The Multifaceted Role of Annexin A1 in Colorectal Cancer: From Molecular Mechanisms to Predictive and Prognostic Implications
Abstract
1. Introduction
2. The Annexin Protein Superfamily
3. ANXA1’s General Structure and Function
4. ANXA1’s Role in Cancer
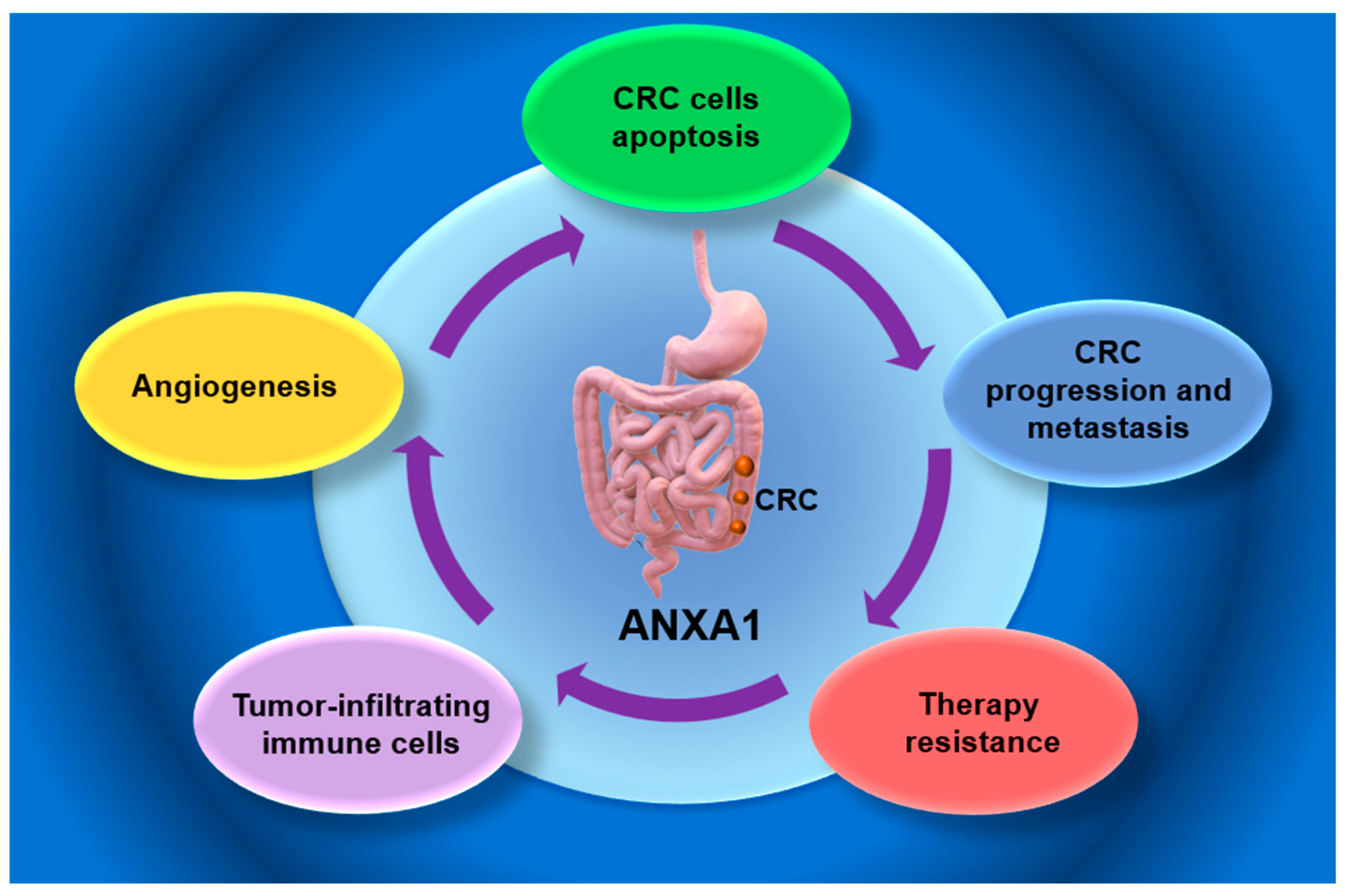
5. ANXA1 Role in CRC
5.1. ANXA1 as a Biomarker in CRC Progression
5.2. Functional Mechanisms of ANXA1 in CRC Progression
5.2.1. ANXA1 in CRC Apoptosis
5.2.2. ANXA1 Involvement in CRC Tumor Microenvironment (TME)
Neutrophils
Macrophages
T Lymphocytes
Dendritic Cells
5.2.3. ANXA 1 Role in CRC Angiogenesis
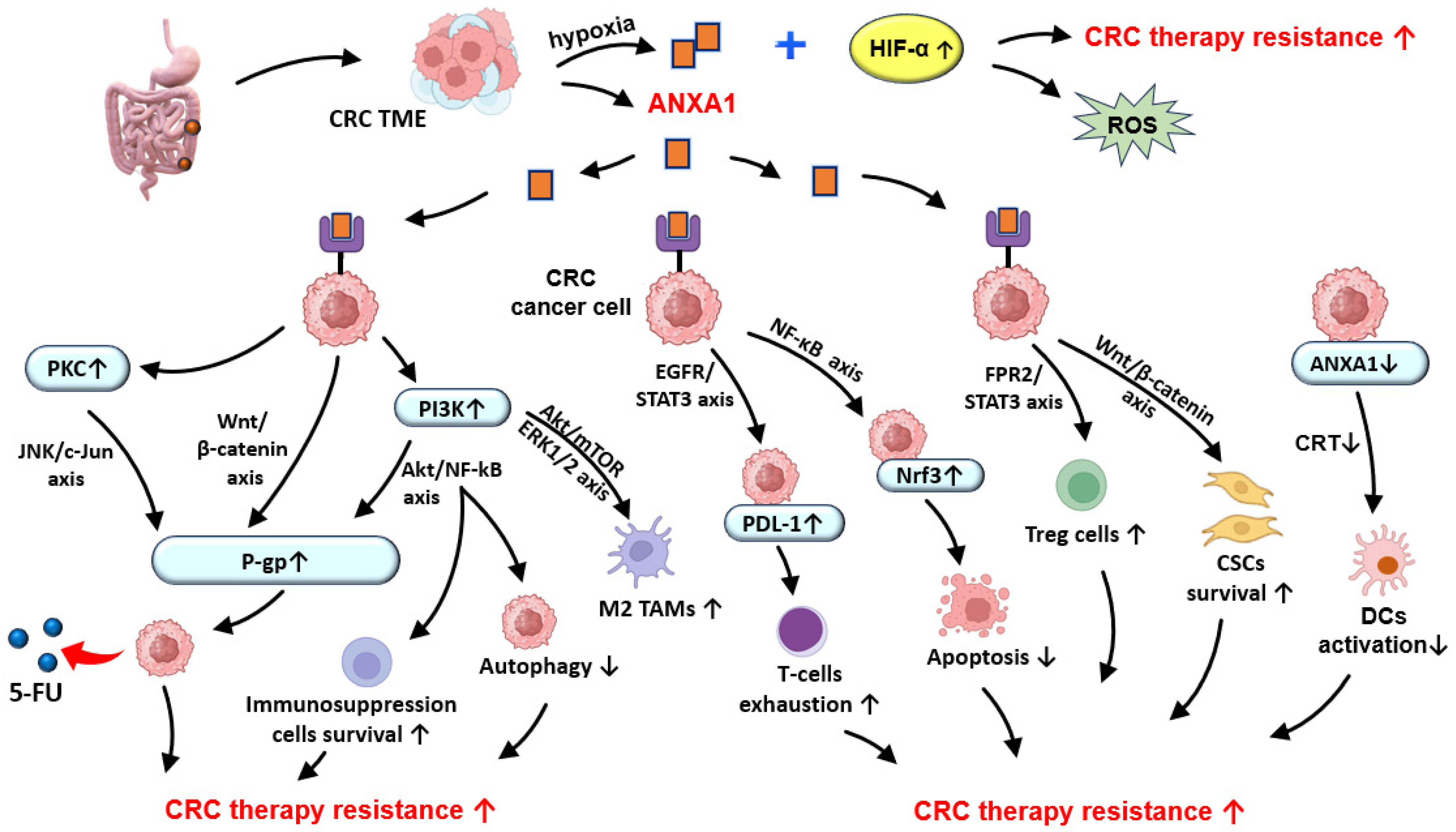
6. ANXA1 in CRC Chemotherapy and Radiotherapy Resistance
6.1. CRC Treatments: Past, Present, and Future
6.2. Perspectives of ANXA1 Exploitation in CRC-Targeted Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, F.; Keshinro, A.; Weiser, M.R. Advances in the treatment of locally advanced rectal cancer. Ann. Gastroenterol. Surg. 2020, 5, 32–38. [Google Scholar] [CrossRef]
- Sullo, F.G.; Passardi, A.; Gallio, C.; Molinari, C.; Marisi, G.; Pozzi, E.; Solaini, L.; Bittoni, A. Advancing personalized medicine in the treatment of locally advanced rectal cancer. J. Clin. Med. 2024, 13, 2562. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Scott, A.J.; Kennedy, E.B.; Berlin, J.; Brown, G.; Chalabi, M.; Cho, M.T.; Cusnir, M.; Dorth, J.; George, M.; Kachnic, L.A.; et al. Management of locally advanced rectal cancer: ASCO guideline. J. Clin. Oncol. 2024, 42, 3355–3375. [Google Scholar] [CrossRef]
- Feeney, G.; Sehgal, R.; Sheehan, M.; Hogan, A.; Regan, M.; Joyce, M.; Kerin, M. Neoadjuvant radiotherapy for rectal cancer management. World J. Gastroenterol. 2019, 25, 4850–4869. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xue, Z.; He, K.; Tian, Y.; Chen, Y.; Zhao, M.; Yu, J.; Yue, J. Strategies to optimize treatment for locally advanced rectal cancer. Cancers 2022, 15, 219. [Google Scholar] [CrossRef]
- Keller, D.S.; Berho, M.; Perez, R.O.; Wexner, S.D.; Chand, M. The multidisciplinary management of rectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 414–429. [Google Scholar] [CrossRef]
- Bunjo, Z.; Sammour, T. The Landmark Series: Neoadjuvant therapy for locally advanced rectal cancer. Ann. Surg. Oncol. 2025, 32, 4935–4944. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Yu, H.; Ke, J.; Ding, P.; Yi, Y.; Jiang, X.; Duan, X.; Tang, J.; Chang, D.T.; Wu, X.; et al. Predicting treatment response from longitudinal images using multi-task deep learning. Nat. Commun. 2021, 12, 1851. [Google Scholar] [CrossRef]
- Qian, C.; Yang, S.; Chen, Y.; Ge, R.; Shi, F.; Liu, C.; Wang, H.; Guo, Y. Predicting pathological response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer with two step feature selection and ensemble learning. Sci. Rep. 2025, 15, 9936. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Xu, M.; Zheng, R.; Guan, G.; Xu, B. Novel biomarkers to predict treatment response and prognosis in locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy. BMC Cancer 2023, 23, 1099. [Google Scholar] [CrossRef]
- Zwart, W.; Temmink, S.; Hospers, G.; Marijnen, C.; Putter, H.; Nagtegaal, I.; Blomqvist, L.; Kranenbarg, E.M.K.; Roodvoets, A.; Martling, A.; et al. Oncological outcomes after a pathological complete response following total neoadjuvant therapy or chemoradiotherapy for high-risk locally advanced rectal cancer in the RAPIDO trial. Eur. J. Cancer 2024, 204, 114044. [Google Scholar] [CrossRef] [PubMed]
- Altıntaş, Y.E.; Bilici, A.; Yıldız, Ö.; Kınıkoğlu, O.; Ölmez, Ö.F. The role of pre-treatment inflammatory biomarkers in predicting tumor response to neoadjuvant chemoradiotherapy in rectal cancer. Medicina 2025, 61, 865. [Google Scholar] [CrossRef]
- Lin, M.; Liu, J.; Lan, C.; Qiu, M.; Huang, W.; Liao, C.; Zhang, S. Factors associated with pathological complete remission after neoadjuvant chemoradiotherapy in locally advanced rectal cancer: A real-world clinical setting. Front. Oncol. 2024, 14, 1421620. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, B.; Chen, M.; Li, Z.; Liao, Z. Biomarkers for predicting the response to radiation-based neoadjuvant therapy in rectal cancer. Front. Biosci. 2022, 27, 201. [Google Scholar] [CrossRef]
- Martins, S.; Veiga, P.; Tralhão, J.G.; Carreira, I.M.; Ribeiro, I.P. Rectal cancer: Exploring predictive biomarkers through molecular pathways involved in carcinogenesis. Biology 2024, 13, 1007. [Google Scholar] [CrossRef]
- Sato, Y.; Kumamoto, K.; Saito, K.; Okayama, H.; Hayase, S.; Kofunato, Y.; Miyamoto, K.; Nakamura, I.; Ohki, S.; Koyama, Y.; et al. Up-regulated Annexin A1 expression in gastrointestinal cancer is associated with cancer invasion and lymph node metastasis. Exp. Ther. Med. 2011, 2, 239–243. [Google Scholar] [CrossRef]
- Han, G.; Lu, K.; Huang, J.; Ye, J.; Dai, S.; Ye, Y.; Zhang, L. Effect of Annexin A1 gene on the proliferation and invasion of esophageal squamous cell carcinoma cells and its regulatory mechanisms. Int. J. Mol. Med. 2017, 39, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Lu, K.J.; Huang, J.X.; Zhang, L.X.; Dai, S.B.; Dai, C.L. Association of serum annexin A1 with treatment response and prognosis in patients with esophageal squamous cell carcinoma. J. Cancer Res. Ther. 2018, 14, S667–S674. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Guo, T.; Chen, G.; Liu, G.; Song, Q.; Li, G.; Xu, F.; Dong, X.; Yang, F.; et al. Annexin A protein family: Focusing on the occurrence, progression and treatment of cancer. Front. Cell Dev. Biol. 2023, 11, 1141331. [Google Scholar] [CrossRef] [PubMed]
- Ydy, L.R.; do Espírito Santo, G.F.; de Menezes, I.; Martins, M.S.; Gnotti, E.; Damazo, A.S. Study of the Annexin A1 and its associations with carcinoembryonic antigen and mismatch repair proteins in colorectal cancer. J. Gastrointest. Cancer 2016, 47, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, L.; Wang, B.; Zhang, S.; Fu, Z.; Cheng, A.; Liang, X. Annexin A1 affects tumor metastasis through epithelial-mesenchymal transition: A narrative review. Transl. Cancer Res. 2022, 11, 4416–4433. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Li, X.; Luo, S.; Zhao, L. An overview of the regulatory role of annexin A1 in the tumor microenvironment and its prospective clinical application (Review). Int. J. Oncol. 2024, 64, 51. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, H.N.; Crichton, S.J.; Fabian, C.; Pepper, C.; Butcher, D.R.; Dempsey, F.C.; Parris, C.N. A therapeutic antibody targeting annexin-A1 inhibits cancer cell growth in vitro and in vivo. Oncogene 2024, 43, 608–614. [Google Scholar] [CrossRef]
