The Role of Angiographic Imaging in the Treatment of Spinal Vascular Malformations
Abstract
1. Introduction
2. Literature Selection
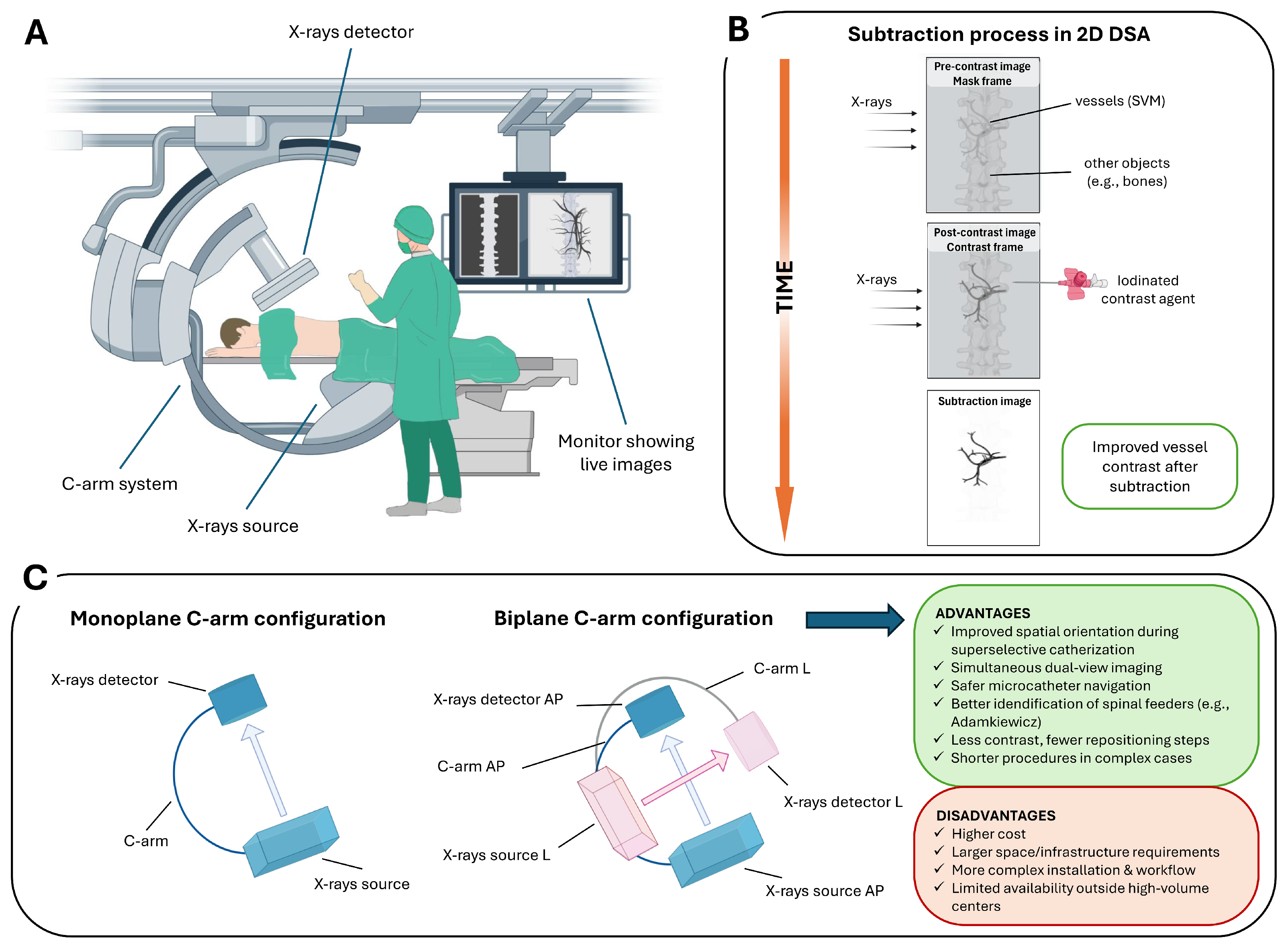
3. Intraoperative Role of 2D DSA
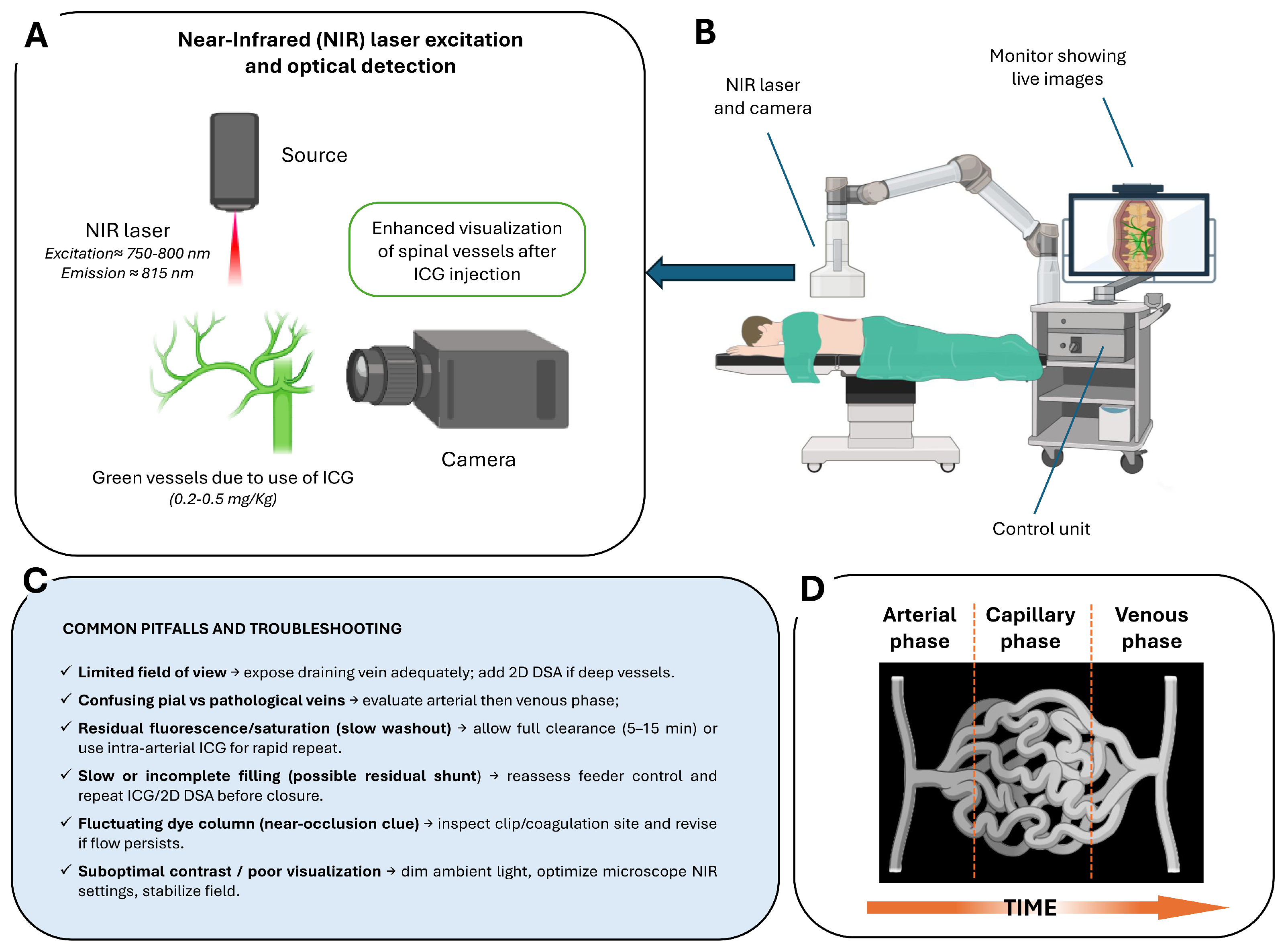
4. Intraoperative Role of ICG–VAG
| SVM Subtype | Key Pathophysiology | Intraoperative Imaging Strategy | Operative Goal and Rationale | Objective Endpoint |
|---|---|---|---|---|
| Dural AVF (Type I) | Intradural venous reflux → venous hypertensive myelopathy; low-flow dural shunt at the nerve root sleeve | Full-spine 2D DSA (gold standard); adjunct CE–trMRA for pre-op level localization; optional feeder coil marking during diagnostic angiography for intra-op guidance | Definitive microsurgical disconnection of the intradural draining vein. Endovascular treatment (EVT) only in favourable anatomy (single feeder, direct shunt) when liquid embolic can penetrate the fistula and proximal outflow vein; avoid proximal-only coil/particulate embolization (high recurrence). Identify AKA; consider IONM | No early venous drainage on final 2D DSA and absent early ICG venous opacification; if EVT used, embolic cast extends into the proximal draining vein; preserved physiologic venous outflow [24,59] |
| Perimedullary (Pial) AVF (Type IV) | Direct pial artery–to–vein shunt; often high-flow; risk to ASA/PSA and cord perfusion; possible hemorrhage | 2D DSA for feeder and ASA/PSA mapping; adjunct CE–trMRA for level targeting; mandatory ICG–VAG intra-op to verify closure and arterial patency | Eliminate pial shunt while preserving ASA/PSA supply. Low-flow: microsurgical disconnection. High-flow/multiple feeders: primary EVT with venous penetration; staged EVT + surgery if incomplete | No residual shunt with venous penetration; preserved ASA/PSA perfusion; no early venous filling on ICG and 2D DSA; angiographic cure at follow-up [60,61] |
| Intramedullary Glomus AVM (Type II) | Compact intramedullary nidus, often fed by ASA/PSA branches; may present with hemorrhage, progressive myelopathy, or rarely radiculopathy from prenidal aneurysm compression | High-resolution spinal 2D DSA mandatory; MRI/MRA to identify intramedullary flow voids and associated aneurysms; intra-op ICG useful only for superficial components | Goal is safe shunt and pressure reduction while preserving cord perfusion. Endovascular embolization preferred when feasible, especially for associated aneurysms; staged or partial embolization when ASA supply places cord at risk; microsurgical resection reserved for select cases with accessible components or incomplete EVT | Reduction or obliteration of nidus/aneurysm with preserved neurological function; complete angiographic cure when safely achievable; durable symptom relief and prevention of rebleeding [62,63] |
| Juvenile / Diffuse AVM (Type III) | Extensive multi-compartment nidus; aggressive hemodynamics | 2D DSA for global flow pattern; ICG–VAG limited to exposed draining veins | Flow reduction and decompression rather than complete cure; staged EVT + surgery as needed | Controlled shunt volume; venous hypertension relieved; neurological stability |
| Extradural AVF (Type V) | Extradural arteriovenous shunt with epidural venous pouch; may exhibit intradural venous reflux causing venous hypertension | 2D DSA to evaluate extradural pouch and confirm/exclude intradural reflux; adjunct intraoperative ICG–VAG if concern for intradural drainage | If intradural venous reflux: target disconnection of intradural draining vein and eliminate venous hypertension; if purely extradural: occlude epidural pouch and arterial feeders endovascularly, preserving spinal arterial supply | Complete occlusion of extradural shunt and epidural venous pouch with durable elimination of intradural reflux; intact spinal cord arterial perfusion; no residual shunt on final 2D DSA and no recurrence on follow-up MRI/MRA [64,65] |
5. Intraoperative Role of Other Angiography Modalities
| Modality | Use Case | Spatial/Temporal Resolution | Quantitative Potential | Radiation/Contrast | When to Choose |
|---|---|---|---|---|---|
| 2D DSA | Intraoperative guidance and confirmation of feeder and venous drainage control. | High spatial/very high temporal resolution (ms scale). | High—dynamic flow and embolic progression assessment. | Radiation + iodinated contrast; arterial catheterization. | Gold standard for intraoperative decision-making, complex fistulas, embolization monitoring, deep lesions [4,75] |
| 3D RA | Pre- or intraoperative 3D vascular mapping and navigation. | Very high spatial/limited temporal. | Moderate—excellent geometry, limited dynamic insight. | Radiation + iodinated contrast. | When detailed 3D vascular anatomy is required (e.g., multi-segment feeders, surgical planning, spinal level uncertainty) [76,77,78]. |
| CTA | Preoperative anatomical survey and vessel localization. | High spatial/no temporal resolution. | Low—structural information only. | Radiation + iodinated contrast. | When MRI/MRA is unavailable/contraindicated or as rapid whole-spine vascular overview with bone context [79,80,81]. |
