Antitumor Immunity and Dietary Compounds
Abstract
:1. Introduction
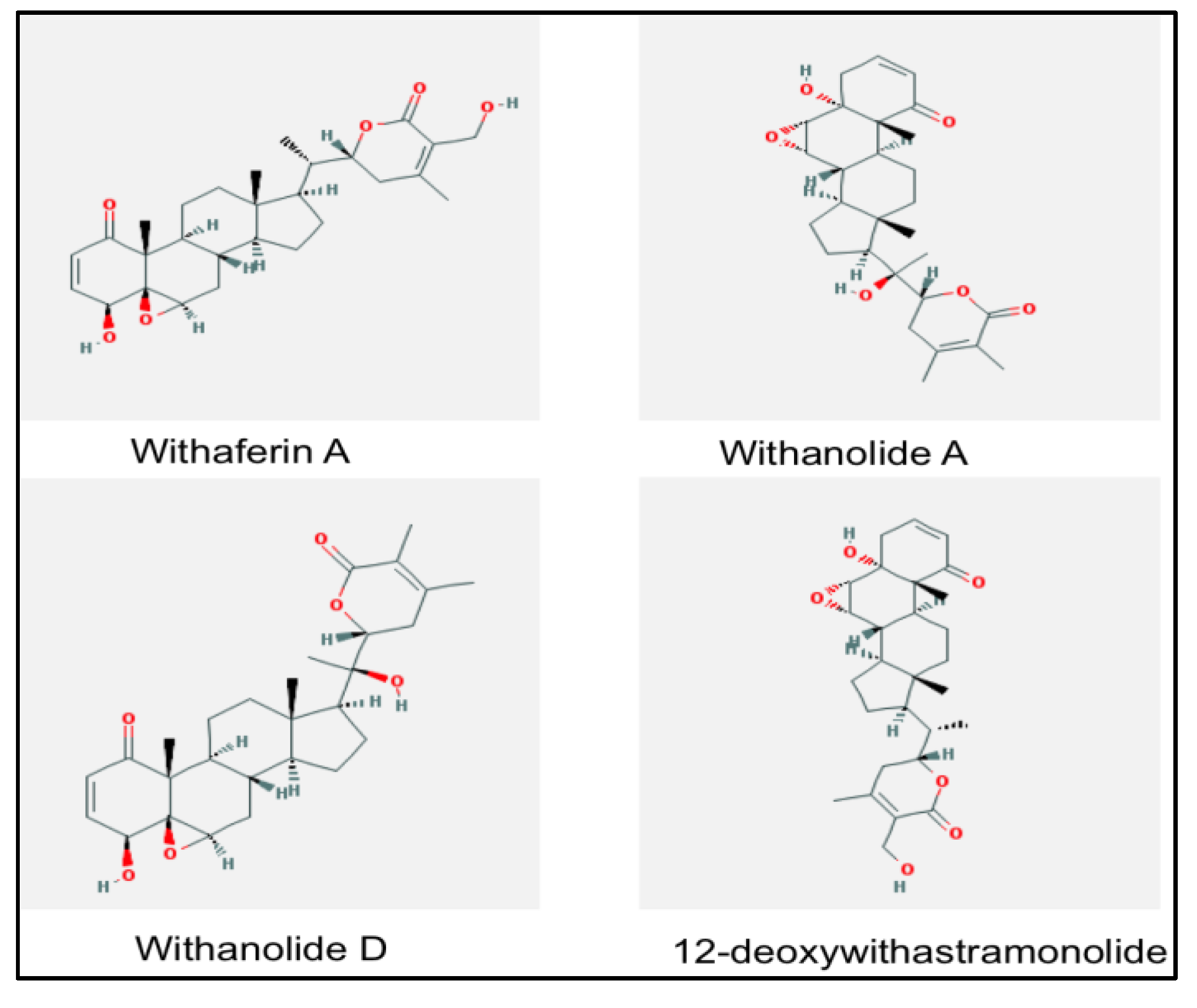
2. Withania somnifera
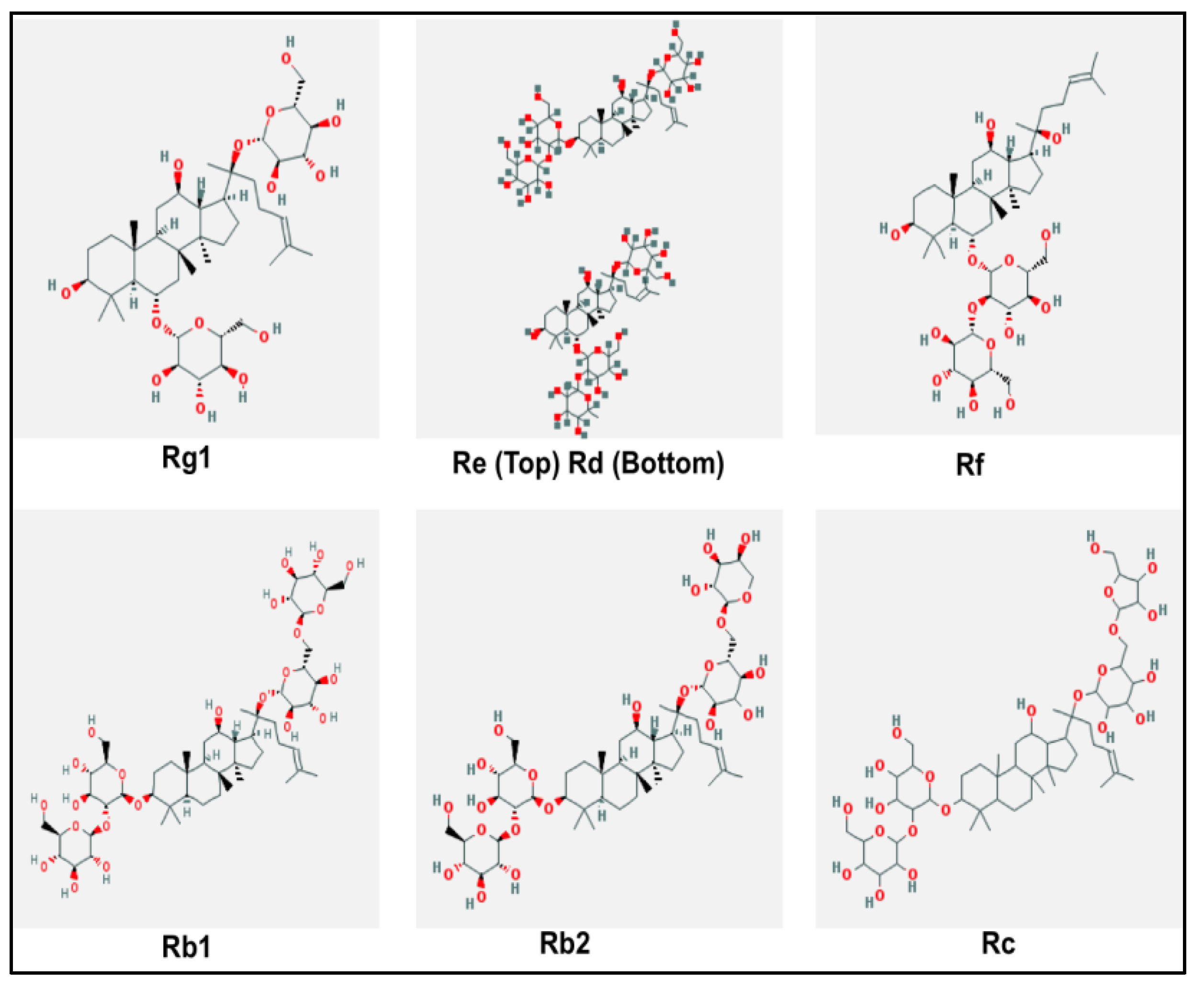
3. Panax ginseng
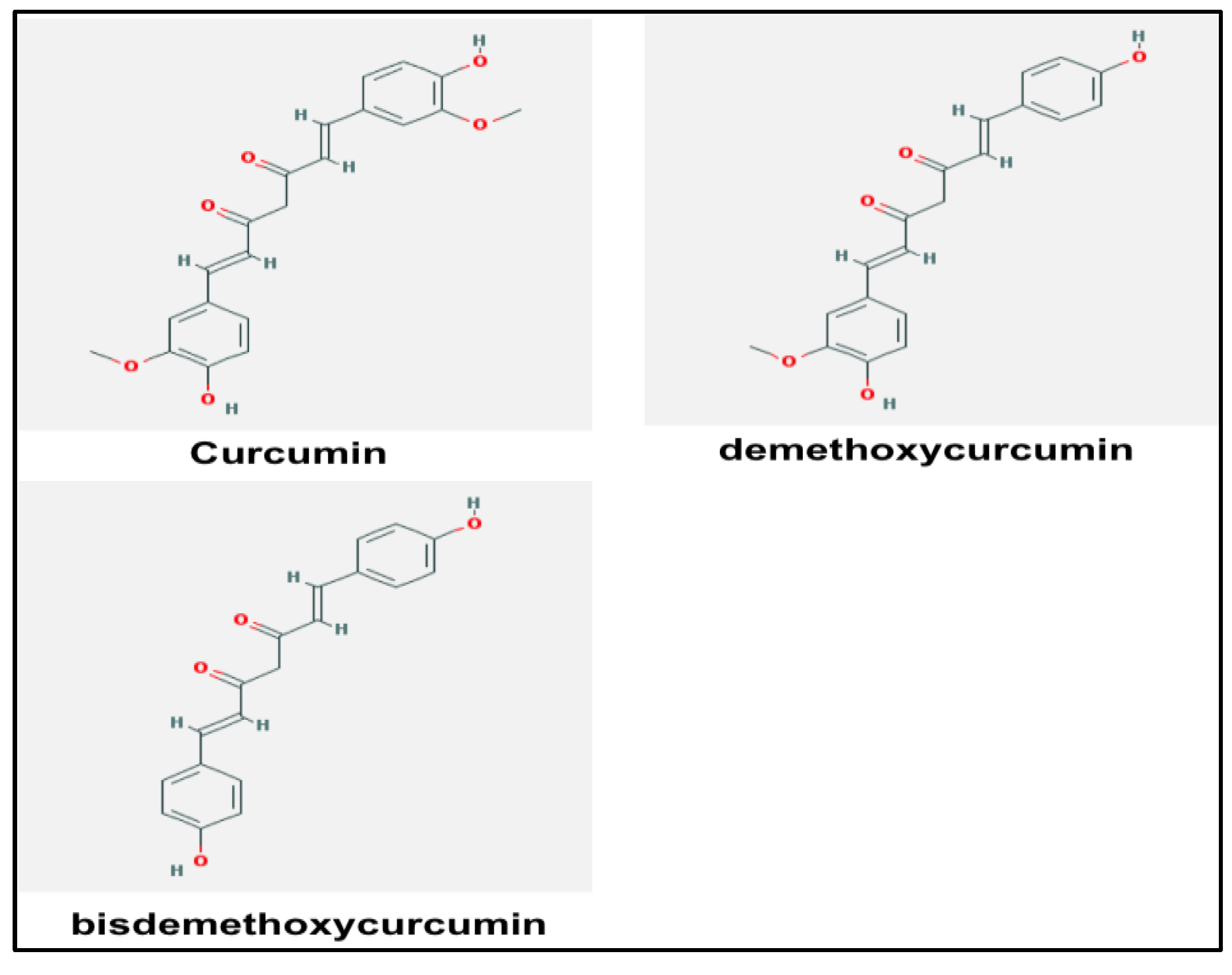
4. Curcumin
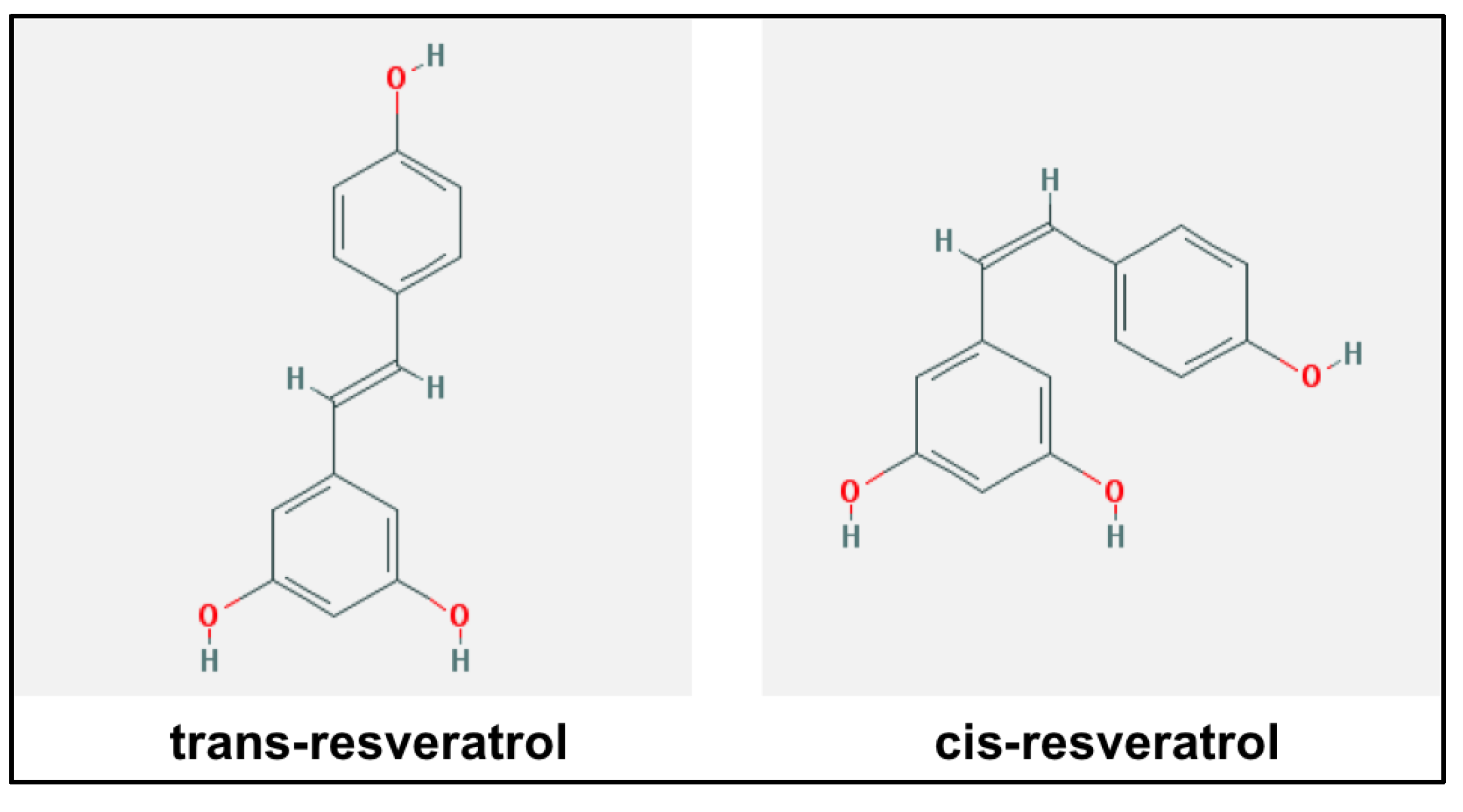
5. Resveratrol
6. Safety Data
6.1. Withania somnifera
6.2. Panax ginseng
6.3. Curcumin
6.4. Resveratrol
7. Perspective and Conclusions
| Compound | Antitumor mechanisms |
|---|---|
| Withania somnifera |
|
| Panax ginseng | |
| Curcumin |
|
| Resveratrol |
|
Acknowledgments
Conflicts of Interest
References and Notes
- Ehrlich, P. Ueber den jetzigen stand der karzinomforschung. J. Am. Chem. Soc. 1909, 42, 17–47. (in German). [Google Scholar]
- Burnet, M. Cancer: A biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br. Med. J. 1957, 1, 841–847. [Google Scholar] [CrossRef]
- Thomas, L. Cellular and Humoral Aspects of the Hypersensitive States; Lawrence, H., Ed.; Hoeber-Harper: New York, NY, USA, 1959. [Google Scholar]
- Vajdic, C.M.; van Leeuwen, M.T. Cancer incidence and risk factors after solid organ transplantation. Int. J. Cancer 2009, 125, 1747–1754. [Google Scholar] [CrossRef]
- Jessy, T. Immunity over inability: The spontaneous regression of cancer. J. Nat. Sci. Biol. Med. 2011, 2, 43–49. [Google Scholar] [CrossRef]
- Disis, M.L.; Calenoff, E.; McLaughlin, G.; Murphy, A.E.; Chen, W.; Groner, B.; Jeschke, M.; Lydon, N.; McGlynn, E.; Livingston, R.B.; et al. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994, 54, 16–20. [Google Scholar]