- Onozawa, H.; Saito, M.; Saito, K.; Kanke, Y.; Watanabe, Y.; Hayase, S.; Sakamoto, W.; Ishigame, T.; Momma, T.; Ohki, S.; et al. Annexin A1 is involved in resistance to 5-FU in colon cancer cells. Oncol. Rep. 2017, 37, 235–240. [Google Scholar] [CrossRef]
- Ganesan, T.; Sinniah, A.; Ramasamy, T.S.; Alshawsh, M.A. Cracking the code of Annexin A1-mediated chemoresistance. Biochem. Biophys. Res. Commun. 2024, 725, 150202. [Google Scholar] [CrossRef]
- Sheu, M.J.; Li, C.F.; Lin, C.Y.; Lee, S.W.; Lin, L.C.; Chen, T.J.; Ma, L.J. Overexpression of ANXA1 confers independent negative prognostic impact in rectal cancers receiving concurrent chemoradiotherapy. Tumour Biol. 2014, 35, 7755–7763. [Google Scholar] [CrossRef]
- Emons, G.; Spitzner, M.; Reineke, S.; Möller, J.; Auslander, N.; Kramer, F.; Hu, Y.; Beissbarth, T.; Wolff, H.A.; Rave-Fränk, M.; et al. Chemoradiotherapy resistance in colorectal cancer cells is mediated by Wnt/β-catenin signaling. Mol. Cancer Res. 2017, 15, 1481–1490. [Google Scholar] [CrossRef]
- Alan, S.; Karadag, N.; Akatlı, A.N.; Tecellioglu, F.S.; Sahin, N.; Huz, M. Evaluation of annexin A1 expression in lung, breast, colon, and prostatic adenocarcinomas and in tumor microenvironment. Indian J. Med. Sci. 2023, 75, 42–46. [Google Scholar] [CrossRef]
- Grewal, T.; Rentero, C.; Enrich, C.; Wahba, M.; Raabe, C.A.; Rescher, U. Annexin animal models—From fundamental principles to translational research. Int. J. Mol. Sci. 2021, 22, 3439. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Gidfar, S.; Vu, A.; Schraufnagel, D. Annexins family: Insights into their functions and potential role in pathogenesis of sarcoidosis. J. Transl. Med. 2016, 14, 89. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Hu, B.; Chen, L.; Jv, M.; Wang, L.; Zhou, C.; Wei, M.; Zhao, L. Advances in cancer treatment: A new therapeutic target, Annexin A2. J. Cancer 2021, 12, 3587–3596. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, L.; Zhu, B.; Huang, Y.; Wang, X.; Lin, X.; Li, M.; Xu, P.; Zhang, X.; Zhang, J.; et al. Annexin A5 is essential for PKCθ translocation during T-cell activation. J. Biol. Chem. 2020, 295, 14214–14221. [Google Scholar] [CrossRef] [PubMed]
- Manai, M.; Doghri, R.; Finetti, P.; Mrad, K.; Bouabsa, R.; Manai, M.; Birnbaum, D.; Bertucci, F.; Charfi, L.; Driss, M. Overexpression of Annexin A1 Is an independent predictor of longer overall survival in epithelial ovarian cancer. In Vivo 2020, 34, 177–184. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Zhang, J.; Lam, E.; Liu, X.; Sun, J.; Feng, L.; Lu, H.; Yu, J.; Jin, H. Annexin A6 is down-regulated through promoter methylation in gastric cancer. Am. J. Transl. Res. 2013, 5, 555–562. [Google Scholar] [PubMed]
- Koese, M.; Rentero, C.; Kota, B.P.; Hoque, M.; Cairns, R.; Wood, P.; Vilà de Muga, S.; Reverter, M.; Alvarez-Guaita, A.; Monastyrskaya, K.; et al. Annexin A6 is a scaffold for PKCα to promote EGFR inactivation. Oncogene 2013, 32, 2858–2872. [Google Scholar] [CrossRef]
- Gerke, V.; Gavins, F.N.E.; Geisow, M.; Grewal, T.; Jaiswal, J.K.; Nylandsted, J.; Rescher, U. Annexins-a family of proteins with distinctive tastes for cell signaling and membrane dynamics. Nat. Commun. 2024, 15, 1574. [Google Scholar] [CrossRef]
- Araújo, T.G.; Mota, S.T.S.; Ferreira, H.S.V.; Ribeiro, M.A.; Goulart, L.R.; Vecchi, L. Annexin A1 as a regulator of immune response in cancer. Cells 2021, 10, 2245. [Google Scholar] [CrossRef]
- Patel, D.R.; Isas, J.M.; Ladokhin, A.S.; Jao, C.C.; Kim, Y.E.; Kirsch, T.; Langen, R.; Haigler, H.T. The conserved core domains of annexins A1, A2, A5, and B12 can be divided into two groups with different Ca2+-dependent membrane-binding properties. Biochemistry 2005, 44, 2833–2844. [Google Scholar] [CrossRef]
- Wang, Y.S.; Li, H.; Li, Y.; Zhu, H.; Jin, Y.H. Identification of natural compounds targeting Annexin A2 with an anti-cancer effect. Protein Cell 2018, 9, 568–579. [Google Scholar] [CrossRef]
- Bai, F.; Zhang, P.; Fu, Y.; Chen, H.; Zhang, M.; Huang, Q.; Li, D.; Li, B.; Wu, K. Targeting ANXA1 abrogates Treg-mediated immune suppression in triple-negative breast cancer. J. Immunother. Cancer 2020, 8, e000169. [Google Scholar] [CrossRef]
- Boudhraa, Z.; Bouchon, B.; Viallard, C.; D’Incan, M.; Degoul, F. Annexin A1 localization and its relevance to cancer. Clin. Sci. 2016, 130, 205–220. [Google Scholar] [CrossRef]
- Häger, S.C.; Nylandsted, J. Annexins: Players of single cell wound healing and regeneration. Commun. Integr. Biol. 2019, 12, 162–165. [Google Scholar] [CrossRef]
- Xi, Y.; Ju, R.; Wang, Y. Roles of Annexin A protein family in autophagy regulation and therapy. Biomed. Pharmacother. 2020, 130, 110591. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Zhou, H.; Zhang, Q.; Jin, Y.; Fu, C. Advancements of Annexin A1 in inflammation and tumorigenesis. OncoTargets Ther. 2019, 12, 3245–3254. [Google Scholar] [CrossRef] [PubMed]
- Weyd, H. More than just innate affairs—On the role of annexins in adaptive immunity. Biol. Chem. 2016, 397, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Grewal, T.; Hoque, M.; Conway, J.R.W.; Reverter, M.; Wahba, M.; Beevi, S.S.; Timpson, P.; Enrich, C.; Rentero, C. Annexin A6-A multifunctional scaffold in cell motility. Cell Adh. Migr. 2017, 11, 288–304. [Google Scholar] [CrossRef]
- Chen, J.J.; Lin, Y.C.; Yao, P.L.; Yuan, A.; Chen, H.Y.; Shun, C.T.; Tsai, M.F.; Chen, C.H.; Yang, P.C. Tumor-associated macrophages: The double-edged sword in cancer progression. J. Clin. Oncol. 2005, 23, 953–964. [Google Scholar] [CrossRef]
- Rosengarth, A.; Gerke, V.; Luecke, H. X-ray structure of full-length Annexin 1 and implications for membrane aggregation. J. Mol. Biol. 2001, 306, 489–498. [Google Scholar] [CrossRef]
- Rosengarth, A.; Luecke, H. A calcium-driven conformational switch of the N-terminal and core domains of Annexin A1. J. Mol. Biol. 2003, 326, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Boudhraa, Z.; Rondepierre, F.; Ouchchane, L.; Kintossou, R.; Trzeciakiewicz, A.; Franck, F.; Kanitakis, J.; Labeille, B.; Joubert-Zakeyh, J.; Bouchon, B.; et al. Annexin A1 in primary tumors promotes melanoma dissemination. Clin. Exp. Metastasis 2014, 31, 749–760. [Google Scholar] [CrossRef]
- Hirata, F.; Thibodeau, L.M.; Hirata, A. Ubiquitination and SUMOylation of annexin A1 and helicase activity. Biochim. Biophys. Acta 2010, 1800, 899–905. [Google Scholar] [CrossRef]
- Moreli, J.B.; Santos, M.R.D.; Calderon, I.M.P.; Hebeda, C.B.; Farsky, S.H.P.; Bevilacqua, E.; Oliani, S.M. The Role of Annexin A1 in DNA damage response in placental cells: Impact on gestational diabetes mellitus. Int. J. Mol. Sci. 2023, 24, 10155. [Google Scholar] [CrossRef]
