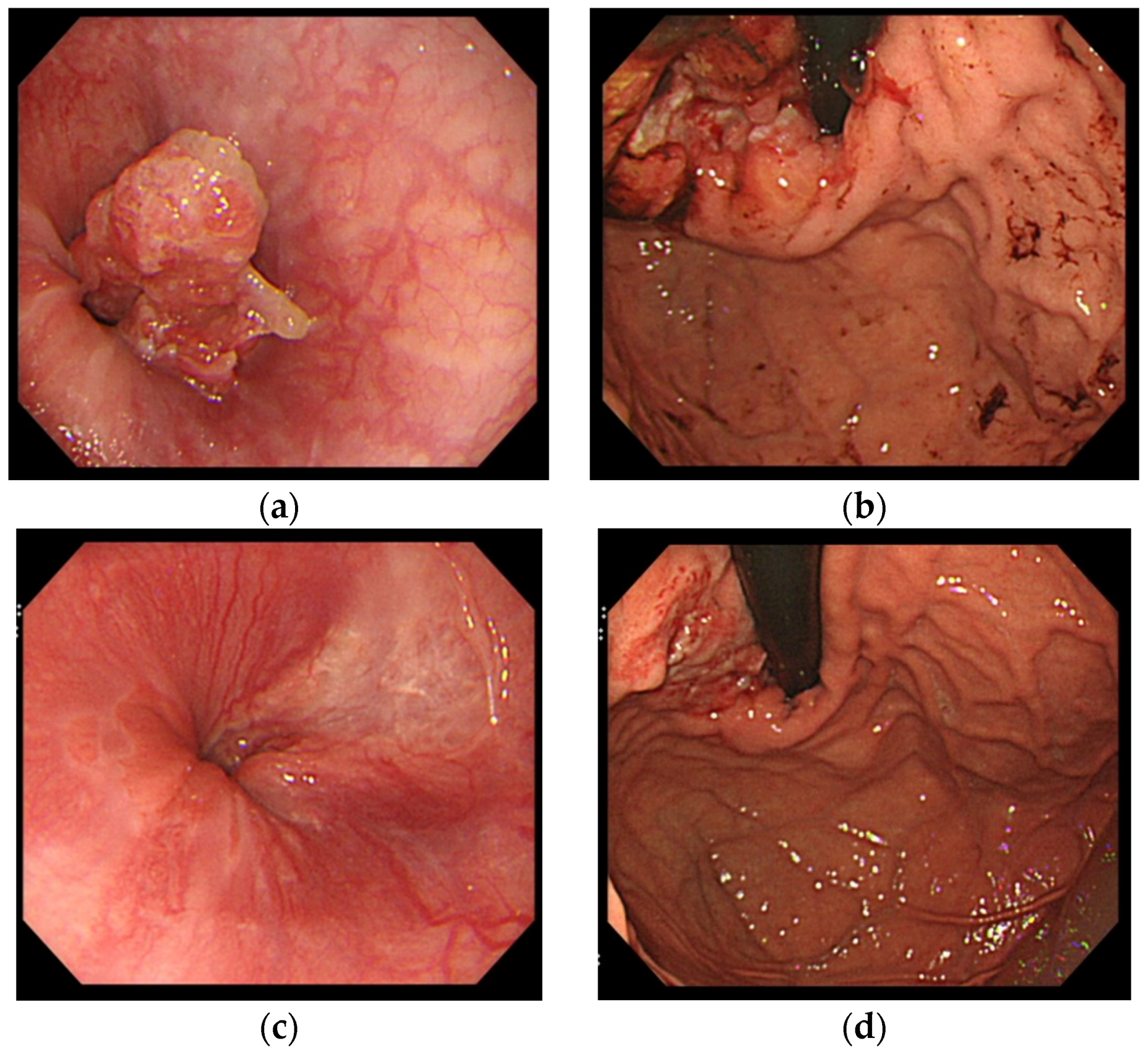
4. Case Report
5. Discussion
6. Conclusions
Author Contributions
Conflicts of Interest
References
- International Agency for Research on Cancer. GLOBOCAN 2012: Cancer Incidence and Mortality Worldwide. Available online: http://globocan.iarc.fr/Default.aspx (accessed on 5 January 2016).