- Old, L.J.; Boyse, E.A. Immunology of experimental tumors. Annu. Rev. Med. 1964, 15, 167–186. [Google Scholar] [CrossRef]
- Shurin, M.R. Dendritic cells presenting tumor antigen. Cancer Immunol. Immunother. 1996, 43, 158–164. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Lindauer, M.; Stanislawski, T.; Haussler, A.; Antunes, E.; Cellary, A.; Huber, C.; Theobald, M. The molecular basis of cancer immunotherapy by cytotoxic T lymphocytes. J. Mol. Med. 1998, 76, 32–47. [Google Scholar] [CrossRef]
- Shresta, S.; Pham, C.T.; Thomas, D.A.; Graubert, T.A.; Ley, T.J. How do cytotoxic lymphocytes kill their targets? Curr. Opin. Immunol. 1998, 10, 581–587. [Google Scholar] [CrossRef]
- Pardoll, D.M.; Topalian, S.L. The role of CD4+ T cell responses in antitumor immunity. Curr. Opin. Immunol. 1998, 10, 588–594. [Google Scholar] [CrossRef]
- Glennie, M.J.; Johnson, P.W. Clinical trials of antibody therapy. Immunol. Today 2000, 21, 403–410. [Google Scholar] [CrossRef]
- Old, L.J. Immunotherapy for cancer. Sci. Am. 1996, 275, 136–143. [Google Scholar] [CrossRef]
- Baselga, J.; Albanell, J.; Molina, M.A.; Arribas, J. Mechanism of action of trastuzumab and scientific update. Semin. Oncol. 2001, 28, 4–11. [Google Scholar]
- Weiner, G.J. Rituximab: Mechanism of action. Semin. Hematol. 2010, 47, 115–123. [Google Scholar] [CrossRef]
