Development of a MassARRAY Genotyping Platform and Its Clinical Application for Venous Thromboembolism Risk Assessment in Thai Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Sample and DNA Preparation
2.3. Candidate Gene and SNP Selection
2.4. Multiplex Polymerase Chain Reaction
2.5. SNPs Genotyping Using MassARRAY
2.6. Polygenic Risk Score Calculation
2.7. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics of 122 VTE Patients
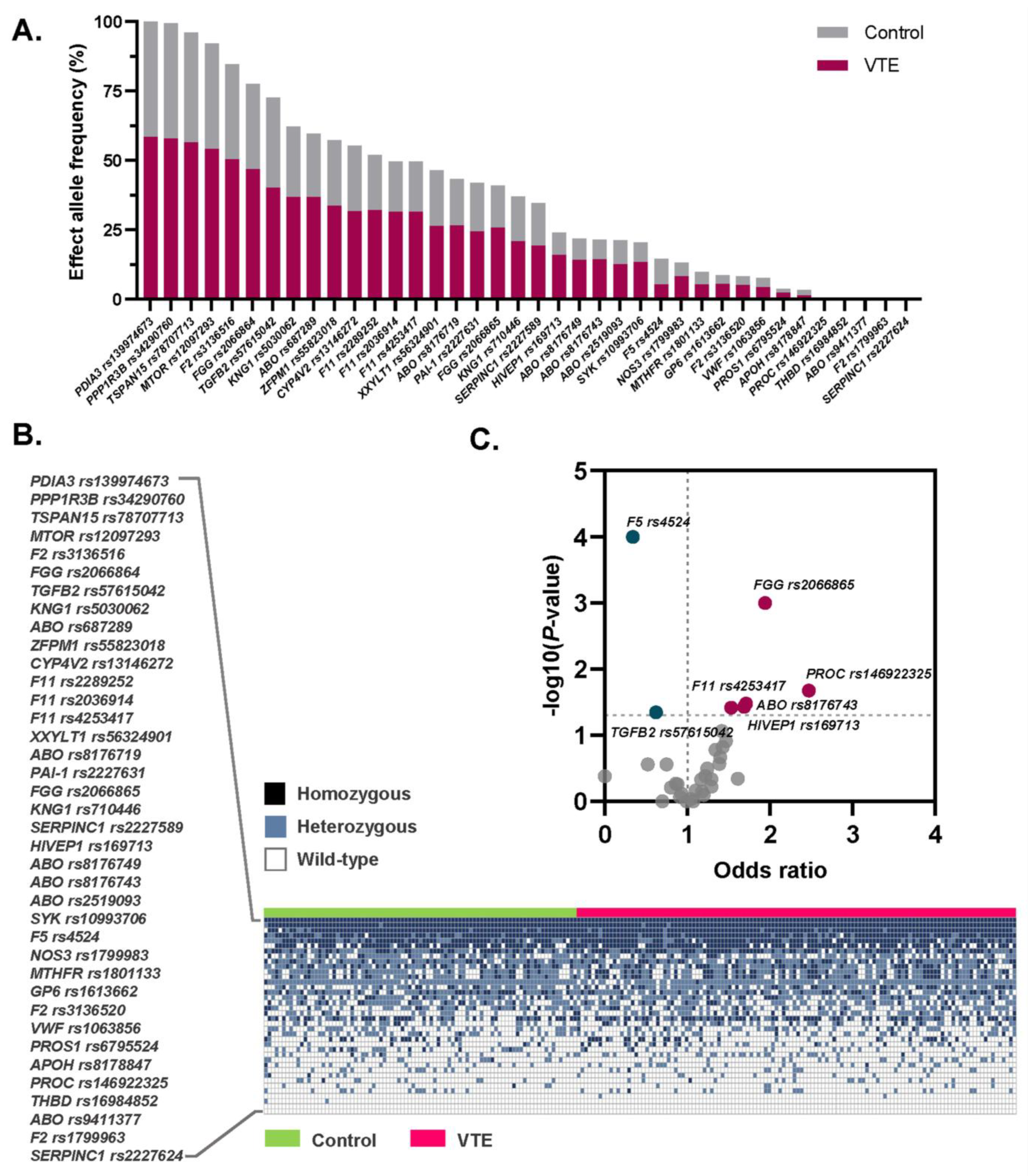
3.2. Allele Frequencies of the 39 VTE Susceptibility SNPs Among Thai Patients and Controls
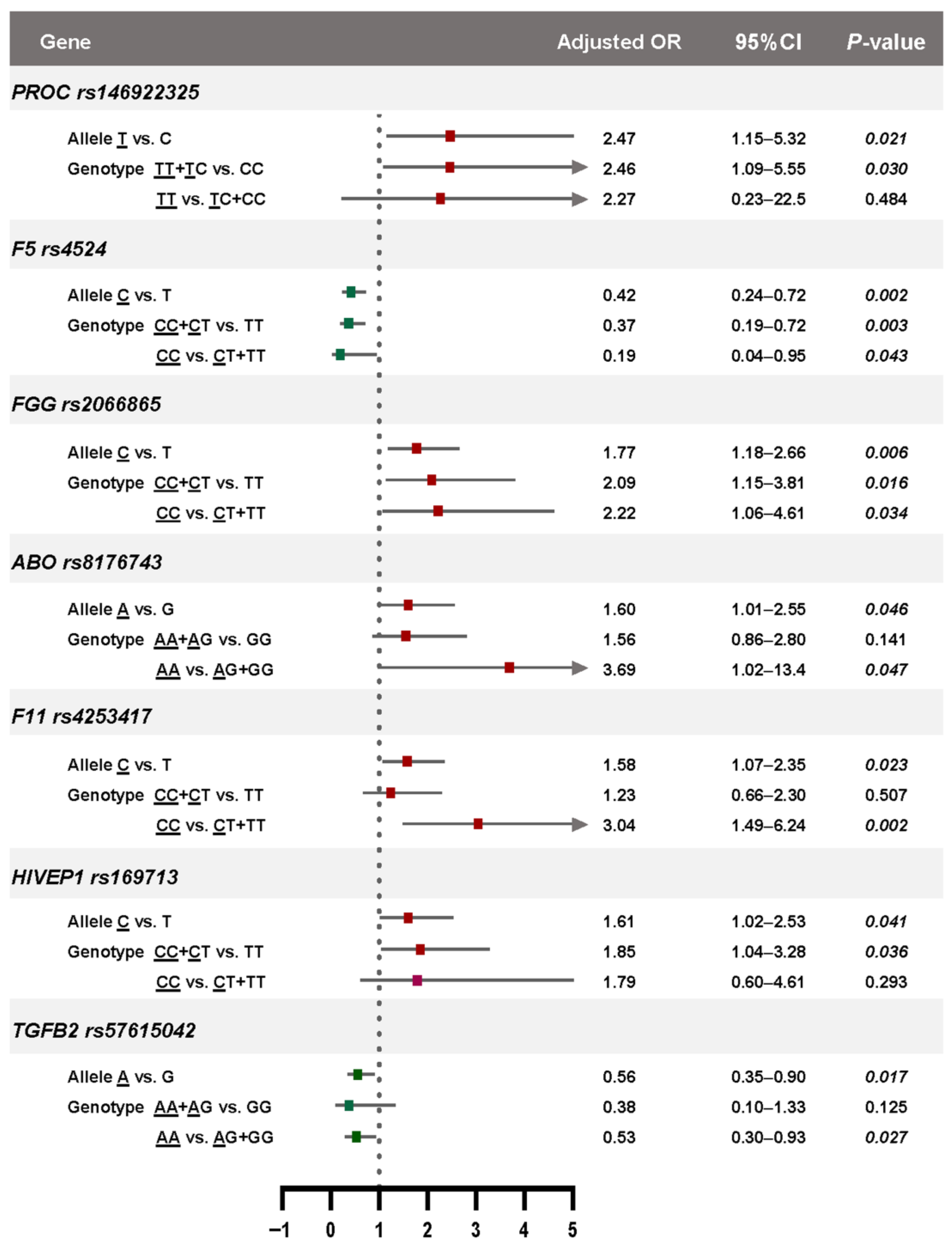
3.3. Association Between the Selected SNPs and Risk of VTE
3.4. Genotype-Phenotype Associations Based on Genetic Models
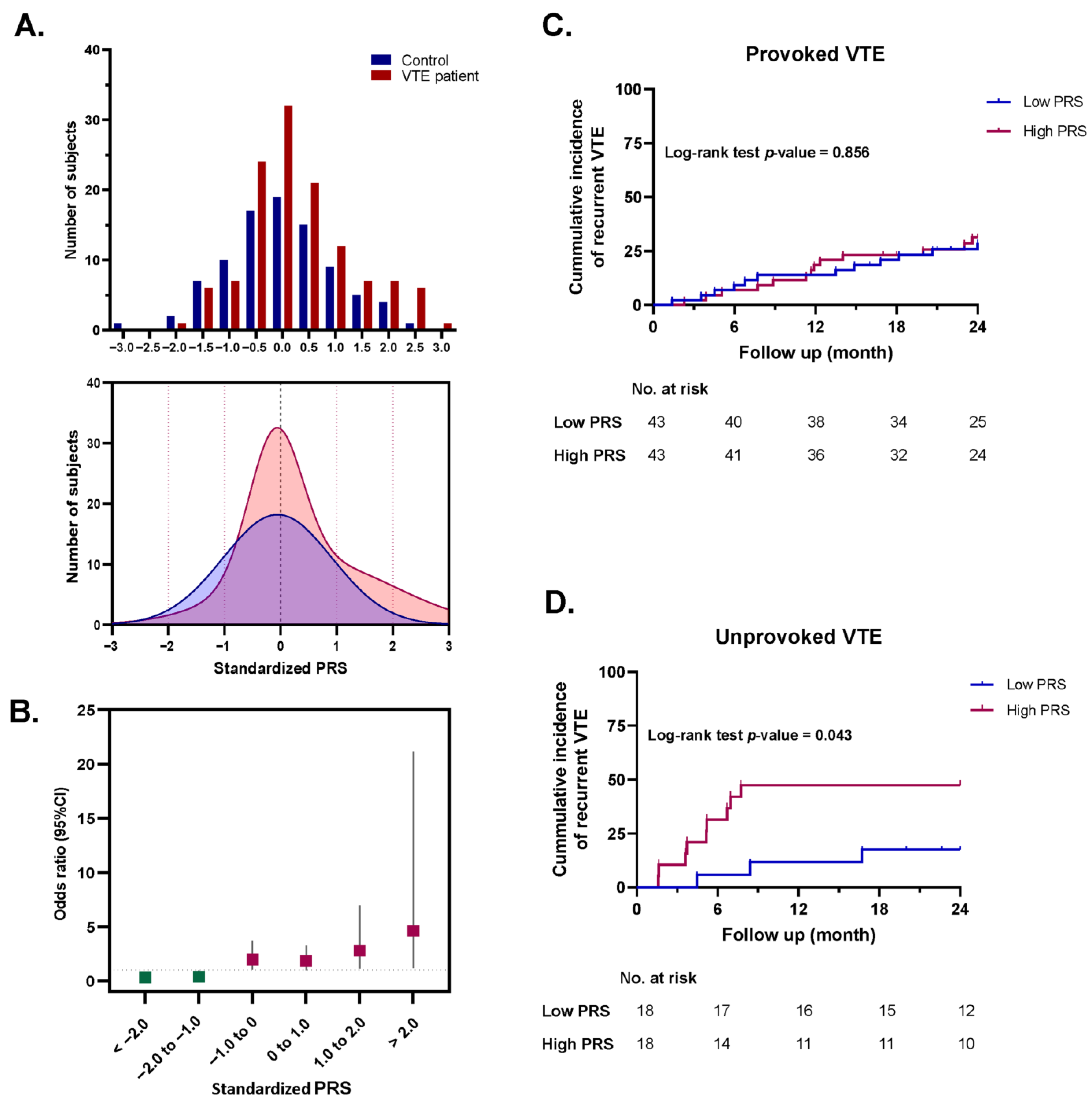
3.5. Polygenic Risk Score and Risk Prediction of VTE
3.6. Association Between the Standardized PRS and Risk of Recurrent VTE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VTE | Venous thromboembolism |
| DVT | Deep vein thrombosis |
| PE | Pulmonary embolism |
| SNPs | Single-nucleotide polymorphisms |
| MALDI-TOF | Matrix-assisted laser desorption/ionization–time of flight |
| PRS | Polygenic risk score |
| CT | Computed tomography |
| BMI | Body mass index |
| EAF | Effect allele frequency |
| PCR | Polymerase chain reaction |
| SAP | Shrimp alkaline phosphatase |
| HPLC | High-performance liquid chromatography |
| DW | Distilled water |
| OR | Odds ratio |
| SBE | Single-base extension |
| MS | Mass spectrometer |
| IQR | Interquartile range |
| AFs | Allele frequencies |
| HR | Hazard ratio |
| CI | Confidence interval |
References
- Yamashita, Y.; Morimoto, T.; Kimura, T. Venous thromboembolism: Recent advancement and future perspective. J. Cardiol. 2022, 79, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Zakai, N.A. Epidemiology and prevention of venous thromboembolism. Nat. Rev. Cardiol. 2023, 20, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.G.; Lee, J.H.; Kim, S.A.; Kim, Y.K.; Yhim, H.Y.; Hong, J.; Bang, S.M. Incidence of venous thromboembolism: The 3(rd) Korean Nationwide study. J. Korean Med. Sci. 2022, 37, e130. [Google Scholar] [CrossRef] [PubMed]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef]
- Cohen, A.T.; Agnelli, G.; Anderson, F.A.; Arcelus, J.I.; Bergqvist, D.; Brecht, J.G.; Greer, I.A.; Heit, J.A.; Hutchinson, J.L.; Kakkar, A.K.; et al. VTE Impact Assessment Group in Europe (VITAE), Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb. Haemost. 2007, 98, 756–764. [Google Scholar]