- Hirata, A.; Hirata, F. DNA chain unwinding and annealing reactions of lipocortin (annexin) I heterotetramer: Regulation by Ca2+ and Mg2+. Biochem. Biophys. Res. Commun. 2002, 291, 205–209. [Google Scholar] [CrossRef]
- Futter, C.E.; White, I.J. Annexins and endocytosis. Traffic 2007, 8, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Sanches, J.M.; Branco, L.M.; Duarte, G.H.B.; Oliani, S.M.; Bortoluci, K.R.; Moreira, V.; Gil, C.D. Annexin A1 regulates NLRP3 inflammasome activation and modifies lipid release profile in isolated peritoneal macrophages. Cells 2020, 9, 926. [Google Scholar] [CrossRef]
- Leoni, G.; Nusrat, A. Annexin A1: Shifting the balance towards resolution and repair. Biol. Chem. 2016, 397, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Kiani-Esfahani, A.; Kazemi Sheykhshabani, S.; Peymani, M.; Hashemi, M.S.; Ghaedi, K.; Nasr-Esfahani, M.H. Overexpression of Annexin A1 suppresses pro-inflammatory factors in PC12 Cells induced by 1-methyl-4-phenylpyridinium. Cell J. 2016, 18, 197–204. [Google Scholar] [PubMed]
- Cao, Y.; Li, Y.; Edelweiss, M.; Arun, B.; Rosen, D.; Resetkova, E.; Wu, Y.; Liu, J.; Sahin, A.; Albarracin, C.T. Loss of annexin A1 expression in breast cancer progression. Appl. Immunohistochem. Mol. Morphol. 2008, 16, 530–534. [Google Scholar] [CrossRef]
- Hnisz, D.; Weintraub, A.S.; Day, D.S.; Valton, A.L.; Bak, R.O.; Li, C.H.; Goldmann, J.; Lajoie, B.R.; Fan, Z.P.; Sigova, A.A.; et al. Activation of proto-oncogenes by disruption of chromosome neighborhoods. Science 2016, 351, 1454–1458. [Google Scholar] [CrossRef]
- Foo, S.L.; Yap, G.; Cui, J.; Lim, L.H.K. Annexin-A1—A blessing or a curse in cancer? Trends Mol. Med. 2019, 25, 315–327. [Google Scholar] [CrossRef]
- Boudhraa, Z.; Merle, C.; Mazzocut, D.; Chezal, J.M.; Chambon, C.; Miot-Noirault, E.; Theisen, M.; Bouchon, B.; Degoul, F. Characterization of pro-invasive mechanisms and N-terminal cleavage of ANXA1 in melanoma. Arch. Dermatol. Res. 2014, 306, 903–914. [Google Scholar] [CrossRef]
- Cheng, S.X.; Tu, Y.; Zhang, S. FoxM1 promotes glioma cells progression by up-regulating Anxa1 expression. PLoS ONE 2013, 8, e72376. [Google Scholar] [CrossRef]
- Sobral-Leite, M.; Wesseling, J.; Smit, V.T.; Nevanlinna, H.; van Miltenburg, M.H.; Sanders, J.; Hofland, I.; Blows, F.M.; Coulson, P.; Patrycja, G.; et al. Annexin A1 expression in a pooled breast cancer series: Association with tumor subtypes and prognosis. BMC Med. 2015, 13, 156. [Google Scholar] [CrossRef]
- Biaoxue, R.; Xiling, J.; Shuanying, Y.; Wei, Z.; Xiguang, C.; Jinsui, W.; Min, Z. Upregulation of Hsp90-beta and annexin A1 correlates with poor survival and lymphatic metastasis in lung cancer patients. J. Exp. Clin. Cancer Res. 2012, 31, 70. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, G.; Fang, W.; Zhu, H.; Chu, K. Increased expression of annexin A1 predicts poor prognosis in human hepatocellular carcinoma and enhances cell malignant phenotype. Med. Oncol. 2014, 31, 327. [Google Scholar] [CrossRef]
- Su, N.; Xu, X.Y.; Chen, H.; Gao, W.C.; Ruan, C.P.; Wang, Q.; Sun, Y.P. Increased expression of annexin A1 is correlated with K-ras mutation in colorectal cancer. Tohoku J Exp. Med. 2010, 222, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shao, G.; Hong, X.; Shi, Y.; Zheng, Y.; Yu, Y.; Fu, C. Targeting Annexin A1 as a druggable player to enhance the anti-tumor role of honokiol in colon cancer through autophagic pathway. Pharmaceuticals 2023, 16, 70. [Google Scholar] [CrossRef]
- Petrella, A.; Festa, M.; Ercolino, S.F.; Zerilli, M.; Stassi, G.; Solito, E.; Parente, L. Annexin-1 downregulation in thyroid cancer correlates to the degree of tumor differentiation. Cancer Biol. Ther. 2006, 5, 643–647. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Garcia-Pedrero, J.M.; Fernandez, M.P.; Morgan, R.O.; Suárez, C.; Herrero, A. Annexin A1 expression in nasopharyngeal carcinoma correlates with squamous differentiation. Am. J. Rhinol. Allergy 2005, 19, 483–487. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, T.; Aladelusi, T.O.; Ju, W.; Zhang, Z.; Zhong, L.; Zhu, D. Decreased Annexin A1 expression enhances sensitivity to docetaxel, cisplatin and 5-fluorouracil combination induction chemotherapy in oral squamous cell carcinoma. J. Oral Pathol. Med. 2021, 50, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Yom, C.K.; Han, W.; Kim, S.W.; Kim, H.S.; Shin, H.C.; Chang, J.N.; Koo, M.; Noh, D.Y.; Moon, B.I. Clinical significance of annexin A1 expression in breast cancer. J. Breast Cancer 2011, 14, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, L.; Zóia, M.A.P.; Santos, T.G.; de Oliveira Beserra, A.; Ramos, C.M.C.; Colombo, B.F.M.; Maia, Y.C.P.; de Andrade, V.P.; Mota, S.T.S.; de Araújo, T.G.; et al. Inhibition of the AnxA1/FPR1 autocrine axis reduces MDA-MB-231 breast cancer cell growth and aggressiveness in vitro and in vivo. Biochim. Biophys. Acta. Mol. Cell Res. 2018, 1865, 1368–1382. [Google Scholar] [CrossRef]
- Anbalagan, D.; Yap, G.; Yuan, Y.; Pandey, V.K.; Lau, W.H.; Arora, S. Annexin-A1 regulates MicroRNA-26b* and MicroRNA-562 to directly target nf-κb and angiogenesis in breast cancer cells. PLoS ONE 2014, 9, e114507. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Wu, M.S.; Lin, J.T.; Lin, M.T.; Shun, C.T.; Huang, H.Y.; Hua, K.T.; Kuo, M.L. Annexin A1 is associated with gastric cancer survival and promotes gastric cancer cell invasiveness through the formyl peptide receptor/extracellular signal-regulated kinase/integrin beta-1-binding protein 1 pathway. Cancer 2012, 118, 5757–5767. [Google Scholar] [CrossRef]
- Berns, K.; Sonnenblick, A.; Gennissen, A.; Brohée, S.; Hijmans, E.; Evers, B.; Fumagalli, D.; Desmedt, C.; Loibl, S.; Denkert, C.; et al. Loss of ARID1A activates ANXA1, which serves as a predictive biomarker for trastuzumab resistance. Clin. Cancer Res. 2016, 22, 5238–5248. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Min, J.; Wu, H.; Zhang, H.; Wang, C.; Tan, G.; Zhang, F. Annexin A1 is a potential biomarker of bone metastasis in small cell lung cancer. Oncol. Lett. 2021, 21, 141. [Google Scholar] [CrossRef]
- Chen, D.L.; Ping, Y.F.; Yu, S.C.; Chen, J.H.; Yao, X.H.; Jiang, X.F.; Zhang, H.R.; Wang, Q.L.; Bian, X.W. Downregulating FPR restrains xenograft tumors by impairing the angiogenic potential and invasive capability of malignant glioma cells. Biochem. Biophys. Res. Commun. 2009, 381, 448–452. [Google Scholar] [CrossRef]
- Snapkov, I.; Öqvist, C.O.; Figenschau, Y.; Kogner, P.; Johnsen, J.I.; Sveinbjørnsson, B. The role of formyl peptide receptor 1 (FPR1) in neuroblastoma tumorigenesis. BMC Cancer 2016, 16, 490. [Google Scholar] [CrossRef]