| ICG–VAG | Intraoperative real-time visualization of exposed vessels. | High spatial/high temporal (seconds). | Low—qualitative surface flow. | No radiation; i.v. ICG. | When direct visualization of intraoperative arterialization/venous drainage is needed; superficial fistulas [52,53,54]. |
| MRA | Non-invasive assessment and localization of suspected SVM; evaluation of venous congestion and cord signal abnormalities. | High spatial/moderate-to-high temporal depending on sequence (seconds). | Moderate—dynamic enhancement, time-of-arrival mapping in advanced protocols. | No radiation; gadolinium contrast may be used depending on the sequence. | When screening for SVMs, for follow-up, or when CTA/2D DSA is inconclusive, first-line non-invasive imaging in suspected venous congestive myelopathy [80,81,82,83]. |
6. Post-Treatment and Follow-Up Role of Angiography
7. Future Directions in Angiographic Imaging
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D DSA | Two-Dimensional Digital Subtraction Angiography |
| 3D RA | Three-Dimensional Rotational Angiography |
| AI | Artificial Intelligence |
| AKA | Artery of Adamkiewicz |
| ASA | Anterior Spinal Artery |
| AVF | Arteriovenous Fistula |
| AVM | Arteriovenous Malformation |
| CE–MRA | Contrast-Enhanced Magnetic Resonance Angiography |
| CE–trMRA | Contrast-Enhanced time resolved Magnetic Resonance Angiography |
| CTA | Computed Tomography Angiography |
| EVT | Endovascular Treatment |
| FL–VAG | Fluorescein Videoangiography |
| i.a. | Intra-arterial |
| ICG | Indocyanine Green |
| ICG–VAG | Indocyanine Green Videoangiography |
| i.v. | Intravenous |
| IONM | Intraoperative Neurophysiological Monitoring |
| MRA | Magnetic Resonance Angiography |
| MRI | Magnetic Resonance Imaging |
| NIR | Near-Infrared |
| PSA | Posterior Spinal Artery |
| SVM | Spinal Vascular Malformation |
| TOF–MRA | Time of Flight Magnetic Resonance Angiography |
| VM | Vascular Malformation |
References
- Schuette, A.J.; Cawley, C.M.; Barrow, D.L. Indocyanine Green Videoangiography in the Management of Dural Arteriovenous Fistulae. Neurosurgery 2010, 67, 658–662. [Google Scholar] [CrossRef]
- Flores, B.C.; Klinger, D.R.; White, J.A.; Batjer, H.H. Spinal vascular malformations: Treatment strategies and outcome. Neurosurg. Rev. 2017, 40, 15–28. [Google Scholar] [CrossRef]
- Ho, W.M.; Gorke, A.; Petr, O.; Thome, C. Treatment strategies of spinal arteriovenous fistulas and malformations: Timing matters. J. Neurosurg. Sci. 2018, 62, 178–186. [Google Scholar] [CrossRef]
- Eddleman, C.S.; Jeong, H.; Cashen, T.A.; Walker, M.; Bendok, B.R.; Batjer, H.H.; Carroll, T.J. Advanced noninvasive imaging of spinal vascular malformations. Neurosurg. Focus 2009, 26, E9. [Google Scholar] [CrossRef]
- Bemporad, J.A.; Sze, G.S. MR imaging of spinal cord vascular malformations with an emphasis on the cervical spine. Magn. Reson. Imaging Clin. N. Am. 2000, 8, 581–596. [Google Scholar] [CrossRef]
- Gailloud, P. Spinal vascular malformations: Angiographic evaluation and endovascular management. Handb. Clin. Neurol. 2021, 176, 267–304. [Google Scholar] [CrossRef] [PubMed]
- Talenti, G.; Vitale, G.; Cester, G.; Della Puppa, A.; Faggin, R.; Causin, F. Rare association between spinal dural arteriovenous fistulas and dysraphisms: Report of two cases and review of the literature with a focus on pitfalls in diagnosis and treatment. Interv. Neuroradiol. 2017, 23, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Zyck, S.; Davidson, C.L.; Sampath, R. Arteriovenous Malformations of the Central Nervous System. StatPearls, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531479/ (accessed on 4 March 2024).
- da Costa, L.; Dehdashti, A.R.; terBrugge, K.G. Spinal cord vascular shunts: Spinal cord vascular malformations and dural arteriovenous fistulas. Neurosurg. Focus 2009, 26, E6. [Google Scholar] [CrossRef] [PubMed]
- Takai, K. Spinal Arteriovenous Shunts: Angioarchitecture and Historical Changes in Classification. Neurol. Med. Chir. 2017, 57, 356–365. [Google Scholar] [CrossRef]
- Krings, T.; Mull, M.; Gilsbach, J.M.; Thron, A. Spinal vascular malformations. Eur. Radiol. 2005, 15, 267–278. [Google Scholar] [CrossRef]
- Udelhoven, A.; Kettner, M.; Reith, W. Spinal arteriovenous malformations. Radiologie 2022, 62, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.H.; Barth, A.; Guzman, R.; Remonda, L.; El-Koussy, M.; Seiler, R.W.; Widmer, H.R.; Schroth, G. Endovascular and surgical treatment of spinal dural arteriovenous fistulas. Neuroradiology 2008, 50, 869–876. [Google Scholar] [CrossRef]
- Kiyosue, H.; Tanoue, S.; Okahara, M.; Hori, Y.; Kashiwagi, J.; Mori, H. Spinal ventral epidural arteriovenous fistulas of the lumbar spine: Angioarchitecture and endovascular treatment. Neuroradiology 2013, 55, 327–336. [Google Scholar] [CrossRef]