- Schultze, J.L.; Michalak, S.; Seamon, M.J.; Dranoff, G.; Jung, K.; Daley, J.; Delgado, J.C.; Gribben, J.G.; Nadler, L.M. CD40-activated human B cells: An alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J. Clin. Investig. 1997, 100, 2757–2765. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Yanaba, K.; Tedder, T.F. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: Therapeutic B cell depletion enhances b16 melanoma growth in mice. J. Immunol. 2010, 184, 4006–4016. [Google Scholar] [CrossRef]
- Fauriat, C.; Long, E.O.; Ljunggren, H.G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Haridas, V.; Sarin, A.; Anandhi, R.; Kochupillai, V.; Saxena, R.K. Effect of gamma interferon on the expression of class i mhc antigens on fresh leukemic cells and their susceptibility to lysis by lymphokine activated killer cells. Indian J. Cancer 1994, 31, 96–102. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Becker, J.C.; Andersen, M.H.; Schrama, D.; Thor Straten, P. Immune-suppressive properties of the tumor microenvironment. Cancer Immunol. Immunother. 2013, 62, 1137–1148. [Google Scholar] [CrossRef]
- Bubenik, J. MHC class I down-regulation: Tumour escape from immune surveillance? (review). Int. J. Oncol. 2004, 25, 487–491. [Google Scholar]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef]
- Rozali, E.N.; Hato, S.V.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. Programmed death ligand 2 in cancer-induced immune suppression. Clin. Dev. Immunol. 2012. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006, 6, 295–307. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Mishra, L.C.; Singh, B.B.; Dagenais, S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): A review. Altern. Med. Rev. 2000, 5, 334–346. [Google Scholar]