- Huang, J.; Chen, K.; Chen, J.; Gong, W.; Dunlop, N.M.; Howard, O.M.; Gao, Y.; Bian, X.W.; Wang, J.M. The G-protein-coupled formylpeptide receptor FPR confers a more invasive phenotype on human glioblastoma cells. Br. J. Cancer 2010, 102, 1052–1060. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, X.; Le, Y.; Gong, W.; Hu, J.; Zhang, X.; Wang, L.; Iribarren, P.; Salcedo, R.; Howard, O.M.; et al. Formylpeptide receptor FPR and the rapid growth of malignant human gliomas. J. Natl. Cancer Inst. 2005, 97, 823–835. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.; Gong, W.; Tang, P.; Yoshimura, T.; Wang, J.M. Regulation of inflammation by members of the formyl-peptide receptor family. J. Autoimmun. 2017, 85, 64–77. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Wu, M.S.; Lin, J.T.; Lin, M.T.; Shun, C.T.; Hua, K.T.; Kuo, M.L. Formyl Peptide receptor 1 expression is associated with tumor progression and survival in gastric cancer. Anticancer Res. 2014, 34, 2223–2229. [Google Scholar]
- Gavins, F.N.; Hickey, M.J. Annexin A1 and the regulation of innate and adaptive immunity. Front. Immunol. 2012, 3, 354. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.A.; Kar, S.; Foo, S.L.; Gu, T.; Toh, Y.Q.; Ampomah, P.B.; Sachaphibulkij, K.; Yap, G.; Zharkova, O.; Lukman, H.M.; et al. Annexin-A1 enhances breast cancer growth and migration by promoting alternative macrophage polarization in the tumour microenvironment. Sci. Rep. 2017, 7, 17925. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Kumamoto, K.; Saito, M.; Onozawa, H.; Saito, K.; Abe, N.; Ohtake, T.; Takenoshita, S. Upregulated Annexin A1 promotes cellular invasion in triple-negative breast cancer. Oncol. Rep. 2015, 33, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.C.; Lung, J.H.; Chen, Y.C.; Lin, Y.C.; Li, Y.C.; Hung, M.-S. The Association of Annexin A1 and chemosensitivity to Osimertinib in lung cancer cells. Cancers 2021, 13, 4106. [Google Scholar] [CrossRef]
- Wan, Y.M.; Tian, J.; Qi, L.; Liu, L.M.; Xu, N. ANXA1 affects cell proliferation, invasion and epithelial-mesenchymal transition of oral squamous cell carcinoma. Exp. Ther. Med. 2017, 14, 5214–5218. [Google Scholar] [CrossRef]
- Feng, J.; Lu, S.S.; Xiao, T.; Huang, W.; Yi, H.; Zhu, W.; Fan, S.; Feng, X.P.; Li, J.Y.; Yu, Z.Z.; et al. ANXA1 binds and stabilizes EphA2 to promote nasopharyngeal carcinoma growth and metastasis. Cancer Res. 2020, 80, 4386–4398. [Google Scholar] [CrossRef]
- Belvedere, R.; Novizio, N.; Pessolano, E.; Tosco, A.; Eletto, D.; Porta, A.; Campiglia, P.; Perretti, M.; Filippelli, A.; Petrella, A. Heparan sulfate binds the extracellular Annexin A1 and blocks its effects on pancreatic cancer cells. Biochem. Pharmacol. 2020, 182, 114252. [Google Scholar] [CrossRef]
- Chen, R.; Chen, C.; Han, N.; Guo, W.; Deng, H.; Wang, Y.; Ding, Y.; Zhang, M. Annexin-1 is an oncogene in glioblastoma and causes tumour immune escape through the indirect upregulation of interleukin-8. J. Cell. Mol. Med. 2020, 26, 4343–4356. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, Y.; Xu, D.; Wang, J.; Yu, G. Differential expression of ANXA1 in benign human gastrointestinal tissues and cancers. BMC Cancer 2014, 14, 520. [Google Scholar] [CrossRef] [PubMed]
- Bizzarro, V.; Belvedere, R.; Migliaro, V.; Romano, E.; Parente, L.; Petrella, A. Hypoxia regulates ANXA1 expression to support prostate cancer cell invasion and aggressiveness. Cell Adh. Migr. 2017, 11, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Ding, M.; Sun, J.M.; Zhu, Z.A. Identification of ANXA1 as a diagnostic and lymphatic metastasis factor in colorectal cancer. J. Tre Bio Res. 2019, 1, 1–4. [Google Scholar] [CrossRef]
- Feliu, J.; Gámez-Pozo, A.; Martínez-Pérez, D.; Pérez-Wert, P.; Matamala-Luengo, D.; Viñal, D.; Kunz, L.; López-Vacas, R.; Dittmann, A.; Rodríguez-Salas, N.; et al. Functional proteomics of colon cancer Consensus Molecular Subtypes. Br. J. Cancer 2024, 130, 1670–1678. [Google Scholar] [CrossRef]
- He, Z.Y.; Wen, H.; Shi, C.B.; Wang, J. Up-regulation of hnRNP A1, Ezrin, tubulin β-2C and Annexin A1 in sentinel lymph nodes of colorectal cancer. World J. Gastroenterol. 2010, 16, 4670–4676. [Google Scholar] [CrossRef]
- Mohammadi, P.; Mohammadi, S.; Eghbalian, A.; Meyabadi, A.J.; Alizadeh, M.; Taefehshokr, S. Potential use of SCAT1, SCAT2, and SCAT8 as diagnostic and prognosis markers in colorectal cancer. Cancer Genet. 2024, 288–289, 106–109. [Google Scholar] [CrossRef]
- Khan, A.; Hasana, U.; Nadeem, I.A.; Khatri, S.P.; Nawaz, S.; Makhdoom, Q.U.; Wazir, S.; Patel, K.; Ghaly, M. Advances in colorectal cancer screening and detection: A narrative review on biomarkers, imaging and preventive strategies. J. Egypt. Natl. Canc. Inst. 2025, 37, 20. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Darmadi, D.; Darabi, R.; Al-Aouadi, R.F.A.; Ivraghi, M.S.; Akkol, E.K. Biomarkers for colorectal cancer detection: An insight into colorectal cancer and FDA-approved biomarkers. Bioimpacts 2025, 15, 31211. [Google Scholar] [CrossRef]
- Faria, P.C.; Sena, A.A.; Nascimento, R.; Carvalho, W.J.; Loyola, A.M.; Silva, S.J.; Durighetto, A.F.; Oliveira, A.D.; Oliani, S.M.; Goulart, L.R. Expression of annexin A1 mRNA in peripheral blood from oral squamous cell carcinoma patients. Oral Oncol. 2010, 46, 25–30. [Google Scholar] [CrossRef]
- Sandri, S.; Hebeda, C.B.; Broering, M.F.; de Paula Silva, M.; Moredo, L.F.; de Barros e Silva, M.J.; Sapata Molina, A.; Lopes Pinto, C.A.; Duprat Neto, J.P.; Reutelingsperger, C.P.; et al. Role of Annexin A1 secreted by neutrophils in melanoma metastasis. Cells 2023, 12, 425. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.S.; Wu, P.F.; Li, Q.; Dai, W.M.; Yuan, S.; Xu, Z.H.; Liu, T.T.; Miao, Z.W.; Fang, W.G.; et al. Brain microvascular endothelium induced-annexin A1 secretion contributes to small cell lung cancer brain metastasis. Int. J. Biochem. Cell Biol. 2015, 66, 11–19. [Google Scholar] [CrossRef]
- Ateş, F.B.; Kaya, D.M. The Investigation of relationship between Annexin A1 gene expression and inflammation in colorectal cancer patients. Acta Haematol. Oncol. Turc. 2018, 51, 159–165. [Google Scholar] [CrossRef]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A comprehensive overview of signaling pathways, morphological changes, and physiological significance and therapeutic implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, D.; Liu, J.; Song, J.T.; Gao, S.L.; Li, H.; Hu, L.H.; Liu, B.R. Effect of NF-κB inhibitors on the chemotherapy-induced apoptosis of the colon cancer cell line HT-29. Exp. Ther. Med. 2012, 4, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhu, X. Knockdown of ANXA10 inhibits proliferation and promotes apoptosis of papillary thyroid carcinoma cells by down-regulating TSG101 thereby inactivating the MAPK/ERK signaling pathway. J. Bioenerg. Biomembr. 2021, 53, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Debret, R.; El Btaouri, H.; Duca, L.; Rahman, I.; Radke, S.; Haye, B.; Sallenave, J.M.; Antonicelli, F. Annexin A1 processing is associated with caspase-dependent apoptosis in BZR cells. FEBS Lett. 2003, 546, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, L.; Liu, L.; Yu, R.; Li, X.; Luo, Z. Knockdown of Annexin-A1 inhibits growth, migration and invasion of glioma cells by suppressing the PI3K/Akt signaling pathway. ASN Neuro 2021, 13, 17590914211001218. [Google Scholar] [CrossRef]
