Insights into Putative Health Implications of Gelam (Melaleuca cajuputi) Honey: Evidence from In-Vivo and In-Vitro Studies
Abstract
:1. Introduction
2. Physicochemical Properties of GH
3. Chemical Composition of GH
4. Antioxidant Activities of GH
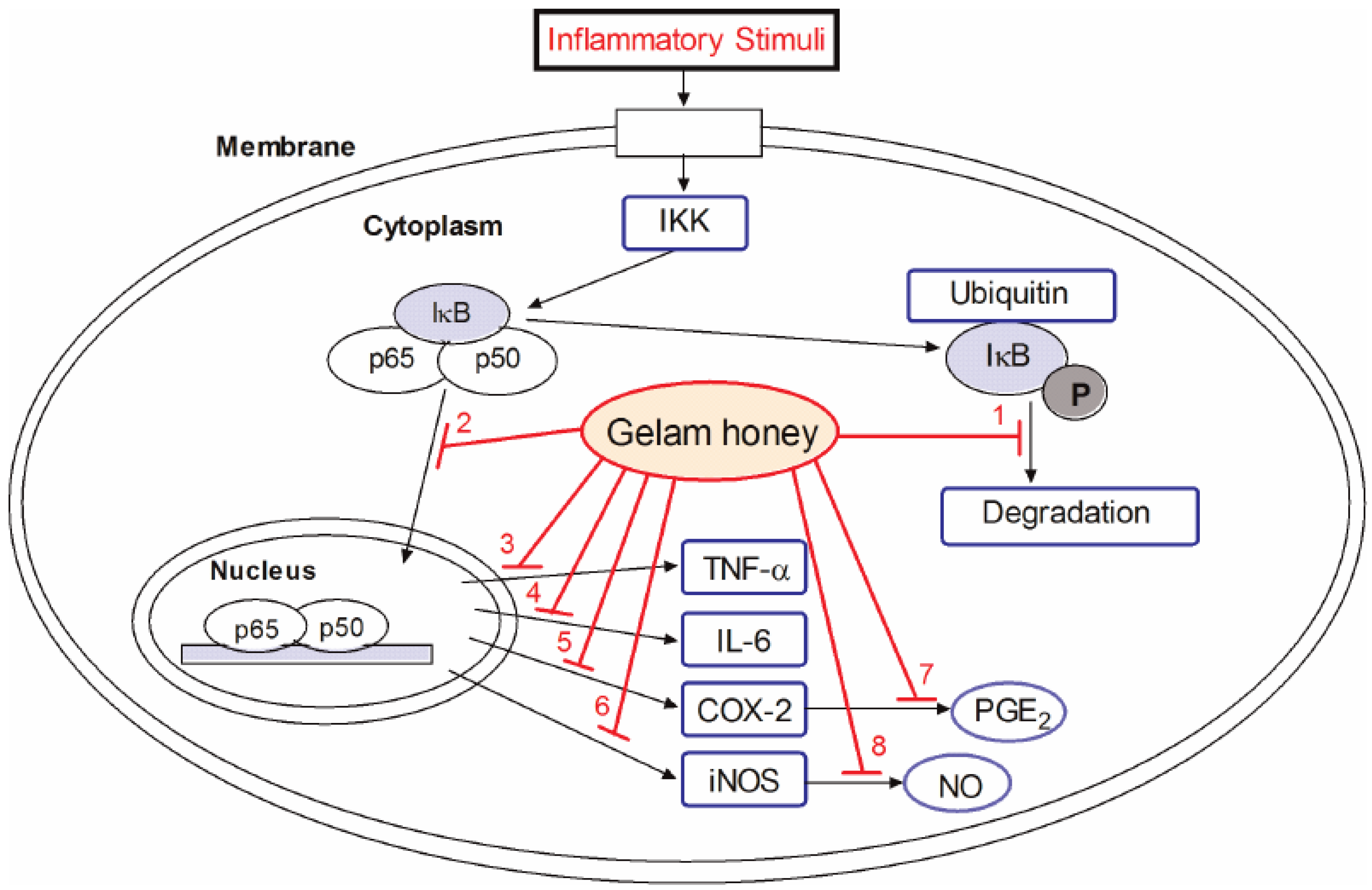
5. Anti-Inflammatory Activities of GH
6. Antimicrobial Properties of GH
7. Anti-Proliferative and Chemo-Preventive Properties of GH
8. Wound Healing Properties of GH
9. Other Health Benefits of GH
10. Conclusions
Acknowledgements
Conflicts of Interest
References
- Othman, N.H. Honey and cancer: Sustainable inverse relationship particularly for developing nations—A review. Evid. Based Complement. Altern. Med. 2012, 2012, 410406. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Singh, O.; Bhagel, P.S.; Moses, S.; Shukla, S.; Mathur, R.K. Honey dressing versus silver sulfadiazene dressing for wound healing in burn patients: A retrospective study. J. Cutan. Aesthet. Surg. 2011, 4, 183. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S. Honey: A Novel Antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Sirajudeen, K.N.; Salleh, M.S.; Gurtu, S. Antioxidant protection of Malaysian tualang honey in pancreas of normal and streptozotocin-induced diabetic rats. Ann. Endocrinol. 2010, 71, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Gurtu, S.; Sulaiman, S.A.; Wahab, M.S.A.; Sirajudeen, K.N.S.; Salleh, M.S.M. Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int. J. Vitam. Nutr. Res. 2010, 80, 74. [Google Scholar] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A.; Sirajudeen, K.N.S.; Salleh, M.S.M.; Gurtu, S. Antioxidant protective effect of glibenclamide and metformin in combination with honey in pancreas of streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2010, 11, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2010, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Swellam, T.; Miyanaga, N.; Onozawa, M.; Hattori, K.; Kawai, K.; Shimazui, T.; Akaza, H. Antineoplastic activity of honey in an experimental bladder cancer implantation model: In vivo and in vitro studies. Int. J. Urol. 2003, 10, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ghashm, A.A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complemet. Altern. Med. 2010, 10, 49. [Google Scholar]
- Inoue, K.; Murayama, S.; Seshimo, F.; Takeba, K.; Yoshimura, Y.; Nakazawa, H. Identification of phenolic compound in manuka honey as specific superoxide anion radical scavenger using electron spin resonance (ESR) and liquid chromatography with coulometric array detection. J. Sci. Food. Agric. 2005, 85, 872–878. [Google Scholar] [CrossRef]
- Hamid, K.A.; Mohd, A.F.; MohdZohdi, R.; Eshak, Z.; Omar, R. Pollen analysis of selected Malaysian honey. Acad. J. Entomol. 2015, 8, 99–103. [Google Scholar]