- Begum, V.H.; Sadique, J. Long term effect of herbal drug Withania somnifera on adjuvant induced arthritis in rats. Indian J. Exp. Biol. 1988, 26, 877–882. [Google Scholar]
- Devi, P.U. Withania somnifera dunal (ashwagandha): Potential plant source of a promising drug for cancer chemotherapy and radiosensitization. Indian J. Exp. Biol. 1996, 34, 927–932. [Google Scholar]
- Agnihotri, A.P.; Sontakke, S.D.; Thawani, V.R.; Saoji, A.; Goswami, V.S. Effects of Withania somnifera in patients of schizophrenia: A randomized, double blind, placebo controlled pilot trial study. Indian J. Pharmacol. 2013, 45, 417–418. [Google Scholar] [CrossRef]
- Withaferin A—Compound Summary (CID 265237). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=265237&loc=ec_rcs#x395/ (accessed on 8 October 2013).
- 3-Rhamnopyranosyl(1-4)-Glucopyranosyl-12-Diacetoxy-20-Hydroxywitha-5,24-Dienolide—Compound Summary (CID 11294368). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=11294368&loc=ec_rcs/ (accessed on 8 October 2013).
- Withanolide D—Substance Summary (SID 163725462). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=163725462&loc=es_rss/ (accessed on 8 October 2013).
- 27-Hydroxywithanolide B—Substance Summary (SID 162251743). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=162251743#x395/ (accessed on 8 October 2013).