- Beckman, M.G.; Hooper, W.C.; Critchley, S.E.; Ortel, T.L. Venous thromboembolism: A public health concern. Am. J. Prev. Med. 2010, 38 (Suppl. 4.), S495–S501. [Google Scholar] [CrossRef]
- Tang, L.; Hu, Y. Ethnic diversity in the genetics of venous thromboembolism. Thromb. Haemost. 2015, 114, 901–909. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Harrington, L.B.; Kabrhel, C. Environmental and genetic risk factors associated with venous thromboembolism. Semin. Thromb. Hemost. 2016, 42, 808–820. [Google Scholar] [CrossRef]
- Zoller, B.; Svensson, P.J.; Dahlback, B.; Lind-Hallden, C.; Hallden, C.; Elf, J. Genetic risk factors for venous thromboembolism. Expert. Rev. Hematol. 2020, 13, 971–981. [Google Scholar] [CrossRef]
- Girardi, L.; Wang, T.F.; Ageno, W.; Carrier, M. Updates in the incidence, pathogenesis, and management of cancer and venous thromboembolism. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 824–831. [Google Scholar] [CrossRef]
- Larsen, T.B.; Sørensen, H.T.; Skytthe, A.; Johnsen, S.P.; Vaupel, J.W.; Christensen, K. Major genetic susceptibility for venous thromboembolism in men: A study of Danish twins. Epidemiology 2003, 14, 328–332. [Google Scholar] [CrossRef]
- Heit, J.A.; Phelps, M.A.; Ward, S.A.; Slusser, J.P.; Petterson, T.M.; De Andrade, M. Familial segregation of venous thromboembolism. J. Thromb. Haemost. 2004, 2, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, J.; Tragante, V.; Ahlberg, G.; Rand, S.A.; Jespersen, J.B.; Leinoe, E.B.; Vissing, C.R.; Trudso, L.; Jonsdottir, I.; Banasik, K.; et al. Genome-wide meta-analysis identifies 93 risk loci and enables risk prediction equivalent to monogenic forms of venous thromboembolism. Nat. Genet. 2023, 55, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M. Thrombophilia testing and venous thrombosis. N. Engl. J. Med. 2017, 377, 2298. [Google Scholar] [CrossRef]
- Morange, P.E.; Suchon, P.; Tregouet, D.A. Genetics of venous thrombosis: Update in 2015. Thromb Haemost. 2015, 114, 910–919. [Google Scholar]
- Tsai, S.H.; Chang, P.Y.; Wen, Y.H.; Lin, W.T.; Hsu, F.P.; Chen, D.P. Screening of single nucleotide polymorphisms within HLA region related to hematopoietic stem cell transplantation using MassARRAY technology. Sci. Rep. 2023, 13, 5913. [Google Scholar] [CrossRef]
- Gabriel, S.; Ziaugra, L.; Tabbaa, D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr. Protoc. Hum. Genet. 2009, 60, 2–12. [Google Scholar] [CrossRef]