- Moniruzzaman, M.; Sulaiman, S.A.; Khalil, M.I.; Gan, S.H. Evaluation of physicochemical and antioxidant properties of sourwood and other Malaysian honeys: A comparison with manuka honey. Chem. Cent. J. 2013, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Mahaneem, M.; Jamalullail, S.M.S.; Alam, N.; Sulaiman, S.A. Evaluation of radical scavenging activity and colour intensity of nine Malaysian honeys of different origin. JAAS 2011, 3, 4–11. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. High 5-hydroxymethylfurfural concentrations are found in Malaysian honey samples stored for more than one year. Food Chem. Toxicol. 2010, 48, 2388–2392. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.Z.; Yusoff, K.M.; Makpol, S.; Mohd, Y.Y. Does gamma irradiation affect physicochemical properties of honey? Clin. Ther. 2014, 165, e125. [Google Scholar]
- Bogdanov, S. Book of honey: Honey composition. Bee Prod. Sci. 2009, 1–9. Available online: http://fantasticflavour.com/yahoo_site_admin/assets/docs/CompositionHoney.20105942.pdf (accessed on 15 December 2015). [Google Scholar]
- Alimentarius Commission. Revised codex standard for honey. Codex Stan 2001, 12, 1982. [Google Scholar]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Chowdhury, M.A.Z.; Rahman, M.A.; Sulaiman, S.A.; Gan, S.H. Determination of Mineral, Trace Element, and Pesticide Levels in Honey Samples Originating from Different Regions of Malaysia Compared to Manuka Honey. Bio Med. Res. Int. 2014, 2014, 359890. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Eddie Tan, T.T. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S.; Adnan, N.A.; Abdul-Rahaman, N.L.; Sarmidi, M.R. Effect of thermal treatment on the biochemical composition of tropical honey samples. Int. Food Res. J. 2014, 21, 773–778. [Google Scholar]
- Hussein, S.Z.; Yusoff, K.M.; Makpol, S.; Yusof, Y.A.M. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules 2011, 16, 6378–6395. [Google Scholar] [CrossRef] [PubMed]
- Kishore, R.K.; Halim, A.S.; Syazana, M.N.; Sirajudeen, K.N.S. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr. Res. 2011, 31, 322–325. [Google Scholar]
- Ginter, E. The role of antioxidants in the prevention of tumors. Bratisl Lek Listy 1995, 96, 195–209. [Google Scholar] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Kromhout, D.; Hollman, P.C.H.; Katan, M.B. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Wana, A. Oxygen free radicals impair wound healing in ischemic rats. Ann. Plast. Surg. 1997, 39, 516–523. [Google Scholar]
- Smirnov, D.A. Acute pancreatitis and biological antioxidants. Khirurgiia 1994, 3, 30–32. [Google Scholar] [PubMed]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic acid composition and antioxidant properties of Malaysian honeys. J. Food Sci. 2011, 76, C921–C928. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.K.; Razak, S.L.A.; Ismail, N.; Fai, N.C.; Asgar, M.H.A.M.; Sharif, N.M.; Aan, G.J.; Jubri, Z. Malaysian gelam honey reduces oxidative damage and modulates antioxidant enzyme activities in young and middle aged rats. J. Med. Plant Res. 2011, 5, 5618–5625. [Google Scholar]