- Vyas, A.R.; Singh, S.V. Molecular targets and mechanisms of cancer prevention and treatment by Withaferin A, a naturally occurring steroidal lactone. AAPS J. 2013. [Google Scholar] [CrossRef]
- Kour, K.; Pandey, A.; Suri, K.A.; Satti, N.K.; Gupta, K.K.; Bani, S. Restoration of stress-induced altered T cell function and corresponding cytokines patterns by withanolide A. Int. Immunopharm. 2009, 9, 1137–1144. [Google Scholar] [CrossRef]
- Malik, F.; Singh, J.; Khajuria, A.; Suri, K.A.; Satti, N.K.; Singh, S.; Kaul, M.K.; Kumar, A.; Bhatia, A.; Qazi, G.N. A standardized root extract of Withania somnifera and its major constituent withanolide-a elicit humoral and cell-mediated immune responses by up regulation of Th1-dominant polarization in BALB/c mice. Life Sci. 2007, 80, 1525–1538. [Google Scholar] [CrossRef]
- Malik, F.; Kumar, A.; Bhushan, S.; Mondhe, D.M.; Pal, H.C.; Sharma, R.; Khajuria, A.; Singh, S.; Singh, G.; Saxena, A.K.; et al. Immune modulation and apoptosis induction: Two sides of antitumoural activity of a standardised herbal formulation of Withania somnifera. Eur. J. Cancer 2009, 45, 1494–1509. [Google Scholar] [CrossRef]
- Davis, L.; Kuttan, G. Effect of Withania somnifera on cell mediated immune responses in mice. J. Exp. Clin. Cancer Res. 2002, 21, 585–590. [Google Scholar]
- Shohat, B.; Joshua, H. Effect of Withaferin A on ehrlich ascites tumor cells. II. Target tumor cell destruction in vivo by immune activation. Int. J. Cancer 1971, 8, 487–496. [Google Scholar]
- Smith, A.R.; Lopez-Rodriguez, D.; Andreansky, S. Withaferin A, a natural plant derived compound targets the stress pathway to induce antitumor immunity. Cancer Res. 2013. submitted for publication. [Google Scholar]
- Inoue, H.; Tani, K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2013, 21, 39–49. [Google Scholar] [CrossRef]
- Sinha, P.; Ostrand-Rosenberg, S. Myeloid-derived suppressor cell function is reduced by Withaferin A, a potent and abundant component of Withania somnifera root extract. Cancer Immunol. Immunother. 2013, 62, 1663–1673. [Google Scholar] [CrossRef]
- Hu, S.Y. A contribution to our knowledge of ginseng. Am. J. Chin. Med. 1977, 5, 1–23. [Google Scholar] [CrossRef]
- Tao, H.C. Shen-Nung-Pen-Tsao-Ching; (in German). Chung Hwa: Taipei, Taiwan, 1955. [Google Scholar]
- Translated and Summarized in Hsu, H.Y. Oriental Materia Medica: A Precise Guide; Oriental Healing Arts Institute: Long Beach, CA, USA, 1986. [Google Scholar]
- Yun, T.K. Brief introduction of Panax ginseng C.A. Meyer. J. Kor. Med. Sci. 2001, 16, S3–S5. [Google Scholar]
- Ginsenoside RG1—Substance Summary (SID 53786785). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=53786785&viewopt=PubChem/ (accessed on 8 October 2013).
- Ginsenoside Rd Mxture w/Re—Substance Summary (SID 8141082). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=8141082&viewopt=PubChem/ (accessed on 8 October 2013).
- Ginsenoside Rf—Compound Summary (CID 441922). Available online: http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=search&db=pccompound&term=441922[uid]/ (accessed on 8 October 2013).
- Ginsenoside Rb1—Compound Summary (CID 9898279). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=9898279&loc=ec_rcs/ (accessed on 8 October 2013).
- Ginsenoside Rb2—Compound Summary (CID 5458674). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=5458674&loc=ec_rcs/ (accessed on 8 October 2013).
- Ginsenoside Rc—Compound Summary (CID 100018). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=100018&loc=ec_rcs/ (accessed on 8 October 2013).
- Kitagawa, I.; Yoshikawa, M.; Yoshihara, M.; Hayashi, T.; Taniyama, T. Chemical studies of crude drugs (1). Constituents of ginseng radix rubra. Yakugaku Zasshi 1983, 103, 612–622. [Google Scholar]
- Choi, K.T. Botanical characteristics, pharmacological effects and medicinal components of korean Panax ginseng c a meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef]
- Gao, Q.P.; Kiyohara, H.; Cyong, J.C.; Yamada, H. Chemical properties and anti-complementary activities of polysaccharide fractions from roots and leaves of Panax ginseng. Planta Med. 1989, 55, 9–12. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kang, K.S.; Kim, S.I. Study on antitumor and immuno-modulating activities of polysaccharide fractions from Panax ginseng: Comparison of effects of neutral and acidic polysaccharide fraction. Arch. Pharm. Res. 1990, 13, 330–337. [Google Scholar] [CrossRef]
- Ni, W.; Zhang, X.; Wang, B.; Chen, Y.; Han, H.; Fan, Y.; Zhou, Y.; Tai, G. Antitumor activities and immunomodulatory effects of ginseng neutral polysaccharides in combination with 5-fluorouracil. J. Med. Food 2010, 13, 270–277. [Google Scholar] [CrossRef]
- Lee, Y.S.; Chung, I.S.; Lee, I.R.; Kim, K.H.; Hong, W.S.; Yun, Y.S. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginsan isolated from Panax ginseng. Anticancer Res. 1997, 17, 323–331. [Google Scholar]
- Kim, K.H.; Lee, Y.S.; Jung, I.S.; Park, S.Y.; Chung, H.Y.; Lee, I.R.; Yun, Y.S. Acidic polysaccharide from Panax ginseng, ginsan, induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Planta Med. 1998, 64, 110–115. [Google Scholar] [CrossRef]
- Shin, J.Y.; Song, J.Y.; Yun, Y.S.; Yang, H.O.; Rhee, D.K.; Pyo, S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol. Immunotoxicol. 2002, 24, 469–482. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, K.H.; Sohn, E.; Park, J.D.; Kim, B.O.; Moon, E.Y.; Rhee, D.K.; Pyo, S. Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-kappab pathway. Biosci. Biotechnol. Biochem. 2008, 72, 1817–1825. [Google Scholar] [CrossRef]
- Park, D.; Bae, D.K.; Jeon, J.H.; Lee, J.; Oh, N.; Yang, G.; Yang, Y.H.; Kim, T.K.; Song, J.; Lee, S.H.; et al. Immunopotentiation and antitumor effects of a ginsenoside Rg(3)-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ. Toxicol. Pharmacol. 2011, 31, 397–405. [Google Scholar] [CrossRef]
- Jeon, C.; Kang, S.; Park, S.; Lim, K.; Hwang, K.W.; Min, H. T cell stimulatory effects of korean red ginseng through modulation of myeloid-derived suppressor cells. J. Ginseng Res. 2011, 35, 462–470. [Google Scholar] [CrossRef]
- Curcumin—Compound Summary (CID 969516). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=969516&loc=ec_rcs/ (accessed on 8 October 2013).