- Nyasinga, J.; Kyany’a, C.; Okoth, R.; Oundo, V.; Matano, D.; Wacira, S.; Sang, W.; Musembi, S.; Musila, L. A six-member SNP assay on the iPlex MassARRAY platform provides a rapid and affordable alternative for typing major African Staphylococcus aureus types. Access Microbiol. 2019, 1, e000018. [Google Scholar] [CrossRef]
- Bakshi, D.; Nagpal, A.; Sharma, V.; Sharma, I.; Shah, R.; Sharma, B.; Bhat, A.; Verma, S.; Bhat, G.R.; Abrol, D.; et al. MassARRAY-based single nucleotide polymorphism analysis in breast cancer of north Indian population. BMC Cancer 2020, 20, 861. [Google Scholar] [CrossRef]
- Shah, R.; Sharma, V.; Bhat, A.; Singh, H.; Sharma, I.; Verma, S.; Bhat, G.R.; Sharma, B.; Bakshi, D.; Kumar, R.; et al. MassARRAY analysis of twelve cancer related SNPs in esophageal squamous cell carcinoma in J&K, India. BMC Cancer 2020, 20, 497. [Google Scholar]
- Venkatesh, S.K.; Siddaiah, A.; Padakannaya, P.; Ramachandra, N.B. Association of SNPs of DYX1C1 with developmental dyslexia in an Indian population. Psychiatr. Genet. 2014, 24, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Thibord, F.; Klarin, D.; Brody, J.A.; Chen, M.H.; Levin, M.G.; Chasman, D.I.; Goode, E.L.; Hveem, K.; Teder-Laving, M.; Martinez-Perez, A.; et al. Cross-ancestry investigation of venous thromboembolism genomic predictors. Circulation 2022, 146, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Lindström, S.; Wang, L.; Smith, E.N.; Gordon, W.; van Hylckama Vlieg, A.; de Andrade, M.; Brody, J.A.; Pattee, J.W.; Haessler, J.; Brumpton, B.M.; et al. Genomic and transcriptomic association studies identify 16 novel susceptibility loci for venous thromboembolism. Blood 2019, 134, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hou, J.; Li, W.; Chen, J.; Li, Y.; Zhang, J.; Zhou, W.; Zhang, W.; Deng, F.; Wang, Y.; et al. Construction and optimization of a polygenic risk model for venous thromboembolism in the Chinese population. J. Vasc. Surg. Venous Lymphat. Disord. 2024, 12, 101666. [Google Scholar] [CrossRef]
- Germain, M.; Chasman, D.I.; de Haan, H.; Tang, W.; Lindstrom, S.; Weng, L.C.; de Andrade, M.; de Visser, M.C.; Wiggins, K.L.; Suchon, P.; et al. Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am. J. Hum. Genet. 2015, 96, 532–542. [Google Scholar] [CrossRef]
- Padda, J.; Khalid, K.; Mohan, A.; Pokhriyal, S.; Batra, N.; Hitawala, G.; Cooper, A.C.; Jean-Charles, G. Factor V Leiden G1691A and Prothrombin gene G20210A mutations on pregnancy outcome. Cureus 2021, 13, e17185. [Google Scholar] [CrossRef]