- Castillo, M. Digital Subtraction Angiography (DSA): Basic Principles. In Vascular Imaging of the Central Nervous System: Physical Principles, Clinical Applications, and Emerging Techniques; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Crone-Münzebrock, W.; Baake, S.; Thoma, G.; Müller, P.; Rehder, U. Comparison of computed tomography and digital subtraction angiography for preoperative evaluation of soft-tissue tumors of the limbs. Arch. Orthop. Trauma. Surg. 1985, 104, 319–324. [Google Scholar] [CrossRef]
- Dardik, H.; Miller, N.; Adler, J.; Ganti, S.R.; Myers, D.; Greweldinger, J.; Ibrahim, I.M.; Sussman, B.; Kahn, M. Primary and adjunctive intra-arterial digital subtraction arteriography of the lower extremities. J. Vasc. Surg. 1986, 3, 599–604. [Google Scholar] [CrossRef]
- Hertzer, N.R.; Flanagan, R.A.; O’Hara, P.J.; Beven, E.G. Surgical Versus Nonoperative Treatment of Symptomatic Carotid Stenosis 211 Patients Documented by Intravenous Angiography. Ann. Surg. 1986, 204, 154–162. [Google Scholar] [CrossRef]
- Pooley, R.A.; McKinney, J.M.; Miller, D.A. The AAPM/RSNA Physics Tutorial for Residents: Digital Fluoroscopy. RadioGraphics 2001, 21, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Crummy, A.B.; Turski, P.A.; Strother, C.M. The History of Digital Subtraction Angiography. J. Vasc. Interv. Radiol. 2018, 29, 1138–1141. [Google Scholar] [CrossRef]
- Manji, F.; Wang, J.; Norman, G.; Wang, Z.; Koff, D. Comparison of dual energy subtraction chest radiography and traditional chest X-rays in the detection of pulmonary nodules. Quant. Imaging Med. Surg. 2016, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yeh, B.M.; FitzGerald, P.F.; Edic, P.M.; Lambert, J.W.; Colborn, R.E.; Marino, M.E.; Evans, P.M.; Roberts, J.C.; Wang, Z.J.; Wong, M.J.; et al. Opportunities for new CT contrast agents to maximize the diagnostic potential of emerging spectral CT technologies. Adv. Drug Deliv. Rev. 2017, 113, 201–222. [Google Scholar] [CrossRef]
- Guthaner, D.F.; Brody, W.R.; Lewis, B.D.; Keyes, G.S.; Belanger, B.F. Clinical Application of Hybrid Subtraction Digital Angiography: Preliminary Results. Cardiovasc. Interv. Radiol. 1983, 6, 290–294. [Google Scholar] [CrossRef]
- Brown, P.A.; Zomorodi, A.R.; Gonzalez, L.F. Endovascular management of spinal dural arteriovenous fistulas. In Arteriovenous and Cavernous Malformations; Elsevier: Amsterdam, The Netherlands, 2017; pp. 199–213. [Google Scholar] [CrossRef]
- Gemmete, J.J.; Pandey, A.S.; Kasten, S.J.; Chaudhary, N. Endovascular Methods for the Treatment of Vascular Anomalies. Neuroimaging Clin. N. Am. 2013, 23, 703–728. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, J.J.; Williamson, E.E. Clinical Pharmacology, Uses, and Adverse Reactions of Iodinated Contrast Agents: A Primer for the Non-radiologist. Mayo Clin. Proc. 2012, 87, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jiang, X.; Hibberd, M.; Sampedro, A.; Rautenbach, J. Estimating the rate of acute adverse reactions to non-ionic low-osmolar contrast media: A systematic review and meta-analysis. Eur. Radiol. 2025, 35, 6240–6249. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, M.V.; Abid, A.; Matheus, M.G. Current Radiographic Iodinated Contrast Agents. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 697–704. [Google Scholar] [CrossRef]
- Kim, K.R. Percutaneous Sclerotherapy of Venous Malformations. Tech. Vasc. Interv. Radiol. 2024, 27, 100960. [Google Scholar] [CrossRef]
- Adler, M.; Mayo, A.; Zhou, X.; Franklin, R.A.; Meizlish, M.L.; Medzhitov, R.; Kallenberger, S.M.; Alon, U. Principles of Cell Circuits for Tissue Repair and Fibrosis. iScience 2020, 23, 100841. [Google Scholar] [CrossRef]
- Prasad, R.; Marotrao, P.S.; Sheorain, V.S.; Gamanagatti, S. Safety and Efficacy of Lipiodol and N-Butyl Cyanoacrylate (N-BCA) Combination for Vascular Embolization. J. Clin. Interv. Radiol. ISVIR 2024, 8, 149–155. [Google Scholar] [CrossRef]
- Lopez, O.; Chevallier, O.; Guillen, K.; Comby, P.O.; Pellegrinelli, J.; Tinel, C.; Falvo, N.; Midulla, M.; Mourey, E.; Loffroy, R. Selective Arterial Embolization with N-Butyl Cyanoacrylate Prior to CT-Guided Percutaneous Cryoablation of Kidney Malignancies: A Single-Center Experience. J. Clin. Med. 2021, 10, 4986. [Google Scholar] [CrossRef]
- Kojima, T.; Maeda, T.; Ito, Y.; Kikuta, H.; Fujii, M. Onyx Liquid Embolic Agent: Basic Knowledge for Its Use in Interventional Neuroradiology. J. Neuroendovascular Ther. 2025, 19, 2024-0073. [Google Scholar] [CrossRef]
- Vollherbst, D.F.; Chapot, R.; Bendszus, M.; Möhlenbruch, M.A. Glue, Onyx, Squid or PHIL? Liquid Embolic Agents for the Embolization of Cerebral Arteriovenous Malformations and Dural Arteriovenous Fistulas. Clin. Neuroradiol. 2022, 32, 25–38. [Google Scholar] [CrossRef]
- Ohlsson, M.; Consoli, A.; DiMaria, F.; Sgreccia, A.; Rodesch, G. Natural history and management of spinal cord arteriovenous shunts in pregnancy: A monocentric series of 10 consecutive cases with emphasis on endovascular treatment. J. Neuroradiol. 2022, 49, 401–408. [Google Scholar] [CrossRef]