- Demethoxycurcumin—Substance Summary (SID 162221237). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=162221237&viewopt=PubChem/ (accessed on 8 October 2013).
- Bisdemethoxycurcumin—Substance Summary (SID 162220513). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=162220513&viewopt=PubChem/ (accessed on 8 October 2013).
- Ammon, H.P.; Wahl, M.A. Pharmacology of curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef]
- Ali, T.; Shakir, F.; Morton, J. Curcumin and inflammatory bowel disease: Biological mechanisms and clinical implication. Digestion 2012, 85, 249–255. [Google Scholar] [CrossRef]
- Yu, W.G.; Xu, G.; Ren, G.J.; Xu, X.; Yuan, H.Q.; Qi, X.L.; Tian, K.L. Preventive action of curcumin in experimental acute pancreatitis in mouse. Indian J. Med. Res. 2011, 134, 717–724. [Google Scholar] [CrossRef]
- Gukovsky, I.; Reyes, C.N.; Vaquero, E.C.; Gukovskaya, A.S.; Pandol, S.J. Curcumin ameliorates ethanol and nonethanol experimental pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G85–G95. [Google Scholar]
- Liu, J.; Chen, S.; Lv, L.; Song, L.; Guo, S.; Huang, S. Recent progress in studying curcumin and its nano-preparations for cancer therapy. Curr. Pharmaceut. Des. 2013, 19, 1974–1993. [Google Scholar]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Ni, X.; Zhang, A.; Zhao, Z.; Shen, Y.; Wang, S. Demethoxycurcumin inhibits cell proliferation, migration and invasion in prostate cancer cells. Oncol. Rep. 2012, 28, 85–90. [Google Scholar]
- Huang, T.Y.; Hsu, C.W.; Chang, W.C.; Wang, M.Y.; Wu, J.F.; Hsu, Y.C. Demethoxycurcumin retards cell growth and induces apoptosis in human brain malignant glioma GBM 8401 cells. Evid. Based Complement Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Li, Y.B.; Gao, J.L.; Zhong, Z.F.; Hoi, P.M.; Lee, S.M.; Wang, Y.T. Bisdemethoxycurcumin suppresses MCF-7 cells proliferation by inducing ROS accumulation and modulating senescence-related pathways. Pharmacol. Rep. 2013, 65, 700–709. [Google Scholar]
- Liu, Y.L.; Yang, H.P.; Zhou, X.D.; Gong, L.; Tang, C.L.; Wang, H.J. The hypomethylation agent bisdemethoxycurcumin acts on the WIF-1 promoter, inhibits the canonical Wnt pathway and induces apoptosis in human non-small-cell lung cancer. Curr. Cancer Drug Targets 2011, 11, 1098–1110. [Google Scholar] [CrossRef]
- Basile, V.; Ferrari, E.; Lazzari, S.; Belluti, S.; Pignedoli, F.; Imbriano, C. Curcumin derivatives: Molecular basis of their anti-cancer activity. Biochem. Pharmacol. 2009, 78, 1305–1315. [Google Scholar] [CrossRef]
- Varalakshmi, C.; Ali, A.M.; Pardhasaradhi, B.V.; Srivastava, R.M.; Singh, S.; Khar, A. Immunomodulatory effects of curcumin: In Vivo. Int. Immunopharm. 2008, 8, 688–700. [Google Scholar] [CrossRef]
- Luo, F.; Song, X.; Zhang, Y.; Chu, Y. Low-dose curcumin leads to the inhibition of tumor growth via enhancing ctl-mediated antitumor immunity. Int. Immunopharm. 2011, 11, 1234–1240. [Google Scholar] [CrossRef]
- Salvadori, S.; Gansbacher, B.; Pizzimenti, A.M.; Zier, K.S. Abnormal signal transduction by T cells of mice with parental tumors is not seen in mice bearing IL-2-secreting tumors. J. Immunol. 1994, 153, 5176–5182. [Google Scholar]
- Otsuji, M.; Kimura, Y.; Aoe, T.; Okamoto, Y.; Saito, T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses. Proc. Natl. Acad. Sci. USA 1996, 93, 13119–13124. [Google Scholar] [CrossRef]
- Gastman, B.R.; Johnson, D.E.; Whiteside, T.L.; Rabinowich, H. Tumor-induced apoptosis of T lymphocytes: Elucidation of intracellular apoptotic events. Blood 2000, 95, 2015–2023. [Google Scholar]
- Ohm, J.E.; Carbone, D.P. Immune dysfunction in cancer patients. Oncology 2002, 16, 11–18. [Google Scholar]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T.; et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell. Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Mandal, D.; Saha, B.; Sen, G.S.; Das, T.; Sa, G. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J. Biol. Chem. 2007, 282, 15954–15964. [Google Scholar] [CrossRef]
- Zhang, H.G.; Kim, H.; Liu, C.; Yu, S.; Wang, J.; Grizzle, W.E.; Kimberly, R.P.; Barnes, S. Curcumin reverses breast tumor exosomes mediated immune suppression of nk cell tumor cytotoxicity. Biochim. Biophys. Acta 2007, 1773, 1116–1123. [Google Scholar]
- Bhattacharyya, S.; Mandal, D.; Sen, G.S.; Pal, S.; Banerjee, S.; Lahiry, L.; Finke, J.H.; Tannenbaum, C.S.; Das, T.; Sa, G. Tumor-induced oxidative stress perturbs nuclear factor-kappab activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: Protection by curcumin. Cancer Res. 2007, 67, 362–370. [Google Scholar]