- Ryu, J.; Ramo, J.T.; Jurgens, S.J.; Niiranen, T.; Sanna-Cherchi, S.; Bauer, K.A.; Haj, A.; Choi, S.H.; Palotie, A.; Daly, M.; et al. Thrombosis risk in single- and double-heterozygous carriers of factor V Leiden and prothrombin G20210A in FinnGen and the UK Biobank. Blood 2024, 143, 2425–2432. [Google Scholar] [CrossRef]
- Angchaisuksiri, P.; Pingsuthiwong, S.; Aryuchai, K.; Busabaratana, M.; Sura, T.; Atichartakarn, V.; Sritara, P. Prevalence of the G1691A mutation in the factor V gene (factor V Leiden) and the G20210A prothrombin gene mutation in the Thai population. Am. J. Hematol. 2000, 65, 119–122. [Google Scholar] [CrossRef]
- Arnutti, P.; Nathalang, O.; Cowawintaweewat, S.; Prayoonwiwat, W.; Choovichian, P. Factor V Leiden and prothrombin G20210A mutations in Thai patients awaiting kidney transplant. Southeast. Asian J. Trop. Med. Public. Health 2002, 33, 869–871. [Google Scholar]
- Hernandez, W.; Gamazon, E.R.; Smithberger, E.; O’Brien, T.J.; Harralson, A.F.; Tuck, M.; Barbour, A.; Kittles, R.A.; Cavallari, L.H.; Perera, M.A. Novel genetic predictors of venous thromboembolism risk in African Americans. Blood 2016, 127, 1923–1929. [Google Scholar] [CrossRef]
- Demirci, F.Y.; Dressen, A.S.; Kammerer, C.M.; Barmada, M.M.; Kao, A.H.; Ramsey-Goldman, R.; Manzi, S.; Kamboh, M.I. Functional polymorphisms of the coagulation factor II gene (F2) and susceptibility to systemic lupus erythematosus. J. Rheumatol. 2011, 38, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Limperger, V.; Kenet, G.; Kiesau, B.; Kother, M.; Schmeiser, M.; Langer, F.; Juhl, D.; Shneyder, M.; Franke, A.; Klostermeier, U.K.; et al. Role of prothrombin 19911 A>G polymorphism, blood group and male gender in patients with venous thromboembolism: Results of a German cohort study. J. Thromb. Thrombolysis 2021, 51, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Guo, T.; Yang, R.; Mei, H.; Wang, H.; Lu, X.; Yu, J.; Wang, Q.; Hu, Y. Genetic background analysis of protein C deficiency demonstrates a recurrent mutation associated with venous thrombosis in Chinese population. PLoS ONE 2012, 7, e35773. [Google Scholar] [CrossRef]
- Tsay, W.; Shen, M.C. R147W mutation of PROC gene is common in venous thrombotic patients in Taiwanese Chinese. Am. J. Hematol. 2004, 76, 8–13. [Google Scholar] [CrossRef]