- Wagner, A.D.; Grothe, W.; Haeting, J.; Kleber, G.; Grothey, A.; Fleig, W.E. Chemotherpay in advanced gastric cancer: A systemic review and meta-analysis based on aggregate data. J. Clin. Oncol. 2006, 24, 2903–2909. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, W.; Narahara, H.; Hara, T.; Takagane, A.; Akiya, T.; Takagi, M.; Miyashita, K.; Nishizaki, T.; Kobayashi, O.; Takiyama, W.; et al. S-1 plus cisplatin versus S-1 alone for first line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008, 9, 215–221. [Google Scholar] [CrossRef]
- Yoshida, M.; Ohtsu, A.; Boku, N.; Miyata, Y.; Shirao, K.; Shimada, Y.; Hyodo, I.; Koizumi, W.; Kurihara, M.; Yoshida, S.; et al. Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn. J. Clin. Oncol. 2004, 34, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Hironaka, S.; Sugimoto, N.; Yamaguchi, K.; Moriwaki, T.; Komatsu, Y.; Nishina, T.; Tsuji, A.; Nakajima, T.E.; Gotoh, M.; Machida, N.; et al. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: A randamised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 99–108. [Google Scholar] [CrossRef]
- Oba, K.; Kobayashi, M.; Matsui, T.; Kodera, Y.; Sakamoto, J. Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res. 2009, 29, 2739–2746. [Google Scholar] [PubMed]
- Ina, K.; Furuta, R.; Kataoka, T.; Kayukawa, S.; Yoshida, T.; Miwa, T.; Yamamura, Y.; Takeuchi, Y. Lentinan prolonged the survival of patients with unresectable or recurrent gastric cancer receiving S-1-based chemotherapy. World J. Clin. Oncol. 2011, 10, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Chihara, G.; Hamuro, J.; Maeda, Y.; Arai, Y.; Fukuoka, F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. Cancer Res. 1970, 30, 2776–2781. [Google Scholar] [PubMed]
- Vetvicka, V. Glucan-immunostimulant, adjuvant, potential drug. World J. Clin. Oncol. 2011, 10, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Ina, K.; Kataoka, T.; Ando, T. The use of lentinan for treating gastric cancer. Anti-Cancer Agents Med. Chem. 2013, 13, 681–688. [Google Scholar] [CrossRef]
- Mushiake, H.; Tsunoda, T.; Nukatsuka, M.; Shimao, K.; Fukushima, M.; Tahara, H. Dendritic cells might be one of key factors for eliciting antitumor effect by chemoimmunotherapy in vivo. Cancer Immunol. Immunother. 2005, 54, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Iwase, H.; Shimada, M.; Tsuzuki, T.; Ina, K.; Sugihara, M.; Haruta, J.; Shinoda, M.; Kumada, T.; Goto, H. A phase II multi-study of triple therapy with paclitaxel, S-1, and cisplatin in patients with advanced gastric cancer. Oncology 2011, 80, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Japanese Research Society for Gastric Cancer. Japanese Classification of Gastric Carcinoma; Japanese Research Society for Gastric Cancer: Tokyo, Japan, 1995. [Google Scholar]