- Lin, Z.; Wen, M.; Yu, E.; Lin, X.; Wang, H.; Chen, J.; Yao, C.; Zhang, H.; Ru, J.; Wang, K.; et al. ANXA1 as a prognostic and immune microenvironmental marker for gliomas based on transcriptomic analysis and experimental validation. Front. Cell Dev. Biol. 2021, 9, 659080. [Google Scholar] [CrossRef]
- Soleimani, A.; Rahmani, F.; Ferns, G.A.; Ryzhikov, M.; Avan, A.; Hassanian, S.M. Role of the NF-κB signaling pathway in the pathogenesis of colorectal cancer. Gene 2020, 726, 144132. [Google Scholar] [CrossRef]
- Karin, M.; Lin, A. NF-κB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef]
- Jani, T.S.; DeVecchio, J.; Mazumdar, T.; Agyeman, A.; Houghton, J.A. Inhibition of NF-κB signaling by quinacrine is cytotoxic to human colon carcinoma cell lines and is synergistic in combination with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or Oxaliplatin. J. Biol. Chem. 2010, 285, 19162–19172. [Google Scholar] [CrossRef]
- Kong, A.S.Y.; Maran, S.; Loh, H.S. Navigating the interplay between BCL-2 family proteins, apoptosis, and autophagy in colorectal cancer. Adv. Cancer Biol. Metast. 2024, 11, 100126. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Stathopoulos, G.P.; Fountzilas, G.; Kalofonos, H.P.; Tsamandas, A.; Vrettou, E.; Scopa, C.; Polychronidis, A.; Simopoulos, K.; et al. Bax protein expression in colorectal cancer: Association with p53, bcl-2 and patterns of relapse. Anticancer Res. 2001, 21, 253–259. [Google Scholar]
- Schulze-Bergkamen, H.; Ehrenberg, R.; Hickmann, L.; Vick, B.; Urbanik, T.; Schimanski, C.C.; Berger, M.R.; Schad, A.; Weber, A.; Heeger, S.; et al. Bcl-x(L) and Myeloid cell leukaemia-1 contribute to apoptosis resistance of colorectal cancer cells. World J. Gastroenterol. 2008, 14, 3829–3840. [Google Scholar] [CrossRef]
- Ramesh, P.; Medema, J.P. BCL-2 family deregulation in colorectal cancer: Potential for BH3 mimetics in therapy. Apoptosis 2020, 25, 305–320. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Shen, M. Tumor microenvironment shapes colorectal cancer progression, metastasis, and treatment responses. Front. Med. 2022, 9, 869010. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Pricope, L.D.; Grigoras, A.; Dimofte, G.M.; Terinte, C.; Amalinei, C. CD133 and CD166 stem cells markers expression, clinicopathological parameters, and fragmentation response patterns of ypt3 rectal cancer following neoadjuvant chemoradiotherapy. Biomedicines 2025, 13, 1300. [Google Scholar] [CrossRef]
- Nallasamy, P.; Nimmakayala, R.K.; Parte, S.; Are, A.C.; Batra, S.K.; Ponnusamy, M.P. Tumor microenvironment enriches the stemness features: The architectural event of therapy resistance and metastasis. Mol. Cancer 2022, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, A.; Amalinei, C. Multi-faceted role of cancer-associated adipocytes in colorectal cancer. Biomedicines 2023, 11, 2401. [Google Scholar] [CrossRef]
- Huang, Y.; Lou, X.Y.; Zhu, Y.X.; Wang, Y.C.; Zhang, L.; Liu, H.L.; Wang, C.; Zhan, H.M.; Cheng, Z.Q.; Tan, W.Y.; et al. Local environment in biopsy better predict the pathological response to neoadjuvant chemoradiotherapy in rectal cancer. Biosci. Rep. 2019, 39, BSR20190003. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Zhao, Y.; Zhao, Y.; Yang, X.; Sun, L.; Chen, Y.; Zhu, S.; Min, L. Distinct tumor microenvironment landscapes of rectal cancer for prognosis and prediction of immunotherapy response. Cell. Oncol. 2022, 45, 1363–1381. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Saxena, M.; Bhardwaj, N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer 2018, 4, 119–137. [Google Scholar] [CrossRef]
- Li, L.; Wang, B.; Zhao, S.; Xiong, Q.; Cheng, A. The role of ANXA1 in the tumor microenvironment. Int. Immunopharmacol. 2024, 131, 111854. [Google Scholar] [CrossRef]
- Novizio, N.; Belvedere, R.; Morretta, E.; Tomasini, R.; Monti, M.C.; Morello, S.; Petrella, A. Role of intracellular and extracellular Annexin A1 in MIA PaCa-2 spheroids formation and drug sensitivity. Cancers 2022, 14, 4764. [Google Scholar] [CrossRef]
- Liang, Z.; Li, X. Identification of ANXA1 as a potential prognostic biomarker and correlating with immune infiltrates in colorectal cancer. Autoimmunity 2021, 54, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Baracco, E.E.; Stoll, G.; Van Endert, P.; Zitvogel, L.; Vacchelli, E.; Kroemer, G. Contribution of annexin A1 to anticancer immunosurveillance. Oncoimmunology 2019, 8, e1647760. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; D’Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Neutrophils as active regulators of the immune system in the tumor microenvironment. J. Leukoc. Biol. 2017, 102, 343–349. [Google Scholar] [CrossRef]
- Wu, L.; Liu, C.; Chang, D.Y.; Zhan, R.; Sun, J.; Cui, S.H.; Eddy, S.; Nair, V.; Tanner, E.; Brosius, F.C.; et al. Annexin A1 alleviates kidney injury by promoting the resolution of inflammation in diabetic nephropathy. Kidney Int. 2021, 100, 107–121. [Google Scholar] [CrossRef]
- Li, S.Q.; Su, N.; Gong, P.; Zhang, H.B.; Liu, J.; Wang, D.; Sun, Y.P.; Zhang, Y.; Qian, F.; Zhao, B.; et al. The Expression of formyl peptide receptor 1 is correlated with tumor invasion of human colorectal cancer. Sci. Rep. 2017, 7, 5918. [Google Scholar] [CrossRef]
- Triner, D.; Devenport, S.N.; Ramakrishnan, S.K.; Ma, X.; Frieler, R.A.; Greenson, J.K.; Inohara, N.; Nunez, G.; Colacino, J.A.; Mortensen, R.M.; et al. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Gastroenterology 2019, 156, 1467–1482. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, Q.; Shen, X.; Lv, Y.; Sun, L.; An, R.; Zhu, H.; Cai, H.; Chen, G.; Liu, A.; et al. Neutrophil extracellular trap is surrogate biomarker for prognosis and response to neoadjuvant therapy in locally advanced rectal cancer. J. Inflamm. Res. 2023, 16, 6443–6455. [Google Scholar] [CrossRef]
- Huang, E.Y.; Chang, J.C.; Chen, H.H.; Hsu, C.Y.; Hsu, H.C.; Wu, K.L. Carcinoembryonic antigen as a marker of radioresistance in colorectal cancer: A potential role of macrophages. BMC Cancer 2018, 18, 321. [Google Scholar] [CrossRef]