- Tsuda, H.; Noguchi, K.; Oh, D.; Bereczky, Z.; Lee, L.H.; Kang, D.; Dusse, L.M.S.; das G Carvalho, M.; Morishita, E.; SSC Subcommittee on Plasma Coagulation Inhibitors of the ISTH. Racial differences in protein S Tokushima and two protein C variants as genetic risk factors for venous thromboembolism. Res. Pract. Thromb. Haemost. 2020, 4, 1295–1300. [Google Scholar] [CrossRef]
- Sirachainan, N.; Chuansumrit, A.; Sasanakul, W.; Yudhasompop, N.; Mahaklan, L.; Vaewpanich, J.; Charoenkwan, P.; Kanjanapongkul, S.; Visudtibhan, A.; Wongwerawattanakoon, P. R147W in PROC gene is a risk factor of thromboembolism in Thai children. Clin. Appl. Thromb. Hemost. 2018, 24, 263–267. [Google Scholar] [CrossRef]
- Tanratana, P.; Seanoon, K.; Payongsri, P.; Kadegasem, P.; Chuansumrit, A.; Sirachainan, N. Unraveling the molecular pathogenesis of protein C deficiency-associated VTE: Insights from protein C mutations C238G and R189W in Thai patients. Thromb. Haemost. 2025, 125, 533–544. [Google Scholar] [CrossRef]
- Goumidi, L.; Thibord, F.; Wiggins, K.L.; Li-Gao, R.; Brown, M.R.; van Hylckama Vlieg, A.; Souto, J.C.; Soria, J.M.; Ibrahim-Kosta, M.; Saut, N.; et al. Association between ABO haplotypes and the risk of venous thrombosis: Impact on disease risk estimation. Blood 2021, 137, 2394–2402. [Google Scholar] [CrossRef]
- Grunbacher, G.; Weger, W.; Marx-Neuhold, E.; Pilger, E.; Koppel, H.; Wascher, T.; Marz, W.; Renner, W. The fibrinogen gamma (FGG) 10034C>T polymorphism is associated with venous thrombosis. Thromb. Res. 2007, 121, 33–36. [Google Scholar] [CrossRef]
- Tavares, V.; Pinto, R.; Assis, J.; Pereira, D.; Medeiros, R. Dataset of GWAS-identified variants underlying venous thromboembolism susceptibility and linkage to cancer aggressiveness. Data Brief. 2020, 30, 105399. [Google Scholar] [CrossRef]
- Tavares, V.; Assis, J.; Pinto, R.; Freitas-Silva, M.; Medeiros, R. Venous thromboembolism-related genetic determinant F11 rs4253417 is a potential prognostic factor in ischaemic stroke. Mol. Cell Probes 2023, 70, 101917. [Google Scholar] [CrossRef]
- de Haan, H.G.; Bezemer, I.D.; Doggen, C.J.; Le Cessie, S.; Reitsma, P.H.; Arellano, A.R.; Tong, C.H.; Devlin, J.J.; Bare, L.A.; Rosendaal, F.R.; et al. Multiple SNP testing improves risk prediction of first venous thrombosis. Blood 2012, 120, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Scicluna, B.P.; Jim, K.K.; Falahi, F.; Qin, W.; Gurkan, B.; Malmstrom, E.; Meijer, M.T.; Butler, J.M.; Khan, H.N.; et al. HIVEP1 Is a negative regulator of NF-kappaB that inhibits systemic inflammation in sepsis. Front. Immunol. 