- Opitz, M.; Zensen, S.; Bos, D.; Li, Y.; Styczen, H.; Wetter, A.; Guberina, N.; Jabbarli, R.; Sure, U.; Forsting, M.; et al. Radiation exposure in the endovascular therapy of cranial and spinal dural arteriovenous fistula in the last decade: A retrospective, single-center observational study. Neuroradiology 2021, 64, 587–595. [Google Scholar] [CrossRef]
- Amarouche, M.; Hart, J.; Siddiqui, A.; Hampton, T.; Walsh, D. Time-Resolved Contrast-Enhanced MR Angiography of Spinal Vascular Malformations. Am. J. Neuroradiol. 2015, 36, 417–422. [Google Scholar] [CrossRef] [PubMed]
- McGraw, J.K. Interventional Radiology of the Spine: Image-Guided Pain Therapy, 1st ed.; Humana Press: Totowa, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Gao, S.J.; Zhang, M.W.; Liu, X.P.; Zhu, Y.S.; Liu, J.H.; Wang, Z.H.; Zang, P.Z.; Shi, Q.; Wang, Q.; Liang, C.S.; et al. The clinical application studies of CT spinal angiography with 64-detector row spiral CT in diagnosing spinal vascular malformations. Eur. J. Radiol. 2009, 71, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Boni, L.; David, G.; Mangano, A.; Dionigi, G.; Rausei, S.; Spampatti, S.; Cassinotti, E.; Fingerhut, A. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg. Endosc. 2015, 29, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A. Indocyanine Green Angiography: A Perspective on Use in the Clinical Setting. Am. J. Ophthalmol. 2011, 151, 745–751.e1. [Google Scholar] [CrossRef]
- Flower, R.W. Injection technique for indocyanine green and sodium fluorescein dye angiography of the eye. Investig. Ophthalmol. 1973, 12, 881–895. [Google Scholar]
- Flower, R.W.; Hochheimer, B.F. Indocyanine green dye fluorescence and infrared absorption choroidal angiography performed simultaneously with fluorescein angiography. Johns Hopkins Med. J. 1976, 138, 33–42. [Google Scholar]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef]
- Norat, P.; Soldozy, S.; Elsarrag, M.; Sokolowski, J.; Yaǧmurlu, K.; Park, M.S.; Tvrdik, P.; Kalani, M.Y.S. Application of Indocyanine Green Videoangiography in Aneurysm Surgery: Evidence, Techniques, Practical Tips. Front. Surg. 2019, 6, 34. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Caglar, Y.S.; Ozdemir, M.; Kahilogullari, G.; Bozkurt, M.; Attar, A. Management of Spinal Arteriovenous Fistulae with Intraarterial Indocyanine Green Angiography: A Case Report. Turk. Neurosurg. 2018, 28, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lindgren, A.; Ahmed, S.U.; Radovanovic, I.; Krings, T.; Andrade-Barazarte, H. Intraoperative intraarterial indocyanine green video-angiography for disconnection of a perimedullary arteriovenous fistula: Illustrative case. J. Neurosurg. Case Lessons 2023, 6, CASE23405. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, S.; Yamazaki, K.; Tokairin, K.; Osanai, T.; Shindo, T.; Seki, T.; Fujimura, M. Intraoperative Identification of the Shunt Point of Spinal Arteriovenous Malformations by a Selective Arterial Injection of Saline to Subtract Signals of Indocyanine Green. World Neurosurg. 2021, 151, 132–137. [Google Scholar] [CrossRef]
- Klingler, J.H.; Gizaw, C.; Blaß, B.I.; Hohenhaus, R.; Neidert, N.; Neumann-Haefelin, E.; Kotsis, F.; Grauvogel, J.; Scheiwe, C.; Beck, J. Intraoperative indocyanine green (ICG) videoangiography in spinal hemangioblastoma surgery: Helpful tool or unnecessary? Clin. Neurol. Neurosurg. 2024, 248, 108661. [Google Scholar] [CrossRef]
- Koyalmantham, V.; Kale, S.S.; Devarajan, L.J.; Phalak, M.; Chandra, P.S.; Suri, A.; Kumar, R.; Tandon, V. Patient Outcomes Following Obliteration of Spinal Dural Arteriovenous Fistula and the Role of Indocyanine Green Angiography Videoangiography (ICG-VA) During Surgery. Neurol. India 2020, 68, 118. [Google Scholar] [CrossRef]
- Foster, C.H.; Morone, P.J.; Tomlinson, S.B.; Cohen-Gadol, A.A. Application of Indocyanine Green During Arteriovenous Malformation Surgery: Evidence, Techniques, and Practical Pearls. Front. Surg. 2019, 6, 70. [Google Scholar] [CrossRef]
- Walsh, D.C.; Zebian, B.; Tolias, C.M.; Gullan, R.W. Intraoperative indocyanine green video-angiography as an aid to the microsurgical treatment of spinal vascular malformations. Br. J. Neurosurg. 2014, 28, 259–266. [Google Scholar] [CrossRef]
- Jing, L.; Su, W.; Guo, Y.; Sun, Z.; Wang, J.; Wang, G. Microsurgical treatment and outcomes of spinal arteriovenous lesions: Learned from consecutive series of 105 lesions. J. Clin. Neurosci. 2017, 46, 141–147. [Google Scholar] [CrossRef]
- Sun, L.; Ren, J.; Wang, L.; Li, J.; He, C.; Ye, M.; Li, G.; Zhang, H. Preservation of Coexisting Normal Superior Petrosal Vein in the Microsurgical Treatment of Superior Petrosal Sinus Dural Arteriovenous Fistulas Assisted by Indocyanine Green Video Angiography. World Neurosurg. 2020, 141, e836–e843. [Google Scholar] [CrossRef] [PubMed]
- Raabe, A.; Fichtner, J.; Gralla, J. Advanced intraoperative imaging: Gold standard in brain and spine surgery? Clin. Transl. Neurosci. 2017, 1, 2514183X17718312. [Google Scholar] [CrossRef]