- Kim, J.; Bae, J.S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediat. Inflamm. 2016, 2016, 6058147. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhu, Y.; Xu, W.; Xu, J.; Yang, M.; Chen, P.; Zhao, J.; Geng, L.; Gong, S. PKCα in colon cancer cells promotes M1 macrophage polarization via MKK3/6-P38 MAPK pathway. Mol. Carcinog. 2018, 57, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, J.E.; Hong, Y.S.; Kim, S.Y.; Kim, J.; Ryu, Y.M.; Kim, S.Y.; Kim, T.W. Comprehensive evaluation of the tumor immune microenvironment and its dynamic changes in patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy: From the phase II ADORE study. Oncoimmunology 2022, 11, 2148374. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.R.; Schmid, M.C. Macrophages as key drivers of cancer progression and metastasis. Mediat. Inflamm. 2017, 2017, 9624760. [Google Scholar] [CrossRef]
- Wang, S.; Sun, J.; Chen, K.; Ma, P.; Lei, Q.; Xing, S.; Cao, Z.; Sun, S.; Yu, Z.; Liu, Y.; et al. Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med. 2021, 19, 140. [Google Scholar] [CrossRef]
- Oshi, M.; Tokumaru, Y.; Mukhopadhyay, S.; Yan, L.; Matsuyama, R.; Endo, I.; Takabe, K. Annexin A1 expression is associated with epithelial–mesenchymal transition (EMT), cell proliferation, prognosis, and drug response in pancreatic cancer. Cells 2021, 10, 653. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Shi, S.; Ye, S.; Mao, J.; Ru, Y.; Lu, Y.; Wu, X.; Xu, M.; Zhu, T.; Wang, Y.; Chen, Y.; et al. CMA1 is potent prognostic marker and associates with immune infiltration in gastric cancer. Autoimmunity 2020, 53, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, S.; Martini, G.; Ciardiello, D.; Del Tufo, S.; Martinelli, E.; Troiani, T.; Ciardiello, F. Targeting the EGFR signalling pathway in metastatic colorectal cancer. Lancet Gastroenterol. Hepatol. 2024, 9, 664–676. [Google Scholar] [CrossRef]
- Doleschal, B.; Petzer, A.; Rumpold, H. Current concepts of anti-EGFR targeting in metastatic colorectal cancer. Front. Oncol. 2022, 12, 1048166. [Google Scholar] [CrossRef]
- Gargalionis, A.N.; Papavassiliou, K.A.; Papavassiliou, A.G. Targeting STAT3 signaling pathway in colorectal cancer. Biomedicines 2021, 9, 1016. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Ballinas, L.; López-Pérez, T.V.; Rocha-Zavaleta, L. STAT3 and the STAT3-regulated inhibitor of apoptosis protein survivin as potential therapeutic targets in colorectal cancer (Review). Biomed. Rep. 2024, 21, 175. [Google Scholar] [CrossRef]
- Sawant, A.; Hensel, J.A.; Chanda, D.; Harris, B.A.; Siegal, G.P.; Maheshwari, A.; Ponnazhagan, S. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J. Immunol. 2012, 189, 4258–4265. [Google Scholar] [CrossRef] [PubMed]
- Raphela-Choma, P.P.; Choene, M.S.; Motadi, L.R. Molecular mechanism of angiogenesis in colorectal cancer. Gene Rep. 2025, 39, 102163. [Google Scholar] [CrossRef]
- Na, S.; Collin, O.; Chowdhury, F.; Tay, B.; Ouyang, M.; Wang, Y.; Wang, N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. USA 2008, 105, 6626–6631. [Google Scholar] [CrossRef]
- Delorme, S.; Privat, M.; Sonnier, N.; Rouanet, J.; Witkowski, T.; Kossai, M.; Mishellany, F.; Radosevic-Robin, N.; Juban, G.; Molnar, I.; et al. New insight into the role of ANXA1 in melanoma progression: Involvement of stromal expression in dissemination. Am. J. Cancer Res. 2021, 11, 1600–1615. [Google Scholar]
- Hatakeyama, S.; Sugihara, K.; Shibata, T.K.; Nakayama, J.; Akama, T.O.; Tamura, N.; Wong, S.M.; Bobkov, A.A.; Takano, Y.; Ohyama, C.; et al. Targeted drug delivery to tumor vasculature by a carbohydrate mimetic peptide. Proc. Natl. Acad. Sci. USA 2011, 108, 19587–19592. [Google Scholar] [CrossRef]
- Hebeda, C.B.; Sandri, S.; Benis, C.M.; Paula-Silva, M.D.; Loiola, R.A.; Reutelingsperger, C.; Perretti, M.; Farsky, S.H.P. Annexin A1/formyl peptide receptor pathway controls uterine receptivity to the blastocyst. Cells 2020, 9, 1188. [Google Scholar] [CrossRef]
- Dianat-Moghadam, H.; Nedaeinia, R.; Keshavarz, M.; Azizi, M.; Kazemi, M.; Salehi, R. Immunotherapies targeting tumor vasculature: Challenges and opportunities. Front. Immunol. 2023, 14, 1226360. [Google Scholar] [CrossRef]
- Sun, W. Angiogenesis in metastatic colorectal cancer and the benefits of targeted therapy. J. Hematol. Oncol. 2012, 5, 63. [Google Scholar] [CrossRef]
- Ortiz-Morales, J.; Toledano-Fonseca, M.; Mena-Osuna, R.; Cano, T.; Gómez-España, A.; De la Haba-Rodríguez, J.; Rodríguez-Ariza, A.; Aranda, E. Basal VEGF-A and ACE plasma levels of metastatic colorectal cancer patients have prognostic value for first-line treatment with chemotherapy plus Bevacizumab. Cancers 2022, 14, 3054. [Google Scholar] [CrossRef] [PubMed]
- Affleck Iv, A.A.; Koprowski, M.A.; Nabavizadeh, N.; Tsikitis, V.L. The evolution of rectal cancer treatment: The journey to total neoadjuvant therapy and organ preservation. Ann. Gastroenterol. 2022, 35, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, B.; Carlsson, G.; Machover, D.; Petrelli, N.; Roth, A.; Schmoll, H.J.; Tveit, K.M.; Gibson, F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin. Color. Cancer 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Formslag, C.R.; Zhao, L.; Heslin, A.J.; Lewis, C.C.; Miller, C.W.; Bai, Q.; Wakefield, M.R.; Fang, Y. The past, present, and future of immunotherapy for colorectal cancer. Med. Oncol. 2023, 40, 95. [Google Scholar] [CrossRef]
- Puzzo, M.; De Santo, M.; Morelli, C.; Leggio, A.; Catalano, S.; Pasqua, L. Colorectal cancer: Current and future therapeutic approaches and related technologies addressing multidrug strategies against multiple level resistance mechanisms. Int. J. Mol. Sci. 2025, 26, 1313. [Google Scholar] [CrossRef]
- Nikolouzakis, T.K.; Chrysos, E.; Docea, A.O.; Fragkiadaki, P.; Souglakos, J.; Tsiaoussis, J.; Tsatsakis, A. Current and Future Trends of Colorectal Cancer Treatment: Exploring advances in immunotherapy. Cancers 2024, 16, 1995. [Google Scholar] [CrossRef]
- Cherri, S.; Libertini, M.; Noventa, S.; Oneda, E.; Meriggi, F.; Zaniboni, A. What is next for refractory colorectal cancer CRC? Looking beyond SUNLIGHT, FRESCO2, RECURSE and CORRECT. Int. J. Mol. Sci. 2025, 26, 2522. [Google Scholar] [CrossRef]
- Romero-Zoghbi, S.E.; Krumina, E.; López-Campos, F.; Couñago, F. Current and future perspectives in the management and treatment of colorectal cancer. World J. Clin. Oncol. 2025, 16, 100807. [Google Scholar] [CrossRef]
- Ashique, S.; Bhowmick, M.; Pal, R.; Khatoon, H.; Kumar, P.; Sharma, H.; Garg, A.; Kumar, S.; Das, U. Multi drug resistance in colorectal cancer- approaches to overcome, advancements and future success. Adv. Cancer Biol. Metastasis 2024, 10, 100114. [Google Scholar] [CrossRef]