- Makpol, S.; Ahmad, T.A.F.T.; Jubri, Z.; Rejab, N.; Yusof, N.; Yusof, Y.A.M. Gelam honey acting as a radioprotectant agent in gamma-irradiated human diploid fibroblasts. J. Med. Plants Res. 2012, 6, 129–138. [Google Scholar]
- Sahhugi, Z.; Hasenan, S.M.; Jubri, Z. Protective effects of gelam honey against oxidative damage in young and aged rats. Oxid. Med. Cell. Longev. 2014, 2014, 673628. [Google Scholar] [CrossRef] [PubMed]
- Batumalaie, K.; Qvist, R.; Yusof, K.M.; Ismail, I.S.; Sekaran, S.D. The antioxidant effect of the Malaysian Gelam honey on pancreatic hamster cells cultured under hyperglycemic conditions. Clin. Exp. Med. 2014, 14, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1 beta generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Lawrencem, T.; Fong, C. The resolution of inflammation: Anti-inflammatory roles for NF-κB. Int. J. Biochem. Cell Biol. 2010, 42, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Iwalewa, E.O.; McGaw, L.J.; Naidoo, V.; Eloff, J.N. Inflammation: The foundation of diseases and disorders. A review of phytomedicines of South African origin used to treat pain and inflammatory conditions. Afr. J. Biotechnol. 2007, 6, 2868–2885. [Google Scholar]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Hung, T.M.; Dang, N.H.; Kim, J.C.; Choi, J.S.; Lee, H.K.; Min, B.S. Phenolicglycosides from Alangiumsalviifolium leaves with inhibitory activity on LPS-inducedNO, PGE2 and TNF-α production. Bioorg. Med. Chem. Lett. 2009, 19, 4389–4393. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Campos, M.M.; Otuki, M.F.; Santos, A.R.S. Anti-inflammatory compounds of plant origin. Part II Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004, 70, 93–103. [Google Scholar] [PubMed]
- Reyes-Gordillo, K.; Segovia, J.; Shibayama, M.; Vergara, P.; Moreno, M.G.; Muriel, P. Curcumin protects against acute liver damage in the rat by inhibiting NFkB, proinflammatory cytokines and oxidative stress. Biochim. Biophys. Acta 2007, 1770, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Himaya, S.W.A.; Ryu, B.; Qian, Z.J.; Li, Y.; Kim, S.K. 1-(5-bromo-2-hydroxy-4-methoxyphenyl)ethanone [SE1] suppresses pro-inflammatory responses by blocking NF-kB and MAPK signaling pathways in activated microglia. Eur. J. Pharmacol. 2001, 670, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Kassim, M.; Achoui, M.; Mansor, M.; Yusoff, K.M. The inhibitory effects of Gelam honey and its extracts on nitric oxide and prostaglandin E2 in inflammatory tissues. Fitoterapia 2010, 81, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.Z.; MohdYusoff, K.; Makpol, S.; MohdYusof, Y.A. Gelam Honey Inhibits the Production of Proinflammatory, Mediators NO, PGE2, TNF-α, and IL-6 in Carrageenan-Induced Acute Paw Edema in Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 109636. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.Z.; MohdYusoff, K.; Makpol, S.; MohdYusof, Y.A. Gelam honey attenuates carrageenan-induced rat paw inflammation via NF-κB pathway. PLoS ONE 2013, 8, 0072365. [Google Scholar] [CrossRef] [PubMed]