- Thorsteinsdottir, J.; Siller, S.; Dorn, F.; Briegel, J.; Tonn, J.C.; Schichor, C. Use of a New Indocyanine Green Pooling Technique for Improved Visualization of Spinal Dural AV Fistula: A Single-Center Case Series. World Neurosurg. 2019, 125, e67–e73. [Google Scholar] [CrossRef]
- Ng, Y.P.; King, N.K.; Wan, K.R.; Wang, E.; Ng, I. Uses and limitations of indocyanine green videoangiography for flow analysis in arteriovenous malformation surgery. J. Clin. Neurosci. 2013, 20, 224–232. [Google Scholar] [CrossRef]
- Fox, S.; Hnenny, L.; Ahmed, U.; Meguro, K.; Kelly, M.E. Spinal dural arteriovenous fistula: A case series and review of imaging findings. Spinal Cord Ser. Cases 2017, 3, 17024. [Google Scholar] [CrossRef]
- Takai, K.; Kurita, H.; Hara, T.; Kawai, K.; Taniguchi, M. Influence of indocyanine green angiography on microsurgical treatment of spinal perimedullary arteriovenous fistulas. Neurosurg. Focus 2016, 40, E10. [Google Scholar] [CrossRef]
- Su, I.C.; terBrugge, K.G.; Willinsky, R.A.; Krings, T. Factors determining the success of endovascular treatments among patients with spinal dural arteriovenous fistulas. Neuroradiology 2013, 55, 1389–1395. [Google Scholar] [CrossRef]
- Daou, B.; Atallah, E.; Al-Saiegh, F.; Alkhalili, K.; Tjoumakaris, S.; Rosenwasser, R.H.; Jabbour, P. Spinal Glomus Arteriovenous Malformation Manifesting with a Subarachnoid Hemorrhage. World Neurosurg. 2017, 98, 874.e1–874.e6. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Kiyosue, H.; Ide, S.; Onishi, K.; Kubo, T.; Tokuyama, K. Spinal intraosseous arteriovenous fistulas with perimedullary drainage associated with vertebral compression fracture: Illustrative case. J. Neurosurg. Case Lessons 2022, 4, CASE22184. [Google Scholar] [CrossRef]
- Takai, K.; Taniguchi, M. Comparative Analysis of Spinal Extradural Arteriovenous Fistulas with or Without Intradural Venous Drainage: A Systematic Literature Review. Neurosurg. Focus 2012, 32, E8. [Google Scholar] [CrossRef]
- Takei, J.; Tochigi, S.; Arai, M.; Tanaka, T.; Kajiwara, I.; Hatano, K.; Ichinose, D.; Sakamoto, H.; Hasegawa, Y.; Ishibashi, T.; et al. Spinal Extradural Arteriovenous Fistula with Cowden Syndrome: A Case Report and Literature Review Regarding Pathogenesis and Therapeutic Strategy. NMC Case Rep. J. 2018, 5, 83–85. [Google Scholar] [CrossRef]
- Ito, A.; Endo, T.; Inoue, T.; Endo, H.; Sato, K.; Tominaga, T. Use of Indocyanine Green Fluorescence Endoscopy to Treat Concurrent Perimedullary and Dural Arteriovenous Fistulas in the Cervical Spine. World Neurosurg. 2017, 101, 814.e1–814.e6. [Google Scholar] [CrossRef]
- Mansour, A.; Endo, T.; Inoue, T.; Sato, K.; Endo, H.; Fujimura, M.; Tominaga, T. Clipping of an anterior spinal artery aneurysm using an endoscopic fluorescence imaging system for craniocervical junction epidural arteriovenous fistula: Technical note. J. Neurosurg. Spine 2019, 31, 279–284. [Google Scholar] [CrossRef]
- Fukuda, N.; Yagi, T.; Kanemaru, K.; Yoshioka, H.; Hashimoto, K.; Senbokuya, N.; Ogiwara, M.; Kinouchi, H. Anterior Approach Combined with Endoscopic Fluorescence Video Angiography for a Cervical Perimedullary Arteriovenous Fistula. World Neurosurg. 2020, 138, 269–273. [Google Scholar] [CrossRef]
- Horiuchi, R.; Kanemaru, K.; Yoshioka, H.; Hashimoto, K.; Murayama, H.; Yagi, T.; Ogiwara, M.; Kinouchi, H. Endoscope-Integrated Fluorescence Video Angiography for the Surgery of Ventrally Located Perimedullary Arteriovenous Fistula at Craniocervical Junction. World Neurosurg. 2020, 137, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Yu, J. Embolization of an epidural arteriovenous fistula of the sacral nerve root with a neural tube defect: A case report. Int. J. Surg. Case Rep. 2024, 123, 110238. [Google Scholar] [CrossRef] [PubMed]
- Simal Julián, J.A.; Miranda Lloret, P.; López González, A.; Evangelista Zamora, R.; Botella Asunción, C. Indocyanine green videoangiography “in negative”: Definition and usefulness in spinal dural arteriovenous fistulae. Eur. Spine J. 2013, 22, 471–477. [Google Scholar] [CrossRef]
- Simal Julián, J.A.; Miranda Lloret, P.; Sanromán Álvarez, P.; Pérez de San Román, L.; Beltrán Giner, A.; Botella Asunción, C. Indocyanine Green Videoangiography in Negative: Spinal Dural Arteriovenous Fistula. Glob. Spine J. 2015, 5, e5–e6. [Google Scholar] [CrossRef]
- Koyanagi, I.; Chiba, Y.; Imamura, H.; Osanai, T. Intradural lumbar radicular arteriovenous malformation mimicking perimedullary arteriovenous malformation of the conus medullaris: Illustrative case. J. Neurosurg. Case Lessons 2021, 2, CASE21551. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Horie, N.; Morofuji, Y.; Fukuda, S.; Yamaguchi, S.; Izumo, T. Intraoperative Angiography Using Portable Fluoroscopy Unit in the Treatment of Vascular Malformation. Neurol. Med.-Chir. 2015, 55, 505–509. [Google Scholar] [CrossRef]
- Da Ros, V.; Picchi, E.; Ferrazzoli, V.; Schirinzi, T.; Sabuzi, F.; Grillo, P.; Muto, M.; Garaci, F.; Muto, M.; Di Giuliano, F. Spinal vascular lesions: Anatomy, imaging techniques and treatment. Eur. J. Radiol. Open 2021, 8, 100369. [Google Scholar] [CrossRef]