- Coelho, D.; Estêvão, D.; Oliveira, M.J.; Sarmento, B. Radioresistance in rectal cancer: Can nanoparticles turn the tide? Mol. Cancer 2025, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Liscu, H.D.; Miron, A.I.; Rusea, A.R.; Oprea, A.N.; Mitre, R.; Herdea, A.; Negreanu, R. Short-course radiotherapy versus long-course radio-chemotherapy as neoadjuvant treatment for locally advanced rectal cancer: Meta-analysis from a toxicity perspective. Maedica 2021, 16, 382–388. [Google Scholar] [PubMed]
- Lișcu, H.D.; Antone-Iordache, I.L.; Atasiei, D.I.; Anghel, I.V.; Ilie, A.T.; Emamgholivand, T.; Ionescu, A.I.; Șandru, F.; Pavel, C.; Ultimescu, F. The impact on survival of neoadjuvant treatment interruptions in locally advanced rectal cancer patients. J. Pers. Med. 2024, 14, 266. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, Y.; Smith, J.J.; Fokas, E.; Watanabe, J.; Cercek, A.; Greten, F.R.; Bando, H.; Hi, Q.; Garcia-Aguilar, J.; Romesser, P.B.; et al. Future direction of total neoadjuvant therapy for locally advanced rectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 444–455. [Google Scholar] [CrossRef]
- Lișcu, H.D.; Verga, N.; Atasiei, D.I.; Ilie, A.T.; Vrabie, M.; Roșu, L.; Poștaru, A.; Glăvan, S.; Lucaș, A.; Dinulescu, M.; et al. Therapeutic management of locally advanced rectal cancer: Existing and prospective approaches. J. Clin. Med. 2025, 14, 912. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.G.R.J.; Park, J.; Helewa, R.M.; Goldenberg, B.A.; Nashed, M.; Hyun, E. Total neoadjuvant therapy for rectal cancer: A guide for surgeons. Can. J. Surg. 2023, 66, E196–E201. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, T.; Xiao, L.; Yang, S.; Liu, Q.; Gao, Y.; Chen, G.; Xiao, W. Total neoadjuvant therapy (TNT) versus standard neoadjuvant chemoradiotherapy for locally advanced rectal cancer: A systematic review and meta-analysis. Oncologist 2021, 26, e1555–e1566. [Google Scholar] [CrossRef]
- Petrelli, F.; Trevisan, F.; Cabiddu, M.; Sgroi, G.; Bruschieri, L.; Rausa, E.; Ghidini, M.; Turati, L. Total neoadjuvant therapy in rectal cancer: A systematic review and meta-analysis of treatment outcomes. Ann. Surg. 2020, 271, 440–448. [Google Scholar] [CrossRef]
- Neugut, A.I.; Lin, A.; Raab, G.T.; Hillyer, G.C.; Keller, D.; O’Neil, D.S.; Accordino, M.K.; Kiran, R.P.; Wright, J.; Hershman, D.L. FOLFOX and FOLFIRI use in stage IV colon cancer: Analysis of SEER-Medicare data. Clin. Colorectal Cancer 2019, 18, 133–140. [Google Scholar] [CrossRef]
- De Marchi, T.; Timmermans, A.M.; Smid, M.; Look, M.P.; Stingl, C.; Opdam, M.; Linn, S.C.; Sweep, C.G.J.; Span, P.N.; Kliffen, M.; et al. Annexin-A1 and caldesmon are associated with resistance to tamoxifen in estrogen receptor positive recurrent breast cancer. Oncotarget 2016, 7, 3098–3110. [Google Scholar] [CrossRef]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A double-edged sword in cancer. ERK-dependent apoptosis as a potential therapeutic strategy for cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef]
- Wang, L.; Xue, M.; Chung, D.C. c-Myc is regulated by HIF-2α in chronic hypoxia and influences sensitivity to 5-FU in colon cancer. Oncotarget 2016, 7, 78910–78917. [Google Scholar] [CrossRef]
- Zichittella, C.; Barreca, M.M.; Cordaro, A.; Corrado, C.; Alessandro, R.; Conigliaro, A. Mir-675-5p supports hypoxia-induced drug resistance in colorectal cancer cells. BMC Cancer 2022, 22, 567. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ma, R.; Zhang, M. CoCl2 increases the expression of hypoxic markers HIF-1α, VEGF and CXCR4 in breast cancer MCF-7 cells. Oncol. Lett. 2018, 15, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ning, X.; Sun, L.; Zhang, H.; Shi, Y.; Guo, C.; Han, S.; Liu, J.; Sun, S.; Han, Z.; et al. Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci. 2008, 99, 121–128. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Z.; Peng, Y.; Pan, F.; Li, J.; Zou, L.; Zhang, Y.; Liang, H. HIF-1α inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS ONE 2014, 9, e98882. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.Q.; Luo, Q.; Wang, L.; Song, G.B.; Sheng, J.P.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Signal Transduct. Target Ther. 2024, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hu, Z.; Niu, G.; Xia, J.; Wang, X.; Hong, R.; Gu, J.; Wang, D.; Ke, C. Annexin A1 induces oxaliplatin resistance of gastric cancer through autophagy by targeting PI3K/AKT/mTOR. FASEB J. 2023, 37, e22790. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Long, G.V.; Scolyer, R.A.; Teng, M.W.; Smyth, M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 2017, 52, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Z.; Liu, Y.Y.; Zhu, W.; Xiao, D.; Huang, W.; Lu, S.S.; Yi, H.; Zeng, T.; Feng, X.P.; Yuan, L.; et al. ANXA1-derived peptide for targeting PD-L1 degradation inhibits tumor immune evasion in multiple cancers. J. Immunother. Cancer 2023, 11, e006345. [Google Scholar] [CrossRef]
- Stojanovska, V.; Samy Sakkal, S.; Nurgali, K. Platinum-based chemotherapy: Gastrointestinal immunomodulation and enteric nervous system toxicity. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G223–G232. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef]
- Alipourgivi, F.; Motolani, A.; Qiu, A.Y.; Qiang, W.; Yang, G.-Y.; Chen, S.; Lu, T. Genetic alterations of NF-κB and its regulators: A rich platform to advance colorectal cancer diagnosis and treatment. Int. J. Mol. Sci. 2024, 25, 154. [Google Scholar] [CrossRef]
- Cai, B.Q.; Chen, W.M.; Zhao, J.; Hou, W.; Tang, J.C. Nrf3 promotes 5-FU resistance in colorectal cancer cells via the NF-κB/BCL-2 signaling pathway in vitro and in vivo. J. Oncol. 2021, 2021, 9355555. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Esbati, N.; Rashidi, M.; Gholami, S.; Raesi, R.; Bidoki, S.S.; Goharrizi, M.A.S.B.; Motlagh, Y.S.M.; Khorrami, R.; Tavakolpournegari, A.; et al. Biological landscape and nanostructural view in development and reversal of oxaliplatin resistance in colorectal cancer. Transl. Oncol. 2024, 40, 101846. [Google Scholar] [CrossRef] [PubMed]
- Pearanpan, L.; Nordin, F.J.; Siew, E.L.; Kumolosasi, E.; Hanif, M.E.A.; Masre, S.F.; Chua, E.W.; Cheng, H.S.; Rajab, N.F. A cell-based systematic review on the role of annexin a1 in triple-negative breast cancers. Int. J. Mol. Sci. 2022, 23, 8256. [Google Scholar] [CrossRef]
- Clay, S.L.; Fonseca-Pereira, D.; Garrett, W.S. Colorectal cancer: The facts in the case of the microbiota. J. Clin. Investig. 2022, 132, e155101. [Google Scholar] [CrossRef]
- Chiarini, F.; Paganelli, F.; Martelli, A.M.; Evangelisti, C. The role played by wnt/β-catenin signaling pathway in acute lymphoblastic leukemia. Int. J. Mol. Sci. 2020, 21, 1098. [Google Scholar] [CrossRef]
