Identifying Cardio-Metabolic Subtypes of Prediabetes Using Latent Class Analysis
Abstract
1. Introduction
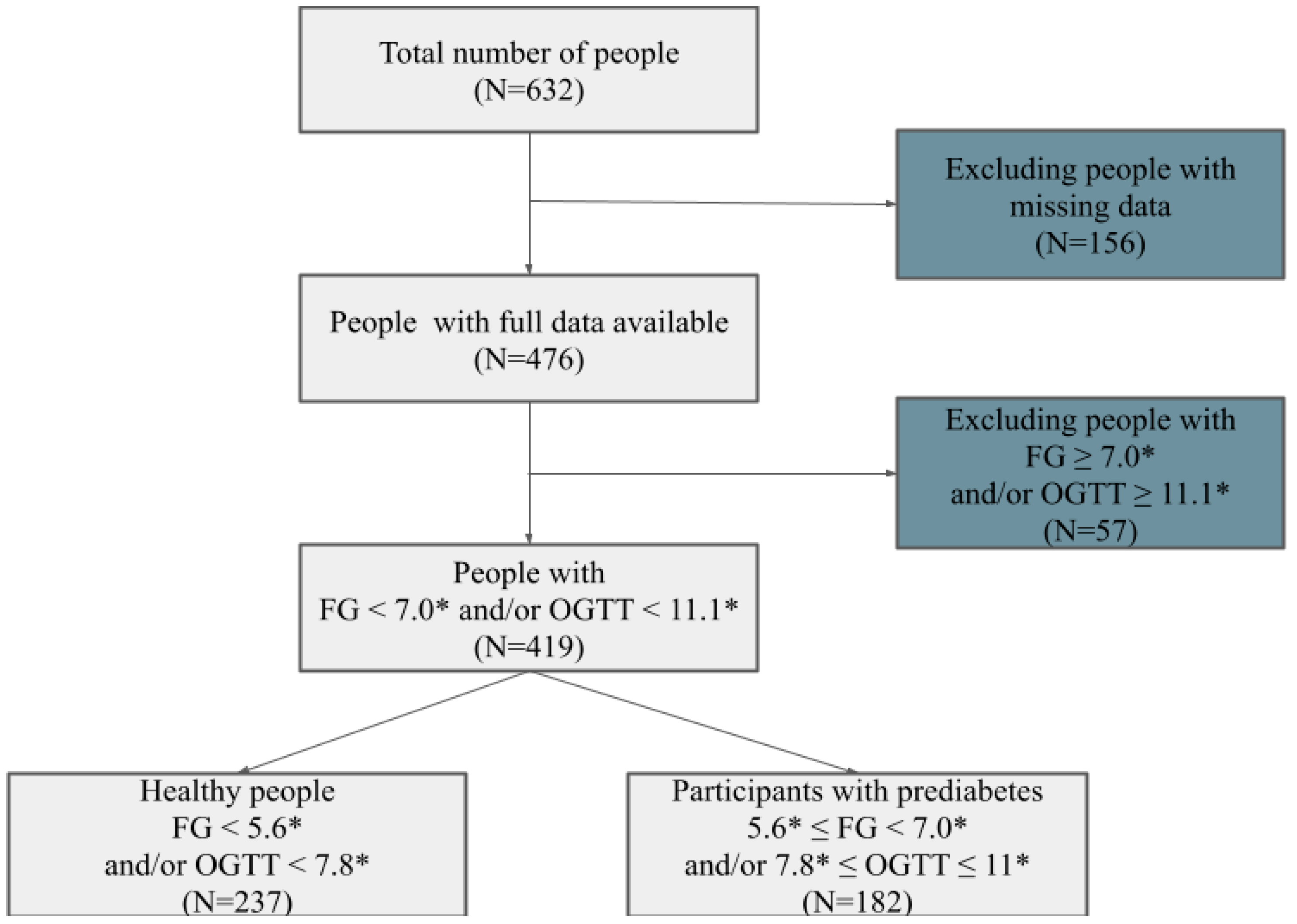
2. Materials and Methods
3. Results
3.1. General Description of the Sample
3.2. LCA Group Definitions and Their Cardio-Metabolic Characteristics
- Class 1: Healthy, low risk (n = 62; 34.1% of PreDM group; median age: 35 years, BMI: 24.8 kg/m2), predominantly IFG (95%, 95% CI: 89–98%).