- Ropper, A.E.; Lin, N.; Gross, B.A.; Zarzour, H.K.; Thiex, C.; Chi, J.H. Rotational angiography for diagnosis and surgical planning in the management of spinal vascular lesions. Neurosurg. Focus 2012, 32, E6. [Google Scholar] [CrossRef]
- Ozpeynirci, Y.; Schmitz, B.; Schick, M.; Konig, R. Role of Three-Dimensional Rotational Angiography in the Treatment of Spinal Dural Arteriovenous Fistulas. Cureus 2017, 9, e1932. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, C.G.; Liu, P.; Zhang, H.; Liu, Z.J.; Liu, B. Three-dimensional rotational angiography for the treatment of spinal cord vascular malformations. Surg. Neurol. 2008, 69, 369–373. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, Z.; Liang, K.; Chen, M.; Zhang, Z.; Zheng, H.; Cheng, G. Application of Spinal Subtraction and Bone Background Fusion CTA in the Accurate Diagnosis and Evaluation of Spinal Vascular Malformations. AJNR Am. J. Neuroradiol. 2024, 45, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Utsunomiya, D.; Hirai, T.; Kai, Y.; Ohmori, Y.; Shigematsu, Y.; Iryo, Y.; Uetani, H.; Azuma, M.; Yamashita, Y. Comparison of dynamic contrast-enhanced 3T MR and 64-row multidetector CT angiography for the localization of spinal dural arteriovenous fistulas. AJNR Am. J. Neuroradiol. 2014, 35, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Endo, T.; Fujimoto, S. Angiographic challenges of spinal dural and epidural arteriovenous fistulas: Report on 45 cases. Neuroradiology 2024, 66, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Bowen, B.C.; Pattany, P.M. MR angiography of the spine. Magn. Reson. Imaging Clin. N. Am. 1998, 6, 165–178. [Google Scholar] [CrossRef]
- Peckham, M.E.; Hutchins, T.A. Imaging of Vascular Disorders of the Spine. Radiol. Clin. N. Am. 2019, 57, 307–318. [Google Scholar] [CrossRef]
- Morris, J.M.; Kaufmann, T.J.; Campeau, N.G.; Cloft, H.J.; Lanzino, G. Volumetric myelographic magnetic resonance imaging to localize difficult-to-find spinal dural arteriovenous fistulas: Report of 3 cases. J. Neurosurg. Spine 2011, 14, 398–404. [Google Scholar] [CrossRef]
- Ikezawa, M.; Izumi, T.; Nishihori, M.; Nagashima, Y.; Nishimura, Y.; Tsukuda, T.; Kropp, A.E.; Goto, S.; Otsuka, T.; Kato, N.; et al. Direct vertebral artery puncture during open surgery for the endovascular treatment of a recurrent vertebro-vertebral arteriovenous fistula. World Neurosurg. 2021, 146, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Love, M.B.; Simeone, F.A. Diagnosis and treatment of a lumbar extradural arteriovenous malformation. Am. J. Neuroradiol. 1987, 8, 1129. [Google Scholar]
- Wakai, S.; Inoh, S.; Iwanaga, H.; Nagai, M.; Sato, T.; Izumi, J. Successful surgical obliteration of a huge intradural arteriovenous fistula of the spinal cord in a child. Child’s Nerv. Syst. 1992, 8, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.A.; Kan, P.; Sharp, L.; Mandel, J.J. Spinal dural arteriovenous fistula and concomitant intramedullary spinal lesion. Can. J. Neurol. Sci. 2018, 45, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ryu, B.; Shima, S.; Mochizuki, T.; Sato, S.; Inoue, T.; Niimi, Y. Pure spinal intraosseous arteriovenous fistula: A case report. Neuroradiol. J. 2023, 36, 755–759. [Google Scholar] [CrossRef]
- Chiang, S.; Pet, D.B.; Talbott, J.F.; LaHue, S.C.; Douglas, V.C.; Rosendale, N. Spinal epidural arteriovenous fistula with nerve root enhancement mimicking myeloradiculitis: A case report. BMC Neurol. 2023, 23, 62. [Google Scholar] [CrossRef]
- Albiña Palmarola, P.; Khanafer, A.; El Mekabaty, A.; Forsting, M.; Ganslandt, O.; Henkes, H. A ruptured craniocervical junction perimedullary arteriovenous fistula successfully treated through flow diversion: A case report. Surg. Neurol. Int. 2024, 15, 381. [Google Scholar] [CrossRef]
- Bishwas, S.; Islam, M.S.; Shiplu, M.H.; Rana, M.S.; Ashfaq, M.; Rashid, M.; Alam, F. Arteriovenous Malformation of Conus Medullaris Fed by the Artery of Desproges-Gotteron. J. Neurosci. Rural Pract. 2022, 13, 550–553. [Google Scholar] [CrossRef]
- Abdalla, R.N.; Shokuhfar, T.; Hurley, M.C.; Ansari, S.A.; Jahromi, B.S.; Potts, M.B.; Batjer, H.H.; Shaibani, A. Metachronous spinal pial arteriovenous fistulas: Case report. J. Neurosurg. Spine SPI 2021, 34, 310–315. [Google Scholar] [CrossRef]
- Ayhan, S.; Palaoglu, S.; Geyik, S.; Saatci, I.; Onal, M.B. Concomitant intramedullary arteriovenous malformation and a vertebral hemangioma of cervical spine discovered by a pathologic fracture during bicycle accident. Eur. Spine J. 2015, 24, 187–192. [Google Scholar] [CrossRef]
- Mizuhashi, S.; Kominami, S.; Fukuda, K. Successful balloon-assisted coil embolization for a diagnostically difficult case of spontaneous vertebrovertebral arteriovenous fistula. Surg. Neurol. Int. 2020, 11, 474. [Google Scholar] [CrossRef]