- Eisenhauer, E.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, S.; Oyama, T.; Hatanaka, N.; Uchikoshi, F.; Yoshidome, K.; Tori, M.; Ueshima, S.; Nakahara, M.; Nakao, K. Unresectable gastric cancer with multiple liver metastases effectively treated with combined paclitaxel and doxifluridine chemotherapy. Int. J. Clin. Oncol. 2006, 11, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Ina, K.; Kataoka, T.; Takeuchi, Y.; Fukuoka, T.; Miwa, T.; Nishio, T.; Furuta, R.; Masaki, A.; Mori, F.; Kayukawa, S.; et al. Pathological complete response induced by the combination therapy of S-1 and 24-h infusion of cisplatin in two cases initially diagnosed as inoperable advanced gastric cancer. Oncol. Rep. 2008, 20, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Chakrabandhu, B.; Yamada, S.; Kato, S.; Hagiwara, N.; Chakrabandhu, T. Complete response of liver metastatic gastric cancer after FOLFOX-4 chemotherapy regimen followed by salvage gastrectomy: A case report. Int. J. Med. Sci. 2012, 2, 278–283. [Google Scholar]
- Goel, G. Long term complete remission in advanced gastric adenocarcinoma with docetaxel, oxaliplatin and capecitabine combination regimen. World J. Oncol. 2012, 3, 124–126. [Google Scholar] [CrossRef]
- Bang, Y.J.; van Custem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-esophageal junction cancer (ToGA): A phase III, open-label, randomized controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomized, multicenter, placebo-controlled, phase 3 trial. Lancet 2013. [Google Scholar] [CrossRef]
- Arnould, L.; Gelly, M.; Penault-Llorca, F.; Benoit, L.; Bonnetain, F.; Migeon, C.; Cabaret, V.; Fermeaux, V.; Bertheau, P.; Garnier, J.; et al. Trastuzumab-based treatment of HER2-positive breast cancer. An antibody-dependent cellular cytotoxicity mechanism? Br. J. Cancer 2006, 94, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Shimamura, T.; Tagami, T.; Takatsuki, F.; Hamuro, J. The skewing to Th1 induced by lentinan is directed through the distinctive cytokine production by macrophages with elevated intracellular glutathione content. Int. Immunophamacol. 2002, 2, 673–689. [Google Scholar] [CrossRef]
- Cheung, N.K.V.; Modak, S.; Vickers, A.; Knuckles, B. Orally administered β-glucans enhance anti-tumor effects of monoclonal antibodies. Cancer Immunol. Immunother. 2002, 51, 557–564. [Google Scholar] [PubMed]
- Herbst, R.S.; Soria, J.C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhu, Y.; Jiang, J.; Zhao, J.; Zhang, X.G.; Xu, N. Immunohistochemical localization of programmed death-1 ligand 1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006, 108, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Nam, K.H.; Ahn, S.H.; Park, D.J.; Kim, H.H.; Kim, S.H.; Chang, H.; Lee, J.O.; Kim, Y.J.; Lee, H.S.; et al. Prognostic implication of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2014, 26. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Liu, C.; Zhou, Y.; Wang, G. Cisplatin induces programmed death ligand-1 (PD-L1) over-expression in hepatoma H22 cells via ERK/MAPK signaling pathway. Cell Mol. Biol. 2010, 56, OL1366–OL1372. [Google Scholar] [PubMed]
- Tel, J.; Hato, S.V.; Torensma, R.; Buschow, S.I.; Figdor, C.G.; Lesterhuis, W.J.; de Vries, I.J.M. The chemotherapeutic drug oxaliplatin differently affects blood DC function dependent on environmental cues. Cancer Immunol. Immunother. 2012, 61, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Su, D.M.; Liang, M.; Fu, J. Chemopreventive agents induce programmed death-1 ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol. Immunol. 2008, 45, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, S.; Tabata, T.; Hazama, S.; Iizuka, N.; Yamamoto, K.; Hirayama, M.; Tangoku, A.; Oka, M. Immunoregulatory effects of the antitumor polysaccharide lentinan on Th1/Th2 balance in patients with digestive cancer. Anticancer Res. 2000, 20, 4707–4712. [Google Scholar] [PubMed]