- Class 2: Overweight, moderate risk (n = 48; 26.4%; median age: 42 years, BMI: 27.5 kg/m2), with 18% IFG + IGT (95% CI: 10–29%).
- Class 3: Older, overweight, high risk (n = 42; 23.1%; median age: 55 years, BMI: 29.2 kg/m2), with 33% IFG + IGT (95% CI: 21–47%) and 40% β-cell dysfunction (HOMA-β ≤ 50, 95% CI: 27–55%).
- Class 4: Obese, middle-aged to older, very high risk (n = 30; 16.5%; median age: 52 years, BMI: 32.1 kg/m2), with 30% IFG + IGT (95% CI: 17–47%) and 50% β-cell dysfunction (95% CI: 34–66%).
4. Discussion
- Age-Related Changes in Glucose Metabolism: Older age in Clusters 3 and 4 is associated with a combined increase in IR and progressive β-cell decline, while higher IFG but lower IGT in younger participants (Cluster 1), may reflect hepatic IR and still-compensating β-cells.
- Obesity and IR Patterns: The worst glycemia is identified in Cluster 4, characterized by high-risk obesity, but with no significant difference in HOMA-IR. This may reflect adipose-related IR affecting both the liver and peripheral tissues, and failing compensatory hyperinsulinemia due to β-cell exhaustion. Despite the lack of statistically significant differences in HOMA-IR between clusters, the observed decline in β-cell function in this cluster suggests that depletion of β-cell insulin secretory capacity, rather than insulin resistance itself, may play a major role in diabetes progression in this population.
- β-cell function and compensation: The declining HOMA-β across clusters indicates progressive β-cell dysfunction, with the lowest values observed in Clusters 3 and 4. Despite similar HOMA-IR values across groups, this suggests insufficient compensation in older and obese groups.
- Glucose dysregulation: suggested by diverse IFG vs. IGT vs. IFG + IGT profiles:
- High IFG: predominantly hepatic IR (Cluster 1);
- IFG + IGT: mixed and advanced hepatic and muscle IR (in Clusters 3 and 4);
- Increasing IGT/IFG + IGT: a transitional group with early decline in β-cell function (Cluster 2).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2DM | Type 2 Diabetes Mellitus |
| PreDM | Prediabetes |
| LCA | Latent Class Analysis |
| BMI | Body Mass Index |
| HOMA | Homeostatic Model Assessment |
| OGTT | Oral Glucose Tolerance Test |
| IR | Insulin Resistance |
| IFG | Impaired Fasting Glucose |
| IGT | Impaired Glucose Tolerance |
References
- The Lancet Diabetes & Endocrinology. Prediabetes: Much More Than Just a Risk Factor. Lancet Diabetes Endocrinol. 2025, 13, 165. [Google Scholar] [CrossRef]
- American Diabetes Association. Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S20–S42. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.; Hemmingsen, B.; Metzendorf, M.I.; Takwoingi, Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst. Rev. 2018, 10, CD012661. [Google Scholar] [CrossRef]
- Rooney, M.R.; He, J.H.; Salpea, P.; Genitsaridi, I.; Magliano, D.J.; Boyko, E.J.; Wallace, A.S.; Fang, M.; Selvin, E. Global and regional prediabetes prevalence: Updates for 2024 and projections for 2050. Diabetes Care 2025, 48, e142–e144. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Hauguel-Moreau, M.; Hergault, H.; Cazabat, L.; Pepin, M.; Beauchet, A.; Aidan, V.; Ouadahi, M.; Josseran, L.; Hage, M.; Rodon, C.; et al. Prevalence of prediabetes and undiagnosed diabetes in a large urban middle-aged population: The CARVAR 92 cohort. Cardiovasc. Diabetol. 2023, 22, 31. [Google Scholar] [CrossRef] [PubMed]