2021, 12, 744358. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Kosta, M.; Suchon, P.; Couturaud, F.; Smadja, D.; Olaso, R.; Germain, M.; Saut, N.; Goumidi, L.; Derbois, C.; Thibord, F.; et al. Minor allele of the factor V K858R variant protects from venous thrombosis only in non-carriers of factor V Leiden mutation. Sci. Rep. 2019, 9, 3750. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Qin, L.; Li, G.; Wang, Z.; Dahlman, J.E.; Malagon-Lopez, J.; Gujja, S.; Cilfone, N.A.; Kauffman, K.J.; Sun, L.; et al. Endothelial TGF-beta signalling drives vascular inflammation and atherosclerosis. Nat. Metab. 2019, 1, 912–926. [Google Scholar] [CrossRef]
- Linnemann, B.; Weingarz, L.; Schindewolf, M.; Schwonberg, J.; Weber, A.; Herrmann, E.; Lindhoff-Last, E. Prevalence of established risk factors for venous thromboembolism according to age. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 131–139. [Google Scholar] [CrossRef]
- Leizorovicz, A.; Turpie, A.G.; Cohen, A.T.; Wong, L.; Yoo, M.C.; Dans, A.; SMART Study Group. Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis. The SMART study. J. Thromb. Haemost. 2005, 3, 28–34. [Google Scholar] [CrossRef]
- Fahrni, J.; Husmann, M.; Gretener, S.B.; Keo, H.H. Assessing the risk of recurrent venous thromboembolism--a practical approach. Vasc. Health Risk Manag. 2015, 11, 451–459. [Google Scholar]
| Characteristics | VTE Patients (n = 122) | Healthy Controls (n = 87) | |
|---|---|---|---|
| Age, year | Median (IQR) | 44.5 (32–57) | 45.0 (36–56) |
| Age < 60 yr, n (%) | 95 (77.9) | 72 (82.8) | |
| Age ≥ 60 yr, n (%) | 27 (22.1) | 15 (17.2) | |
| BMI, kg/m2 | Mean ± SD | 24.5 ± 4.0 | 24.9 ± 4.4 |
| BMI < 30.0 kg/m2, n (%) | 109 (89.3) | 77 (88.5) | |
| BMI ≥ 30.0 kg/m2, n (%) | 13 (10.7) | 10 (11.5) | |
| Sex, n (%) | |||
| Male | 67 (54.9) | 51 (58.6) | |
| Female | 55 (45.1) | 36 (41.4) | |
| Diagnosis, n (%) | |||
| DVT | 97 (79.5) | N/A | |
| PE | 16 (13.1) | N/A | |
| PE + DVT | 9 (7.4) | N/A | |
| Risk group, n (%) | |||
| Provoked VTE | 86 (70.5) | N/A | |
| Unprovoked VTE | 36 (29.5) | N/A | |
| VTE risk factors, n (%) | |||
| Antiphospholipid syndrome | 17 (13.9) | 0 (0.0) | |
| Active cancer | 11 (9.0) | 0 (0.0) | |
| Recent trauma or surgery (≤1 month) | 9 (7.4) | 0 (0.0) | |
| Protein C deficiency | 8 (6.6) | 0 (0.0) | |
| Protein S deficiency | 7 (5.7) | 0 (0.0) | |
| Oral contraceptive used | 7 (5.7) | 0 (0.0) | |
| Immobility | 3 (2.5) | 0 (0.0) | |