- Liu, C.; Yu, S.; Zinn, K.; Wang, J.; Zhang, L.; Jia, Y.; Kappes, J.C.; Barnes, S.; Kimberly, R.P.; Grizzle, W.E.; et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 2006, 176, 1375–1385. [Google Scholar]
- Stuelten, C.H.; DaCosta Byfield, S.; Arany, P.R.; Karpova, T.S.; Stetler-Stevenson, W.G.; Roberts, A.B. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNV-alpha and TGF-beta. J. Cell. Sci. 2005, 118, 2143–2153. [Google Scholar] [CrossRef]
- Chang, Y.F.; Chuang, H.Y.; Hsu, C.H.; Liu, R.S.; Gambhir, S.S.; Hwang, J.J. Immunomodulation of curcumin on adoptive therapy with T cell functional imaging in mice. Cancer Prev. Res. 2012, 5, 444–452. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Puccetti, P. Indoleamine 2,3-dioxygenase: From catalyst to signaling function. Euro. J. Immunol. 2012, 42, 1932–1937. [Google Scholar] [CrossRef]
- Tu, S.P.; Jin, H.; Shi, J.D.; Zhu, L.M.; Suo, Y.; Lu, G.; Liu, A.; Wang, T.C.; Yang, C.S. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev. Res. 2012, 5, 205–215. [Google Scholar]
- Zhao, G.J.; Lu, Z.Q.; Tang, L.M.; Wu, Z.S.; Wang, D.W.; Zheng, J.Y.; Qiu, Q.M. Curcumin inhibits suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. Int. Immunopharm. 2012, 14, 99–106. [Google Scholar] [CrossRef]
- Resveratrol—Substance Summary (SID 164216570). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=164216570&viewopt=PubChem/ (accessed on 8 October 2013).
- cis-Resveratrol—Substance Summary (SID 164233321). Available online: http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=164233321&viewopt=PubChem/ (accessed on 8 October 2013).
- Sanders, T.H.; McMichael, R.W., Jr.; Hendrix, K.W. Occurrence of resveratrol in edible peanuts. J. Agric. Food Chem. 2000, 48, 1243–1246. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Wang, Y.; Catana, F.; Yang, Y.; Roderick, R.; van Breemen, R.B. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 2002, 50, 431–435. [Google Scholar] [CrossRef]
- Lyons, M.M.; Yu, C.; Toma, R.B.; Cho, S.Y.; Reiboldt, W.; Lee, J.; van Breemen, R.B. Resveratrol in raw and baked blueberries and bilberries. J. Agric. Food Chem. 2003, 51, 5867–5870. [Google Scholar] [CrossRef]
- Carrizzo, A.; Forte, M.; Damato, A.; Trimarco, V.; Salzano, F.; Bartolo, M.; Maciag, A.; Puca, A.A.; Vecchione, C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem. Toxicol. 2013, 61, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.O.; Mullin, G.E. A review of complementary and alternative approaches to immunomodulation. Nutr. Clin. Pract. 2008, 23, 49–62. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Ramprasath, V.R.; Aluko, R.E.; Jones, P.J. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr. Rev. 2008, 66, 445–454. [Google Scholar] [CrossRef]
- Li, T.; Fan, G.X.; Wang, W.; Li, T.; Yuan, Y.K. Resveratrol induces apoptosis, influences IL-6 and exerts immunomodulatory effect on mouse lymphocytic leukemia both in vitro and in vivo. Int. Immunopharm. 2007, 7, 1221–1231. [Google Scholar] [CrossRef]
- Bergman, M.; Levin, G.S.; Bessler, H.; Djaldetti, M.; Salman, H. Resveratrol affects the cross talk between immune and colon cancer cells. Biomed. Pharmacother. 2013, 67, 43–47. [Google Scholar] [CrossRef]
- Yang, Y.; Paik, J.H.; Cho, D.; Cho, J.A.; Kim, C.W. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory t cells. Int. Immunopharm. 2008, 8, 542–547. [Google Scholar] [CrossRef]
- Guan, H.; Singh, N.P.; Singh, U.P.; Nagarkatti, P.S.; Nagarkatti, M. Resveratrol prevents endothelial cells injury in high-dose interleukin-2 therapy against melanoma. PLoS One 2012, 7, e35650. [Google Scholar]
- Takikawa, O.; Habara-Ohkubo, A.; Yoshida, R. IFN-gamma is the inducer of indoleamine 2,3-dioxygenase in allografted tumor cells undergoing rejection. J. Immunol. 1990, 145, 1246–1250. [Google Scholar]
- Noh, K.T.; Chae, S.H.; Chun, S.H.; Jung, I.D.; Kang, H.K.; Park, Y.M. Resveratrol suppresses tumor progression via the regulation of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2013, 431, 348–353. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Kim, S.W.; Jung, I.D.; Lee, J.S.; Chang, J.H.; Lee, C.M.; Chun, S.H.; Yoon, M.S.; Kim, G.T.; Ryu, S.W.; et al. Curcumin suppresses the induction of indoleamine 2,3-dioxygenase by blocking the janus-activated kinase-protein kinase Cdelta-STAT1 signaling pathway in interferon-gamma-stimulated murine dendritic cells. J. Biol. Chem. 2009, 284, 3700–3708. [Google Scholar]