- Orazumbekova, B.; Issanov, A.; Atageldiyeva, K.; Berkinbayev, S.; Junusbekova, G.; Danyarova, L.; Shyman, Z.; Tashmanova, A.; Sarria-Santamera, A. Prevalence of Impaired Fasting Glucose and Type 2 Diabetes in Kazakhstan: Findings from Large Study. Front. Public Health 2022, 10, 810153. [Google Scholar] [CrossRef] [PubMed]
- Aleman-Vega, G.; Garrido-Elustondo, S.; Del Cura-Gonzalez, I.; Sarria-Santamera, A. Is a maintained glycemia between 110/125 mg/dl a risk factor in the development of diabetes? Aten. Primaria 2017, 49, 557–558. [Google Scholar]
- Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; Kubik, M.; et al. US Preventive Services Task Force Screening for Prediabetes Type 2 Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 326, 736–743. [Google Scholar] [CrossRef]
- Hollander, P.; Spellman, C. Controversies in prediabetes: Do we have a diagnosis? Postgrad. Med. 2012, 124, 109–118. [Google Scholar] [CrossRef]
- Barbu, E.; Popescu, M.R.; Popescu, A.C.; Balanescu, S.M. Phenotyping the prediabetic population-a closer look at intermediate glucose status and cardiovascular disease. Int. J. Mol. Sci. 2021, 22, 6864. [Google Scholar] [CrossRef] [PubMed]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Barthow, C.; Pullon, S.; McKinlay, E.; Krebs, J. It is time for a more targeted approach to prediabetes in primary care in Aotearoa New Zealand. J. Prim. Health Care 2022, 14, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Geloneze, B.; Vasques, A.C.J.; Stabe, C.F.C.; Pareja, J.C.; Rosado, L.E.F.P.; Queiroz, E.C.; Tambascia, M.A. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome—Brazilian Metabolic Syndrome Study (BRAMS). Arq. Bras. Endocrinol. Metabol. 2009, 53, 281–287. [Google Scholar] [CrossRef]
- Kim, B.; Choi, H.Y.; Kim, W.; Ahn, C.; Lee, J.; Kim, J.G.; Kim, J.; Shin, H.; Yu, J.M.; Moon, S. The cut-off values of surrogate measures for insulin resistance in the Korean population according to the Korean Genome and Epidemiology Study (KOGES). PLoS ONE 2018, 13, e0206994. [Google Scholar] [CrossRef]
- Basukala, P.; Jha, B.; Yadav, B.K.; Shrestha, P.K. Determination of insulin resistance and Beta-Cell function using homeostatic model assessment in Type 2 diabetic patients at diagnosis. J. Diabetes Metab. 2018, 9, 1000790. [Google Scholar] [CrossRef]
- Lanza, S.T.; Rhoades, B.L. Latent class analysis: An alternative perspective on subgroup analysis in prevention and treatment. Prev. Sci. 2013, 14, 157–168. [Google Scholar] [CrossRef]
- Akter, S.; Rahman, M.M.; Abe, S.K.; Sultana, P. Prevalence of diabetes and prediabetes and their risk factors among Bangladeshi adults: A nationwide survey. Bull. World Health Organ. 2014, 92, 204–213A. [Google Scholar] [CrossRef]