- Ogbu, I.I.; Tzerakis, N.; Al-Shamary, Z. Sudden-onset paraplegia in a 72-year-old male with a spinal dural arteriovenous fistula: Illustrative case. JNS Case Lessons 2021, 2, CASE21283. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, M.J.; Boogaarts, H.D.; de Vries, J.; Verbeek, A.L.; Meijer, F.J.; Prokop, M.; Bartels, R.H. MRA versus DSA for follow-up of coiled intracranial aneurysms: A meta-analysis. Am. J. Neuroradiol. 2014, 35, 1655–1661. [Google Scholar] [CrossRef]
- Schmidt, V.F.; Masthoff, M.; Czihal, M.; Cucuruz, B.; Häberle, B.; Brill, R.; Wohlgemuth, W.A.; Wildgruber, M. Imaging of peripheral vascular malformations—Current concepts and future perspectives. Mol. Cell. Pediatr. 2021, 8, 19. [Google Scholar] [CrossRef]
- Lindenholz, A.; TerBrugge, K.; van Dijk, J.; Farb, R. The accuracy and utility of contrast-enhanced MR angiography for localization of spinal dural arteriovenous fistulas: The Toronto experience. Eur. Radiol. 2014, 24, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Kannath, S.; Mandapalu, S.; Thomas, B.; Enakshy Rajan, J.; Kesavadas, C. Comparative Analysis of Volumetric High-Resolution Heavily T2-Weighted MRI and Time-Resolved Contrast-Enhanced MRA in the Evaluation of Spinal Vascular Malformations. Am. J. Neuroradiol. 2019, 40, 1601–1606. [Google Scholar] [CrossRef]
- Khalafallah, A.M.; Tigre, J.Y.; Rady, N.; Starke, R.M.; Saraf-Lavi, E.; Levi, A.D. Evaluating the diagnostic accuracy of 3D contrast-enhanced magnetic resonance angiography versus digital subtraction angiography in spinal dural arteriovenous fistulas. Neurosurg. Focus 2024, 56, E10. [Google Scholar] [CrossRef]
- Block, K.T.; Chandarana, H.; Milla, S.; Bruno, M.; Mulholland, T.; Fatterpekar, G.; Hagiwara, M.; Grimm, R.; Geppert, C.; Kiefer, B.; et al. Towards Routine Clinical Use of Radial Stack-of-Stars 3D Gradient-Echo Sequences for Reducing Motion Sensitivity. J. Korean Soc. Magn. Reson. Med. 2014, 18, 87. [Google Scholar] [CrossRef]
- Calastra, C.; Kleban, E.; Helfenstein, F.; Haupt, F.; Peters, A.; Huber, A.; von Tengg-Kobligk, H.; Jung, B. Dynamic contrast-enhanced MRA of the aorta using a Golden-angle RAdial Sparse Parallel (GRASP) sequence: Comparison with conventional time-resolved cartesian MRA (TWIST). Int. J. Cardiovasc. Imaging 2024, 40, 2523–2534. [Google Scholar] [CrossRef]
- Kim, A.Y.; Khil, E.K.; Choi, I.; Choi, J.A. Spinal extradural arteriovenous fistula after lumbar epidural injection: CT angiographic diagnosis using 3D-volume rendering. Skelet. Radiol. 2020, 49, 2073–2079. [Google Scholar] [CrossRef]
- Shimizu, T.; Nagoshi, N.; Akiyama, T.; Suzuki, S.; Nori, S.; Tsuji, O.; Okada, E.; Yagi, M.; Watanabe, K.; Nakamura, M.; et al. Surgical resection of arteriovenous fistula at the cauda equina. Spinal Cord Ser. Cases 2021, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Kular, S.; Tse, G.; Budu, A.; Bacon, A.; Choudhari, K.; Nagaraja, S. Transarterial CT angiography for surgical planning of spinal dural arteriovenous fistula. Br. J. Radiol. 2020, 93, 20200020. [Google Scholar] [CrossRef]
- Abdelazim, A.; Hartman, C.; Hooten, K.; Cutler, A.; Blackburn, S. Neurologic decline after spinal angiography for dural arteriovenous fistula and improvement with emergent surgical ligation. World Neurosurg. 2016, 92, 587.e15–587.e18. [Google Scholar] [CrossRef] [PubMed]
- Paolini, S.; Severino, R.; Cardarelli, G.; Missori, P.; Bartolo, M.; Esposito, V. Indocyanine Green Videoangiography in the Surgical Treatment of Spinal Dural Arterovenous Fistula: A Useful Application. World Neurosurg. 2019, 122, 508–511. [Google Scholar] [CrossRef]
- Subramaniam, S.M.; Ishii, K.; Sheng, C.J.; Nakatomi, H.; Takai, K.; Saito, N. Successful surgical strategy for ventral thoracic spinal perimedullary spinal arteriovenous fistulas: Case report. Surg. Neurol. Int. 2019, 10, 251. [Google Scholar] [CrossRef]
- Zhao, J.; Esemen, Y.; Rane, N.; Nair, R. Intracranial subarachnoid haemorrhage caused by cervical spinal dural arteriovenous fistulas: Case report. Front. Neurol. 2021, 12, 685332. [Google Scholar] [CrossRef]
- Meder, J.F.; Devaux, B.; Merland, J.J.; Frédy, D. Spontaneous disappearance of a spinal dural arteriovenous fistula. AJNR. Am. J. Neuroradiol. 1995, 16, 2058–2062. [Google Scholar] [PubMed] [PubMed Central]
- Iampreechakul, P.; Chuntaroj, S.; Wattanasen, Y.; Hangsapruek, S.; Lertbutsayanukul, P.; Siriwimonmas, S. Spontaneous regression of extradural high-flow vascular malformation in spinal arteriovenous metameric syndrome (SAMS): A unique case report. Surg. Neurol. Int. 2023, 14, 163. [Google Scholar] [CrossRef]
- Ionita, C.N.; Garcia, V.L.; Bednarek, D.R.; Snyder, K.V.; Siddiqui, A.H.; Levy, E.I.; Rudin, S. Effect of injection technique on temporal parametric imaging derived from digital subtraction angiography in patient specific phantoms. In Proceedings of the Medical Imaging 2014: Biomedical Applications in Molecular, Structural, and Functional Imaging, San Diego, CA, USA, 6–18 February 2014; Molthen, R.C., Weaver, J.B., Eds.; Volume 9038, p. 90380L. [Google Scholar] [CrossRef]