- Lee-Chang, C.; Bodogai, M.; Martin-Montalvo, A.; Wejksza, K.; Sanghvi, M.; Moaddel, R.; de Cabo, R.; Biragyn, A. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J. Immunol. 2013, 191, 4141–4151. [Google Scholar] [CrossRef]
- Olkhanud, P.B.; Damdinsuren, B.; Bodogai, M.; Gress, R.E.; Sen, R.; Wejksza, K.; Malchinkhuu, E.; Wersto, R.P.; Biragyn, A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011, 71, 3505–3515. [Google Scholar] [CrossRef]
- Bani, S.; Gautam, M.; Sheikh, F.A.; Khan, B.; Satti, N.K.; Suri, K.A.; Qazi, G.N.; Patwardhan, B. Selective th1 up-regulating activity of Withania somnifera aqueous extract in an experimental system using flow cytometry. J. Ethnopharmacol. 2006, 107, 107–115. [Google Scholar] [CrossRef]
- Sharada, A.C.; Solomon, F.E.; Devi, P.U.; Udupa, N.; Srinivasan, K.K. Antitumor and radiosensitizing effects of Withaferin A on mouse ehrlich ascites carcinoma in vivo. Acta Oncol. 1996, 35, 95–100. [Google Scholar] [CrossRef]
- Thaiparambil, J.T.; Bender, L.; Ganesh, T.; Kline, E.; Patel, P.; Liu, Y.; Tighiouart, M.; Vertino, P.M.; Harvey, R.D.; Garcia, A.; et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer 2011, 129, 2744–2755. [Google Scholar]
- Raut, A.A.; Rege, N.N.; Tadvi, F.M.; Solanki, P.V.; Kene, K.R.; Shirolkar, S.G.; Pandey, S.N.; Vaidya, R.A.; Vaidya, A.B. Exploratory study to evaluate tolerability, safety, and activity of ashwagandha (Withania somnifera) in healthy volunteers. J. Ayurveda Integr. Med. 2012, 3, 111–114. [Google Scholar]
- Fahim, M.S.; Fahim, Z.; Harman, J.M.; Clevenger, T.E.; Mullins, W.; Hafez, E.S. Effect of Panax ginseng on testosterone level and prostate in male rats. Arch. Androl. 1982, 8, 261–263. [Google Scholar] [CrossRef]
- Hess, F.G., Jr.; Parent, R.A.; Stevens, K.R.; Cox, G.E.; Becci, P.J. Effects of subchronic feeding of ginseng extract G115 in beagle dogs. Food Chem. Toxicol. 1983, 21, 95–97. [Google Scholar]
- Lee, N.H.; Yoo, S.R.; Kim, H.G.; Cho, J.H.; Son, C.G. Safety and tolerability of Panax ginseng root extract: A randomized, placebo-controlled, clinical trial in healthy korean volunteers. J. Altern. Complement Med. 2012, 18, 1061–1069. [Google Scholar]
- Hsu, C.H.; Cheng, A.L. Clinical studies with curcumin. Adv. Exp. Med. Biol. 2007, 595, 471–480. [Google Scholar]
- National Institute of Health. Curcumin clnical trials. Available online: http://clinicaltrials.gov/ct2/results?term=Curcumin+Clinical+Trials&Search=Search/ (accessed on 6 October 2013).
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar]
- Tome-Carneiro, J.; Larrosa, M.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharmaceut. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef]
- Johnson, W.D.; Morrissey, R.L.; Usborne, A.L.; Kapetanovic, I.; Crowell, J.A.; Muzzio, M.; McCormick, D.L. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem. Toxicol. 2011, 49, 3319–3327. [Google Scholar]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar]
- Crowell, J.A.; Korytko, P.J.; Morrissey, R.L.; Booth, T.D.; Levine, B.S. Resveratrol-associated renal toxicity. Toxicol. Sci. 2004, 82, 614–619. [Google Scholar]
- National Institute of Health. Resveratrol Clinical Trials. Available online: http://clinicaltrials.gov/ct2/results?term=Resveratrol+Clinical+Trials&Search=Search/ (accessed on 6 October 2013).
- Cottart, C.H.; Nivet-Antoine, V.; Beaudeux, J.L. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol. Nutr. Food Res. 2013. [Google Scholar] [CrossRef]
- Fong, M.Y.; Jin, S.; Rane, M.; Singh, R.K.; Gupta, R.; Kakar, S.S. Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer. PLoS One 2012, 7, e42265. [Google Scholar]
- Sharma, S.; Chopra, K.; Kulkarni, S.K.; Agrewala, J.N. Resveratrol and curcumin suppress immune response through CD28/CTLA-4 and CD80 co-stimulatory pathway. Clin. Exp. Immunol. 2007, 147, 155–163. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Smith, A.R.; Andreansky, S. Antitumor Immunity and Dietary Compounds. Med. Sci. 2014, 2, 1-22. https://doi.org/10.3390/medsci2010001
Smith AR, Andreansky S. Antitumor Immunity and Dietary Compounds. Medical Sciences. 2014; 2(1):1-22. https://doi.org/10.3390/medsci2010001
Chicago/Turabian StyleSmith, Annalise R., and Samita Andreansky. 2014. "Antitumor Immunity and Dietary Compounds" Medical Sciences 2, no. 1: 1-22. https://doi.org/10.3390/medsci2010001
APA StyleSmith, A. R., & Andreansky, S. (2014). Antitumor Immunity and Dietary Compounds. Medical Sciences, 2(1), 1-22. https://doi.org/10.3390/medsci2010001