- Kassim, M.; Yusoff, K.M.; Ong, G.; Sekaran, S.; Yusof, M.Y.B.M.; Mansor, M. Gelam honey inhibits lipopolysaccharide-induced endotoxemia in rats through the induction of heme oxygenase-1 and the inhibition of cytokines, nitric oxide, and high-mobility group protein B1. Fitoterapia 2012, 83, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.A.; Hamzah, N.; Fauzi, A.R.; Yusof, Y.A.M.; Ibrahim, N.; Abdul Rahman, M.; AbdulGhafar, N.; Baharin, B. The Effect of Gelam (Melaleuca cajuputi) Honey on Inflammatory Mediators in Periodontitis-Induced Sprague-Dawley Rats. Int. J. Appl. Res. Nat. Prod. 2014, 7, 7–16. [Google Scholar]
- White, J.W.; Subers, M.H.; Schepartz, A.I. Theidentification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta 1963, 73, 57–70. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mandal, M. Antiproliferative effects of honey and of its polyphenols: A review. J. Biomed. Biotechnol. 2009, 2009. Available online: http://www.hindawi.com/journals/jbb/2009/830616 (accessed on 20 December 2015). [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. Available online: http://apjtb.com/zz/April/16.pdf (accessed on 21 December 2015). [Google Scholar] [CrossRef]
- Zainol, M.I.; Yusoff, K.M.; Yusof, M.Y.M. Antibacterial activity of selected Malaysian honey. BMC Complement. Altern. Med. 2013, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.J.; Ken, K.W.; Kumar, R.V.; Gunasagaran, H.; Chandramogan, V.; Lee, Y.Y. In-Vitro Screening Of Malaysian Honey From Different Floral Sources For Antibacterial Activity On Human Pathogenic Bacteria. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.J.; Lim, K.Y.; Chong, J.Y.; Low, K.L. In vitro Screening of Honey against Enterococcus spp. Biofilm. J. Med. Bioeng. 2014, 3, 23–28. [Google Scholar] [CrossRef]
- Tendolkar, P.M.; Baghdayan, A.S.; Gilmore, M.S.; Shankar, N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect. Immun. 2004, 72, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Aljadi, A.M.; Yusoff, K.M. Isolation and identification of phenolic acids in Malaysian honey with antibacterial properties. Turk. J. Med. Sci. 2003, 33, 229–236. [Google Scholar]
- Jubri, Z.; Narayanan, N.N.N.; Karim, N.A.; Ngah, W.Z.W. Antiproliferative activity and apoptosis induction by gelam honey on liver cancer cell line. Int. J. Appl. 2012, 2, 135–141. [Google Scholar]
- Wen, C.T.P.; Hussein, S.Z.; Abdullah, S.; Karim, N.A.; Makpol, S.; Yusof, Y.A.M. Gelam and nenas honeys inhibit proliferation of HT 29 colon cancer cells by inducing DNA damage and apoptosis while suppressing inflammation. Asian Pac. J. Cancer Prev. 2012, 13, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Abu, M.N.; Salleh, M.A.M.; Eshak, Z.; Hasan, M.H.; Hassan, H.F.; Ismail, W.I.W. Anti-proliferative effect of Tinasporacrispa (L.) Hook. F. & Thompson and Gelam (Melaleuca sp.) honey on several cancer cell lines. In Proceedings of the IEEE Symposium on Business, Engineering and Industrial Applications (ISBEIA), Langkawi, Malaysia, 25–28 September 2011; pp. 545–548.