- Frey, S.; Haine, A.; Kammer, R.; von Tengg-Kobligk, H.; Obrist, D.; Baumgartner, I. Hemodynamic Characterization of Peripheral Arterio-venous Malformations. Ann. Biomed. Eng. 2017, 45, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Calastra, C.G.; Bono, M.; Granada, A.B.; Tuleja, A.; Bernhard, S.M.; Diaz-Zuccarini, V.; Balabani, S.; Obrist, D.; von Tengg-Kobligk, H.; Jung, B. Hemodynamic Characterization of Peripheral Arterio-Venous Malformations Using Rapid Contrast-Enhanced MR Imaging: An In Vitro and In Vivo Study. Ann. Biomed. Eng. 2025, 53, 2147–2163. [Google Scholar] [CrossRef]
- Schubert, T.; Wu, Y.; Johnson, K.M.; Wieben, O.; Maksimovic, J.; Mistretta, C.; Turski, P. Time-of-Arrival Parametric Maps and Virtual Bolus Images Derived From Contrast-Enhanced Time-Resolved Radial Magnetic Resonance Angiography Improve the Display of Brain Arteriovenous Malformation Vascular Anatomy. Investig. Radiol. 2016, 51, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.; McGuire, L.S.; Abou-Mrad, T.; Saalfeld, S.; Behme, D.; Alaraj, A.; Berg, P. Feasibility Study for Multimodal Image-Based Assessment of Patient-Specific Intracranial Arteriovenous Malformation Hemodynamics. J. Clin. Med. 2025, 14, 2638. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Qin, W.; Ge, L.; Zhang, X.; Ding, G.; Wang, S. Enhancing cerebral arteriovenous malformation analysis: Development and application of patient-specific lumped parameter models based on 3D imaging data. Comput. Biol. Med. 2024, 180, 108977. [Google Scholar] [CrossRef]
- Ganjkhanlou, M.R.; Shahidian, A.; Shahmohammadi, M.R. Hemodynamic Study of Cerebral Arteriovenous Malformation: Newtonian and Non-Newtonian Blood Flow. World Neurosurg. 2024, 185, e317–e341. [Google Scholar] [CrossRef]
- Franzetti, G.; Bonfanti, M.; Tanade, C.; Lim, C.S.; Tsui, J.; Hamilton, G.; Díaz-Zuccarini, V.; Balabani, S. A Computational Framework for Pre-Interventional Planning of Peripheral Arteriovenous Malformations. Cardiovasc. Eng. Technol. 2021, 13, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Forkert, N.D.; Illies, T.; Goebell, E.; Fiehler, J.; Säring, D.; Handels, H. Computer-aided nidus segmentation and angiographic characterization of arteriovenous malformations. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 775–786. [Google Scholar] [CrossRef]
- Forkert, N.D.; Säring, D.; Handels, H. Automatic Analysis of the Anatomy of Arteriovenous Malformations using 3D and 4D MRA Image Sequences. In Proceedings of the 13th World Congress on Medical Informatics, Cape Town, South Africa, 12–15 September 2010; IOS Press: Amsterdam, The Netherlands, 2010; Volume 160, pp. 1268–1272. [Google Scholar] [CrossRef]
- Nico, E.; Hossa, J.; McGuire, L.S.; Alara, A. Rupture-Risk Stratifying Patients with Cerebral Arteriovenous Malformations Using Quantitative Hemodynamic Flow Measurements. World Neurosurg. 2023, 179, e167–e175. [Google Scholar] [CrossRef]
- Illies, T.; Forkert, N.D.; Ries, T.; Regelsberger, J.; Fiehler, J. Classification of Cerebral Arteriovenous Malformations and Intranidal Flow Patterns by Color-Encoded 4D-Hybrid-MRA. Am. J. Neuroradiol. 2013, 34, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Loecher, M.W.; Wu, Y.; Niemann, D.B.; Ciske, B.; Aagaard-Kienitz, B.; Kecskemeti, S.; Johnson, K.M.; Wieben, O.; Mistretta, C.; et al. Hemodynamic Changes in Patients with Arteriovenous Malformations Assessed Using High-Resolution 3D Radial Phase-Contrast MR Angiography. Am. J. Neuroradiol. 2012, 33, 1565–1572. [Google Scholar] [CrossRef]
- von Tengg-Kobligk, H.; Frey, S.; Obrist, D.; Baumgartner, I. Arteriographic assessment: Is it still the gold standard for diagnosis of arteriovenous malformations? In Vascular Malformations, 1st ed.; Lee, B., Baumgartner, I., Loose, D., Yakes, W., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 69–72. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calastra, C.G.; Ayechu Abendaño, A.; Barna, R.-A.-M.; Orellana, F.; Baffelli, S.; Aiyangar, A.; Parrilli, A. The Role of Angiographic Imaging in the Treatment of Spinal Vascular Malformations. Med. Sci. 2025, 13, 266. https://doi.org/10.3390/medsci13040266
Calastra CG, Ayechu Abendaño A, Barna R-A-M, Orellana F, Baffelli S, Aiyangar A, Parrilli A. The Role of Angiographic Imaging in the Treatment of Spinal Vascular Malformations. Medical Sciences. 2025; 13(4):266. https://doi.org/10.3390/medsci13040266
Chicago/Turabian StyleCalastra, Camilla Giulia, Ada Ayechu Abendaño, Raluca-Ana-Maria Barna, Federica Orellana, Simone Baffelli, Ameet Aiyangar, and Annapaola Parrilli. 2025. "The Role of Angiographic Imaging in the Treatment of Spinal Vascular Malformations" Medical Sciences 13, no. 4: 266. https://doi.org/10.3390/medsci13040266
APA StyleCalastra, C. G., Ayechu Abendaño, A., Barna, R.-A.-M., Orellana, F., Baffelli, S., Aiyangar, A., & Parrilli, A. (2025). The Role of Angiographic Imaging in the Treatment of Spinal Vascular Malformations. Medical Sciences, 13(4), 266. https://doi.org/10.3390/medsci13040266