| Antithrombin deficiency | 1 (0.8) | 0 (0.0) | |
| Ongoing hormone therapy | 1 (0.8) | 0 (0.0) | |
| Recurrent VTE a, n (%) | |||
| Recurrence | 60 (49.2) | N/A | |
| No recurrence | 62 (50.8) | N/A | |
| Gene | rs ID | Alt/Ref | Literature OR [9,13,15,22,23,24,25] | EAF Cases/Controls | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | Adjusted OR a (95%CI) | p-Value | |||||
| PROC | rs146922325 | T/C | 6.91 | 0.16/0.09 | 1.94 (1.08–3.60) | 0.041 | 1.71 (1.05–2.79) | 0.032 |
| THBD | rs16984852 | A/C | 2.80 | 0.01/0.00 | Infinity (0.33–infinity) | 0.513 | - | - |
| F2 | rs1799963 | A/G | 1.88 | 0.00/0.00 | Infinity (0.00–infinity) | >0.999 | - | - |
| F2 | rs191945074 | T/C | 1.86 | 0.09/0.08 | 1.07 (0.54–2.21) | >0.999 | - | - |
| ABO | rs8176719 | C/- | 1.85 | 0.46/0.40 | 1.24 (0.83–1.83) | 0.317 | - | - |
| ABO | rs8176743 | T/C | 1.76 | 0.26/0.17 | 1.71 (1.07–2.80) | 0.033 | 1.60 (1.01–2.55) | 0.046 |
| FGG | rs2066865 | A/G | 1.56 | 0.52/0.36 | 1.94 (1.30–2.90) | 0.001 | 1.77 (1.18–2.66) | 0.006 |
| PAI-1 | rs2227631 | A/G | 1.55 | 0.42/0.42 | 0.99 (0.66–1.46) | >0.999 | - | - |
| APOH | rs8178847 | T/C | 1.55 | 0.02/0.05 | 0.52 (0.18–1.56) | 0.275 | - | - |
| NOS3 | rs1799983 | T/G | 1.41 | 0.14/0.14 | 1.29 (0.71–2.32) | 0.464 | - | - |
| ABO | rs2519093 | T/C | 1.40 | 0.24/0.21 | 1.22 (0.76–1.98) | 0.411 | - | - |
| ABO | rs9411377 | A/C | 1.36 | 0.00/0.00 | 0.00 (0.00–6.42) | 0.416 | - | - |
| ABO | rs687289 | A/G | 1.34 | 0.63/0.55 | 1.42 (0.97–2.10) | 0.086 | - | - |
| F11 | rs2036914 | T/C | 1.32 | 0.19/0.24 | 0.75 (0.47–1.20) | 0.275 | - | - |
| MTHFR | rs1801133 | T/C | 1.30 | 0.09/0.11 | 0.80 (0.42–1.51) | 0.617 | - | - |
| F11 | rs2289252 | T/C | 1.26 | 0.55/0.48 | 1.34 (0.90–1.99) | 0.165 | - | - |
| ABO | rs8176749 | T/C | 1.23 | 0.24/0.18 | 1.43 (0.89–2.31) | 0.151 | - | - |
| F11 | rs4253417 | C/T | 1.22 | 0.54/0.43 | 1.53 (1.02–2.20) | 0.038 | 1.58 (1.07–2.35) | 0.023 |
| SERPINC1 | rs2227589 | T/C | 1.20 | 0.33/0.37 | 1.17 (0.77–1.76) | 0.467 | - | - |
| HIVEP1 | rs169713 | C/T | 1.20 | 0.28/0.19 | 1.69 (1.05–2.66) | 0.037 | 1.61 (1.02–2.53) | 0.041 |
| TSPAN15 | rs78707713 | T/C | 1.20 | 0.97/0.95 | 1.61 (0.66–4.18) | 0.452 | - | - |
| FGG | rs2066864 | A/G | 1.20 | 0.80/0.74 | 1.47 (0.94–2.30) | 0.122 | - | - |
| KNG1 | rs710446 | G/A | 1.19 | 0.36/0.39 | 0.86 (0.58–1.29) | 0.537 | - | - |
| F2 | rs3136516 | G/A | 1.19 | 0.86/0.82 | 1.39 (0.82–2.34) | 0.270 | - | - |
| PROS1 | rs6795524 | G/A | 1.19 | 0.04/0.03 | 1.20 (0.44–3.31) | 0.801 | - | - |