- Tahir, A.A.; Sani, N.F.A.; Murad, N.A.; Makpol, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Combined ginger extract & Gelam honey modulate Ras/ERK and PI3K/AKT pathway genes in colon cancer HT29 cells. Nutr. J. 2015, 14, 31. [Google Scholar] [PubMed]
- Wee, L.H.; Morad, N.A.; Aan, G.J.; Makpol, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Mechanism of chemoprevention against colon cancer cells using combined Gelam honey and Ginger extract via mTOR and Wnt/β-catenin pathways. Asian Pac. J. Cancer Prev. 2015, 16, 6549–6556. [Google Scholar] [CrossRef] [PubMed]
- Aljadi, A.M.; Kamaruddin, M.Y.; Jamal, A.M.; MohdYassim, M.Y. Biochemical study on the efficacy of Malaysian honey on inflicted wounds: An animal model. Med. J. Islam. Acad. Sci. 2000, 13, 125–132. [Google Scholar]
- Salmah, I.; Mahmood, A.A.; Sidik, K. Synergistic effects of alcoholic extract of sweetbasil (Ocimumbasilicum L.) leaves and honey on cutaneous wound healing in rats. Int. J. Mol. Med. Adv. Sci. 2005, 1, 220–224. [Google Scholar]
- Suguna, L.; Chandrakasan, G.; Thomas Joseph, K. Influence of honey on collagenmetabolism during wound healing in rats. J. Clin. Biochem. Nutr. 1992, 13, 7–12. [Google Scholar] [CrossRef]
- Rozaini, M.Z.; Zuki, A.; Noordin, M.; Norimah, Y.; Hakim, A.N. Tensile Strength Evaluation on Burns Wound Healing Treated With Nenas (Ananascomosus spp.) and Gelam (Melaleuca spp.) Honey. In Proceedings of the 11th International Conference of The Association of Institutions for Tropical Veterinary Medicine, Petaling Jaya, Malaysia, 23–27 August 2004; p. 338.
- Rozaini, M.Z.; Zuki, A.B.Z.; Noordin, M.; Norimah, Y.; Hakim, A.N. Histological Evaluation on Burns Wound Healing Treated with Nenas (Ananascomosus spp.) and Gelam (Melaleuca spp.) Honey. In Proceedings of the 11th International Conference of The Association of Institutions for Tropical Veterinary Medicine, Petaling Jaya, Malaysia, 23–27 August 2004; p. 381.
- Rozaini, M.Z.; Zuki, A.B.Z.; Noordin, M.M.; Norimah, Y.; Nazrul Hakim, A. Macroscopic evaluation of burn wounds healing progress treated with different types of honey. Pak. J. Biol. Sci. 2005, 8, 672–678. [Google Scholar]
- Yusof, N.; Hafiza, A.A.; Zohdi, R.M.; Bakar, M.Z.A. Development of honey hydrogel dressing for enhanced wound healing. Radiat. Phys. Chem. 2007, 76, 1767–1770. [Google Scholar] [CrossRef]
- Tan, M.K.; HasanAdli, D.S.; Tumiran, M.A.; Abdulla, M.A.; Yusoff, K.M. The efficacy of Gelam honey dressing towards excisional wound healing. Evid. Based Complement. Altern. Med. 2012, 2012, 805932. [Google Scholar]
- Medhi, B.; Puri, A.; Upadhyay, S.; Kaman, L. Topical application of honey in the treatment of wound healing: A meta-analysis. JK Sci. 2008, 10, 166–169. [Google Scholar]
- Raf, C. Wound Repair: Overview and General Considerations. In The Molecular and Cellular Biology of Wound Repair, 2nd ed.; Raf, C., Ed.; Plenumpress: New York, NY, USA, 1996; pp. 3–50. [Google Scholar]
- Al-jadi, A.M.; Enchang, F.K.; Yusoff, K.M. The effect of Malaysian honey and its major components on the proliferation of cultured fibroblasts. Turk. J. Med. Sci. 2014, 44, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Rozaini, M.Z.; Md. Zuki, A.B.Z.; Norimah, Y.; Noordin, M.M.; Muhammad Nazrul, H.S.; Asnah, H. Honey hydrogel dressing to treat burn wound in rats-a preliminary report. Pertan. J. Trop. Agric. Sci. 2012, 35, 67–74. [Google Scholar]