| Characteristics | Number of Patients | |
|---|---|---|
| Gender | Male | 8 |
| Female | 4 | |
| Age (years) | Range | 42–82 |
| Median | 67 | |
| Performance status | 0 | 5 |
| 1 | 3 | |
| 2 | 4 | |
| HER2 status | High | 2 |
| Low | 10 | |
| Target lesions | Primary | 9 |
| Liver | 12 | |
| Lung | 2 | |
| Peritoneum | 4 | |
| Lymph nodes | 12 | |
| Original chemotherapy | S-1 alone | 1 |
| S-1/cisplatin | 8 | |
| PSC | 3 | |
| Case | Age | Gender | HER2 | S-1 | Cisplatin | Taxanes | Trastsuzumab | Start | Last | OR | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | Male | Low | + | + | + | − | 07.09.2010 | 31.01.2016 | CR | Alive |
| 2 | 67 | Female | Low | + | + | − | − | 21.05.2015 | 31.01.2016 | PR | Alive |
| 3 | 74 | Female | Low | + | + | + | − | 07.12.2011 | 06.11.2013 | PR | Dead (liver failure) |
| 4 | 71 | Male | High | + | + | + | + | 18.09.2015 | 31.01.2016 | PR | Alive |
| 5 | 42 | Male | High | + | + | + | + | 07.07.2015 | 31.01.2016 | PR | Alive |
| 6 | 58 | Male | Low | + | + | + | − | 01.10.2010 | 29.07.2012 | SD | Dead (obstructive jaundice) |
| 7 | 67 | Male | Low | + | + | − | − | 27.12.2013 | 21.11.2014 | SD | Dead (liver failure) |
| 8 | 52 | Female | Low | + | + | + | − | 27.02.2013 | 22.09.2013 | SD | Dead (meningitis) |
| 9 | 76 | Male | Low | + | + | − | − | 11.09.2014 | 31.12.2015 | SD | Dead (liver failure) |
| 10 | 75 | Male | Low | + | + | + | − | 08.03.2014 | 19.04.2015 | SD | Dead (liver failure) |
| 11 | 82 | Male | Low | + | − | − | − | 02.03.2011 | 13.03.2012 | PD | Dead (liver failure) |
| 12 | 72 | Female | Low | + | + | − | − | 07.12.2011 | 23.02.2012 | PD | Dead (multiple organ failure) |
| Age | Gender | Before | Original Regimen | CR Duration (Months) | Recurrence |
|---|---|---|---|---|---|
| 72 | Male | T4 N3 H3 | Paclitaxel/Doxifluridine | 14 | + |
| 56 | Male | T4 N3 H1 | S-1/Cisplatin | 84 | − |
| 48 | Male | T4 N1 H1 | FOLFOX4 | 43 | − |
| 65 | Female | T4 NX H1 | DOX | 9 | + |
| 65 | Male | T4 N3 H3 | PSC plus lentinan | 33 | − |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ina, K.; Furuta, R.; Kataoka, T.; Kayukawa, S.; Ina, H.; Yoneda, M. Chemo-Immunotherapy Using Lentinan for the Treatment of Gastric Cancer with Liver Metastases. Med. Sci. 2016, 4, 8. https://doi.org/10.3390/medsci4020008
Ina K, Furuta R, Kataoka T, Kayukawa S, Ina H, Yoneda M. Chemo-Immunotherapy Using Lentinan for the Treatment of Gastric Cancer with Liver Metastases. Medical Sciences. 2016; 4(2):8. https://doi.org/10.3390/medsci4020008
Chicago/Turabian StyleIna, Kenji, Ryuichi Furuta, Takae Kataoka, Satoshi Kayukawa, Hiroko Ina, and Masahiko Yoneda. 2016. "Chemo-Immunotherapy Using Lentinan for the Treatment of Gastric Cancer with Liver Metastases" Medical Sciences 4, no. 2: 8. https://doi.org/10.3390/medsci4020008
APA StyleIna, K., Furuta, R., Kataoka, T., Kayukawa, S., Ina, H., & Yoneda, M. (2016). Chemo-Immunotherapy Using Lentinan for the Treatment of Gastric Cancer with Liver Metastases. Medical Sciences, 4(2), 8. https://doi.org/10.3390/medsci4020008