| CYP4V2 | rs13146272 | A/C | 1.17 | 0.54/0.56 | 0.93 (0.64–1.38) | 0.765 | - | - |
| VWF | rs1063856 | C/T | 1.16 | 0.07/0.08 | 0.91 (0.45–1.93) | 0.853 | - | - |
| GP6 | rs1613662 | G/A | 1.15 | 0.09/0.07 | 1.29 (0.65–2.62) | 0.596 | - | - |
| F5 | rs4524 | C/T | 0.88 | 0.09/0.22 | 0.34 (0.19–0.60) | < 0.001 | 0.42 (0.24–0.72) | 0.002 |
| SERPINC1 | rs2227624 | A/T | 0.99 | 0.00/0.00 | Infinity (0.00–infinity) | > 0.999 | - | - |
| PDIA3 | rs139974673 | T/C | 0.98 | 1.00/1.00 | Infinity (0.00–infinity) | > 0.999 | - | - |
| PPP1R3B | rs34290760 | C/G | 0.96 | 0.99/0.99 | 0.70 (0.05–6.06) | > 0.999 | - | - |
| SYK | rs10993706 | A/G | 0.93 | 0.23/0.17 | 1.40 (0.85–2.34) | 0.215 | - | - |
| TGFB2 | rs57615042 | A/G | 0.89 | 0.69/0.78 | 0.62 (0.40–0.98) | 0.045 | 0.56 (0.35–0.90) | 0.017 |
| MTOR | rs12097293 | A/G | 0.77 | 0.93/0.91 | 1.18 (0.58–2.48) | 0.714 | - | - |
| KNG1 | rs5030062 | A/C | 0.63 | 0.63/0.61 | 1.10 (0.74–1.63) | 0.683 | - | - |
| ZFPM1 | rs55823018 | T/C | 0.31 | 0.58/0.57 | 1.04 (0.71–1.55) | 0.920 | - | - |
| XXYLT1 | rs56324901 | A/G | 0.20 | 0.45/0.48 | 0.88 (0.59–1.31) | 0.551 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apipongrat, D.; Laoruangroj, C.; Nathalang, O.; Arnutti, P.; Theeraapisakkun, M.; Chantkran, W. Development of a MassARRAY Genotyping Platform and Its Clinical Application for Venous Thromboembolism Risk Assessment in Thai Patients. Med. Sci. 2025, 13, 282. https://doi.org/10.3390/medsci13040282
Apipongrat D, Laoruangroj C, Nathalang O, Arnutti P, Theeraapisakkun M, Chantkran W. Development of a MassARRAY Genotyping Platform and Its Clinical Application for Venous Thromboembolism Risk Assessment in Thai Patients. Medical Sciences. 2025; 13(4):282. https://doi.org/10.3390/medsci13040282
Chicago/Turabian StyleApipongrat, Dollapak, Chonlada Laoruangroj, Oytip Nathalang, Pasra Arnutti, Montalee Theeraapisakkun, and Wittawat Chantkran. 2025. "Development of a MassARRAY Genotyping Platform and Its Clinical Application for Venous Thromboembolism Risk Assessment in Thai Patients" Medical Sciences 13, no. 4: 282. https://doi.org/10.3390/medsci13040282
APA StyleApipongrat, D., Laoruangroj, C., Nathalang, O., Arnutti, P., Theeraapisakkun, M., & Chantkran, W. (2025). Development of a MassARRAY Genotyping Platform and Its Clinical Application for Venous Thromboembolism Risk Assessment in Thai Patients. Medical Sciences, 13(4), 282. https://doi.org/10.3390/medsci13040282