- Rozaini, M.Z.; Zuki, A.B.Z.; Norimah, Y.; Noordin, M.M.; Muhammad Nazrul, H.A. Gelam (Melaleuca spp.) honey-based hydrogel as burn wound dressing. Evid. Based Complement. Altern. Med. 2012, 2012, 843025. [Google Scholar]
- Samat, S.; Nor, N.A.M.; Hussein, F.N.; Ismail, W.I.W. Effects of Gelam and Acacia honey acute administration on some biochemical parameters of Sprague Dawley rats. BMC Complement. Altern. Med. 2014, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Asiyah, H.A.; Syazana, N.S.; Hashida, N.H.; DurriyyahSharifah, H.A.; Kamaruddin, M.Y. Effects of nicotine and Gelam honey on testis parameters and sperm qualities of juvenile rats. Sci. Res. Essays 2011, 6, 5471–5474. [Google Scholar] [CrossRef]
| Physicochemical Characteristics | Values |
|---|---|
| pH | 3.55–3.91 |
| Free acids (meq/kg) | 32.33–50.93 |
| Lactones (meq/kg) | 5.34–9.00 |
| Moisture content (%) | 17.93–20.76 |
| Electrical conductivity (mS/cm) | 0.74 |
| Total dissolved solids (ppm) | 368.33 |
| Color intensity/ ABS450 (mAU) | 500.30–1355.00 |
| Color characteristic (mm Pfund) | 122.00, Dark amber |
| HMF content (mg/kg) | 8.52–66.00 |
| Chemical Compound | Values | Reference |
|---|---|---|
| Carbohydrate | [12,15] | |
| Total sugar content (%) | 64.93–69.60 | |
| Reducing sugar (%) | 62.17–69.16 | |
| Sucrose (%) | 0.41–2.77 | |
| Protein/Amino Acid Content | [12] | |
| Protein content (g/kg) | 3.14 | |
| Proline content (mg/kg) | 261.33 | |
| Mineral Content (mg/kg) | [15,19] | |
| Sodium | 17.37–196.84 | |
| Potassium | 23.04–1363.40 | |
| Calcium | 21.63–275.77 | |
| Iron | 2.37–142.37 | |
| Magnesium | 4.94–31.63 | |
| Zinc | 4.91–29.23 | |
| Copper | 0.29–2.21 | |
| Selenium | 16.20 | |
| Vitamin Content (mg/kg) | [15,20,21] | |
| Thiamin | 13.85 | |
| Riboflavin | 94.21 | |
| Nicotinic acid | 355.38 | |
| Panthotenic acid | 12.93 | |
| Ascorbic acid | 22.90–67.36 | |
| Vitamin E (µg/g) | 55.59–70.70 | |
| Polyphenol Content | [12,13,20,22,23] | |
| Total phenolic content (TPC) | 34.30–159.74 mg GAE/100 g; 8.47–71.51 mg RE/100 g | |
| Total flavonoid content (TFC) | 1.47–32.89 mg RE/100g; 3.24–4.30 mg CE/100g; 46.11mg QE /100 g | |
| Phenolic Compounds (µg/100 g) | [22] | |
| Gallic acid | 859.43–876.80 | |
| Chlorogenic acid | 502.77–528.08 | |
| Caffeic acid | 428.84–442.01 | |
| p-coumaric acid | 301.45–308.31 | |
| Ferulic acid | 356.93–381.37 | |
| Ellagic acid | 558.78–575.67 | |
| Quercetin | 1588.90–1594.30 | |
| Hesperetin | 1475.20–1477.78 | |
| Chrysin | 1498.60–1504.60 | |
| Enzymes | [21] | |
| Invertase (U/L) † | 85.56 | |
| Diastase (DN) * | 0.57 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, B.K.; Haron, H. Insights into Putative Health Implications of Gelam (Melaleuca cajuputi) Honey: Evidence from In-Vivo and In-Vitro Studies. Med. Sci. 2016, 4, 3. https://doi.org/10.3390/medsci4010003
Chan BK, Haron H. Insights into Putative Health Implications of Gelam (Melaleuca cajuputi) Honey: Evidence from In-Vivo and In-Vitro Studies. Medical Sciences. 2016; 4(1):3. https://doi.org/10.3390/medsci4010003
Chicago/Turabian StyleChan, Boon Keng, and Hasnah Haron. 2016. "Insights into Putative Health Implications of Gelam (Melaleuca cajuputi) Honey: Evidence from In-Vivo and In-Vitro Studies" Medical Sciences 4, no. 1: 3. https://doi.org/10.3390/medsci4010003
APA StyleChan, B. K., & Haron, H. (2016). Insights into Putative Health Implications of Gelam (Melaleuca cajuputi) Honey: Evidence from In-Vivo and In-Vitro Studies. Medical Sciences, 4(1), 3. https://doi.org/10.3390/medsci4010003