- Satman, I.; Omer, B.; Tutuncu, Y.; Kalaca, S.; Gedik, S.; Dinccag, N.; Karsidag, K.; Genc, S.; Telci, A.; Canbaz, B.; et al. Twelve-year trends in the prevalence and risk factors of diabetes and prediabetes in Turkish adults. Eur. J. Epidemiol. 2013, 28, 169–180. [Google Scholar] [CrossRef]
- Bocquet, V.; Ruiz-Castell, M.; de Beaufort, C.; Barré, J.; de Rekeneire, N.; Michel, G.; Donahue, R.P.; Kuemmerle, A.; Stranges, S. Public health burden of pre-diabetes and diabetes in Luxembourg: Finding from the 2013–2015 European Health Examination Survey. BMJ Open 2019, 9, e022206. [Google Scholar] [CrossRef]
- Vera-Ponce, V.J.; Zuzunaga-Montoya, F.E.; Vásquez-Romero, L.E.M.; Loayza-Castro, J.A.; Paucar, C.R.I.; De Carrillo, C.I.G.; Valladares-Garrido, M.J.; Medina, M.P. Anthropometric measures of obesity as risk indicators for prediabetes. A systematic review and meta-analysis. Diabetes Epidemiol. Manag. 2024, 16, 100230. [Google Scholar] [CrossRef]
- Beulens, J.W.J.; Rutters, F.; Ryden, L.; Schnell, O.; Mellbin, L.; Hart, H.E.; Vos, R.C. Risk and management of pre-diabetes. Eur. J. Prev. Cardiol. 2019, 26 (Suppl. S2), 47–54. [Google Scholar] [CrossRef]
- Gupta, A.K.; Greenway, F.L.; Cornelissen, G.; Pan, W.; Halberg, F. Prediabetes is associated with abnormal circadian blood pressure variability. J. Hum. Hypertens. 2008, 22, 627–633. [Google Scholar] [CrossRef]
- Nanri, A.; Nakagawa, T.; Kuwahara, K.; Yamamoto, S.; Honda, T.; Okazaki, H.; Uehara, A.; Yamamoto, M.; Miyamoto, T.; Kochi, T.; et al. Development of Risk Score for Predicting 3-Year Incidence of Type 2 Diabetes: Japan Epidemiology Collaboration on Occupational Health Study. PLoS ONE 2015, 10, e0142779. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, Y.; Li, M.; Wu, J.H.; Mai, L.; Li, J.; Yang, Y.; Hu, Y.; Huang, Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ 2020, 370, m2297. [Google Scholar] [CrossRef] [PubMed]
- Armato, J.P.; DeFronzo, R.A.; Abdul-Ghani, M.; Ruby, R.J. Successful treatment of prediabetes in clinical practice using physiological assessment (STOP DIABETES). Lancet Diabetes Endocrinol. 2018, 6, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Hjellvik, V.; Strøm, H.; Sakshaug, S. Body mass index, triglycerides, glucose, and blood pressure as predictors of type 2 diabetes in a middle-aged Norwegian cohort of men and women. Clin. Epidemiol. 2012, 4, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS ONE 2018, 13, e0194127. [Google Scholar] [CrossRef]
- Glechner, A.; Keuchel, L.; Affengruber, L.; Titscher, V.; Sommer, I.; Matyas, N.; Wagner, G.; Kien, C.; Klerings, I.; Gartlehner, G. Effects of lifestyle changes on adults with prediabetes: A systematic review and meta-analysis. Prim. Care Diabetes 2018, 12, 393–408. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.-F.; Chen, J.; Xia, L.; Cao, A.; Zhang, Y.; Wang, J.; Li, H.; Yang, K.; Guo, K.; et al. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Diabetologia 2020, 63, 21–33. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, H.; Zhou, X.; Chang, C.; Chen, W.; Guo, X.; Yang, J.; Ji, L.; Paul, S.K. A randomized controlled clinical trial of lifestyle intervention and pioglitazone for normalization of glucose status in Chinese with prediabetes. J. Diabetes Res. 2022, 2022, 2971382. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, H.K.; Kim, E.H.; Bae, S.J.; Choe, J.; Park, J.Y. Risk of progression to diabetes from prediabetes defined by HbA1c or fasting plasma glucose criteria in Koreans. Diabetes Res. Clin. Pract. 2016, 118, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bennasar-Veny, M.; Fresneda, S.; López-González, A.; Busquets-Cortés, C.; Aguiló, A.; Yañez, A.M. Lifestyle and progression to type 2 diabetes in a cohort of workers with prediabetes. Nutrients 2020, 12, 1538. [Google Scholar] [CrossRef]
- Giráldez-García, C.; Cea-Soriano, L.; Albaladejo, R.; Franch-Nadal, J.; Mata-Cases, M.; Díez-Espino, J.; Artola, S.; Serrano, R.; Regidor, E.; PREDAPS Study Group. The heterogeneity of reversion to normoglycemia according to prediabetes type is not explained by lifestyle factors. Sci. Rep. 2021, 11, 9667. [Google Scholar] [CrossRef]
- Sentell, T.L.; He, G.; Gregg, E.W.; Schillinger, D. Racial/ethnic variation in prevalence estimates for United States prediabetes under alternative 2010 American Diabetes Association criteria: 1988–2008. Ethn. Dis. 2012, 22, 451–458. [Google Scholar] [PubMed]
- Vicks, W.S.; Lo, J.C.; Guo, L.; Rana, J.S.; Zhang, S.; Ramalingam, N.D.; Gordon, N.P. Prevalence of prediabetes and diabetes vary by ethnicity among U.S. Asian adults at healthy weight, overweight, and obesity ranges: An electronic health record study. BMC Public Health 2022, 22, 1954. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Heni, M.; Tabák, A.G.; Machann, J.; Schick, F.; Randrianarisoa, E.; de Angelis, M.H.; Birkenfeld, A.L.; Stefan, N.; Peter, A.; et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat. Med. 2021, 27, 49–57. [Google Scholar] [CrossRef]
- Prystupa, K.; Delgado, G.E.; Moissl, A.P.; Kleber, M.E.; Birkenfeld, A.L.; Heni, M.; Fritsche, A.; März, W.; Wagner, R. Clusters of prediabetes and type 2 diabetes stratify all-cause mortality in a cohort of participants undergoing invasive coronary diagnostics. Cardiovasc. Diabetol. 2023, 22, 211. [Google Scholar] [CrossRef]
- Cho, S.B.; Kim, S.C.; Chung, M.G. Identification of novel population clusters with different susceptibilities to type 2 diabetes and their impact on the prediction of diabetes. Sci. Rep. 2019, 9, 3329. [Google Scholar] [CrossRef] [PubMed]
- Méndez, D.Y.; Zhou, M.; Lagerros, Y.T.; Velasco, D.V.G.; Tynelius, P.; Gudjonsdottir, H.; de Leon, A.P.; Eeg-Olofsson, K.; Östenson, C.-G.; Brynedal, B.; et al. Characterization of data-driven clusters in diabetes-free adults and their utility for risk stratification of type 2 diabetes. BMC Med. 2022, 20, 356. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.C.; Moon, J.Y.; Arthur, R.; Sotres-Alvarez, D.; Daviglus, M.L.; Pirzada, A.; Mattei, J.; Perreira, K.M.; Rotter, J.I.; et al. Genetic Subtypes of Prediabetes, Healthy Lifestyle, and Risk of Type 2 Diabetes. Diabetes 2024, 73, 1178–1187. [Google Scholar] [CrossRef]
- Huemer, M.-T.; Spagnuolo, M.C.; Maalmi, H.; Wagner, R.; Bönhof, G.J.; Heier, M.; Koenig, W.; Rathmann, W.; Prystupa, K.; Nano, J.; et al. Phenotype-based clusters, inflammation and cardiometabolic complications in older people before the diagnosis of type 2 diabetes: KORA F4/FF4 cohort study. Cardiovasc. Diabetol. 2025, 24, 83. [Google Scholar] [CrossRef]