- Hervieu, C.; Christou, N.; Battu, S.; Mathonnet, M. The role of cancer stem cells in colorectal cancer: From the basics to novel clinical trials. Cancers 2021, 13, 1092. [Google Scholar] [CrossRef]
- Bizzarro, V.; Belvedere, R.; Milone, M.R.; Pucci, B.; Lombardi, R.; Bruzzese, F.; Popolo, A.; Parente, L.; Budillon, A.; Petrella, A. Annexin A1 is involved in the acquisition and maintenance of a stem cell-like/aggressive phenotype in prostate cancer cells with acquired resistance to zoledronic acid. Oncotarget 2015, 6, 25076–25092. [Google Scholar] [CrossRef]
- Cardin, L.T.; Prates, J.; da Cunha, B.R.; Tajara, E.H.; Oliani, S.M.; Rodrigues-Lisoni, F.C. Annexin A1 peptide and endothelial cell-conditioned medium modulate cervical tumorigenesis. FEBS Open Bio 2019, 9, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Fang, Y.; Long, J.; Zhang, Y. Annexin 1-nuclear factor-κB-microRNA-26a regulatory pathway in the metastasis of non-small cell lung cancer. Thorac. Cancer 2019, 10, 665–675. [Google Scholar] [CrossRef]
- Kelly, L.; McGrath, S.; Rodgers, L.; McCall, K.; Tulunay Virlan, A.; Dempsey, F.; Crichton, S.; Goodyear, C.S. Annexin-A1: The culprit or the solution? Immunology 2022, 166, 2–16. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, B.; Zhu, L.; Yi, L.; Jin, X. RRM2 regulates sensitivity to Sunitinib and PD-1 blockade in renal cancer by stabilizing ANXA1 and activating the AKT pathway. Adv. Sci. 2021, 8, e2100881. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Fujishita, T. Oncogenic roles of the PI3K/AKT/mTOR axis. Curr. Top. Microbiol. Immunol. 2017, 407, 153–189. [Google Scholar]
- Leite, L.F.; Noronha, M.M.; de Menezes, J.S.A.; da Conceição, L.D.; Almeida, L.F.C.; Cappellaro, A.P.; Belotto, M.; Biachi de Castria, T.; Peixoto, R.D.; Megid, T.B.C. Anti-EGFR Therapy in Metastatic Colorectal Cancer: Identifying, Tracking, and Overcoming Resistance. Cancers 2025, 17, 2804. [Google Scholar] [CrossRef]
- Brand, T.M.; Iida, M.; Luthar, N.; Starr, M.M.; Huppert, E.J.; Wheeler, D.L. Nuclear EGFR as a molecular target in cancer. Radiother. Oncol. 2013, 108, 370–377. [Google Scholar] [CrossRef]
- Ganesan, T.; Sinniah, A.; Chik, Z.; Alshawsh, M.A. Punicalagin regulates apoptosis-autophagy switch via modulation of Annexin A1 in colorectal cancer. Nutrients 2020, 12, 2430. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Khyeam, S.; Zhang, Z.J.; Zhang, K.Y.B. Granatin B and punicalagin from Chinese herbal medicine pomegranate peels elicit reactive oxygen species-mediated apoptosis and cell cycle arrest in colorectal cancer cells. Phytomedicine 2022, 97, 153923. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.; Symeonides, S.; Dempsey, F.; Crichton, S.; Tennant, C.; Wood, C.; Knight, R.; Balabanova, S.; Upton, C.; Low, S.; et al. ATTAINMENT: A phase Ib trial of MDX-124, a first-in-class annexin-A1 targeting antibody, alone and in combination with anti-cancer treatments, in patients with advanced solid tumors. J. Clin. Oncol. 2024, 42, TPS2671. [Google Scholar] [CrossRef]
- Dempsey, F.; Crichton, S.; Parris, C.; Ibrahem, R.; Al-Ali, H.; Pepper, C.; Patel, A. MDX-124, a novel anti-ANXA1 antibody, has significant anti-cancer activity in preclinical models of osteosarcoma. ESMO Open 2025, 10, 104406. [Google Scholar] [CrossRef]
| Cancer Type | Study Models | ANXA1 Expression | Roles and Clinical Signification | References |
|---|---|---|---|---|
| Breast cancer | 4T1 breast cancer cell lines | −/+++ |
| [85] |
| ANXA1 blocker Boc1 administration in balb/c mice and in TNBC patient tissue samples | −/+ |
| [41] | |
| MDA-MB-231 cell line and TNBC patient tissue samples | + |
| [86] | |
| Lung cancer | A549, H1703, H1650, H460, H1975, and H157 tumor cell lines | +++ |
| [87] |
| Mice xenograft lung cancer |
| |||
| OSCC | SCC-9 and Tca-8113 cell lines | + |
| [88] |
| NPC | NPC patient tissue samples | +++ |
| [89] |
| 6–10B and 5–8F NPC cell lines | [76] | |||
| Pancreatic cancer | Human MIA PaCa-2 cells | +++ |
| [90] |
| Melanoma | M4Beu, SK-MEL-3, M3Dau, and A375 cells lines | −/+ |
| [62] |
| Glioblastoma | Human glioblastoma cell lines U251 and U87 | +++ |
| [91] |
| Gastric cancer | Patient tissue samples AGS and N87 cell lines | −/+ |
| [92] |
| Prostate cancer | DU145, LNCaP, and PC3 cells lines | − |
| [93] |
| Parameters | Study Model | Colon Cancer and CRC | RC | References | ||
|---|---|---|---|---|---|---|
| ANXA1 Expression | Functional Correlation | ANXA1 Expression | Functional Correlation | |||
| TNM status | Patients’ tissue samples | Positive | T3 and T4 stages | Positive | T3 and T4 stages | [17,21,27,94] |
| Lymph node metastasis | Patients’ tissue samples | Positive | Positive | Positive | N2 and N3 stages | [17,27,94] |
| Tumor angiogenesis and vascular invasion | Patients’ tissue samples | Positive | Vascular invasion | Positive | Vascular invasion | [17,27] |
| Metastasis | Patients’ tissue sample | Positive | Variable positive | Positive | MeFS ↓ | [17,27] |
| Prognosis | Patients’ tissue samples | Positive | DSS ↓ OS ↓ | Positive | DSS ↓ OS ↓ LRFR ↓ | [17,27,68,128] |
| Serological marker | Patients’ serum samples | Value ↑ Value ↓ |
| - | - | [94,103] |
| Tumor cells’ cycle |
| Positive |
| - | - | [25,68,94,112] |
| TME hypoxia | SW620, HCT116, and SW48 colon carcinoma cell lines | Positive | HIF-1α ↑ | - | - | [25] |
| Tumor-infiltrating immune cells | Patients’ tissue samples | Positive |
| - | - | [128] |
| Negative | [29] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pricope, D.L.; Grigoraș, A.; Dimofte, G.M.; Amalinei, C. The Multifaceted Role of Annexin A1 in Colorectal Cancer: From Molecular Mechanisms to Predictive and Prognostic Implications. Med. Sci. 2025, 13, 263. https://doi.org/10.3390/medsci13040263
Pricope DL, Grigoraș A, Dimofte GM, Amalinei C. The Multifaceted Role of Annexin A1 in Colorectal Cancer: From Molecular Mechanisms to Predictive and Prognostic Implications. Medical Sciences. 2025; 13(4):263. https://doi.org/10.3390/medsci13040263
Chicago/Turabian StylePricope, Diana Lavinia, Adriana Grigoraș, Gabriel Mihail Dimofte, and Cornelia Amalinei. 2025. "The Multifaceted Role of Annexin A1 in Colorectal Cancer: From Molecular Mechanisms to Predictive and Prognostic Implications" Medical Sciences 13, no. 4: 263. https://doi.org/10.3390/medsci13040263
APA StylePricope, D. L., Grigoraș, A., Dimofte, G. M., & Amalinei, C. (2025). The Multifaceted Role of Annexin A1 in Colorectal Cancer: From Molecular Mechanisms to Predictive and Prognostic Implications. Medical Sciences, 13(4), 263. https://doi.org/10.3390/medsci13040263