- Fowler, L.A.; Fernández, J.R.; O’Neil, P.M.; Parcha, V.; Arora, P.; Shetty, N.S.; Cardel, M.I.; Foster, G.D.; Gower, B.A. Genetic Risk Phenotypes for Type 2 Diabetes Differ with Ancestry in US Adults with Diabetes and Overweight/Obesity. Arch. Med. Res. 2025, 56, 103128. [Google Scholar] [CrossRef] [PubMed]
- Onthoni, D.D.; Chen, Y.E.; Lai, Y.H.; Li, G.H.; Zhuang, Y.S.; Lin, H.M.; Hsiao, Y.P.; Onthoni, A.I.; Chiou, H.Y.; Chung, R.H. Clustering-based risk stratification of prediabetes populations: Insights from the Taiwan and UK Biobanks. J. Diabetes Investig. 2025, 16, 25–35. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; McDowell, C.; Laffey, J.G.; O’Kane, C.M.; McAuley, D.F.; et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018, 6, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, H.; Cai, J.; Chen, R.; Zuo, X.; Cheng, H.; Yan, D. Applying latent class analysis to risk stratification of incident diabetes among Chinese adults. Diabetes Res. Clin. Pract. 2021, 174, 108742. [Google Scholar] [CrossRef] [PubMed]
- Lanza, S.T.; Rhoades, B.L.; Nix, R.L.; Greenberg, M.T. Modeling the interplay of multilevel risk factors for future academic and behavior problems: A person-centered approach. Dev. Psychopathol. 2010, 22, 313–335. [Google Scholar] [CrossRef]
- Færch, K.; Borch-Johnsen, K.; Holst, J.J.; Vaag, A. Pathophysiology and etiology of impaired fasting glycaemia and impaired glucose tolerance: Does it matter for prevention and treatment of type 2 diabetes. Diabetologia 2009, 52, 1714–1723. [Google Scholar] [CrossRef]
- Franks, P.W.; Sargent, J.L. Diabetes and obesity: Leveraging heterogeneity for precision medicine. Eur. Heart J. 2024, 45, 5146–5155. [Google Scholar] [CrossRef]
- Nathan, D.M.; Davidson, M.B.; DeFronzo, R.A.; Heine, R.J.; Henry, R.R.; Pratley, R.; Zinman, B. Impaired Fasting Glucose and Impaired Glucose Tolerance: Implications for care. Diabetes Care 2007, 30, 753–759. [Google Scholar] [CrossRef]
- Faerch, K.; Vaag, A.; Holst, J.J.; Hansen, T.; Jorgensen, T.; Borch-Johnsen, K. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance-the Inter99 study. Diabetes Care 2009, 32, 439–444. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Kodama, K.; Tojjar, D.; Yamada, S.; Toda, K.; Patel, C.J.; Butte, A.J. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care 2013, 36, 1789–1796. [Google Scholar] [CrossRef]
- Yabe, D.; Seino, Y.; Fukushima, M.; Seino, S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr. Diabetes Rev. 2015, 15, 602. [Google Scholar] [CrossRef]
- Sikhayeva, N.; Talzhanov, Y.; Iskakova, A.; Dzharmukhanov, J.; Nugmanova, R.; Zholdybaeva, E.; Ramanculov, E. Type 2 diabetes mellitus: Distribution of genetic markers in Kazakh population. Clin. Interv. Aging 2018, 13, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Saruarov, Y.; Nuskabayeva, G.; Gencer, M.Z.; Sadykova, K.; Zhunissova, M.; Tatykayeva, U.; Iskandirova, E.; Sarsenova, G.; Durmanova, A.; Gaipov, A.; et al. Associations of Clusters of Cardiovascular Risk Factors with Insulin Resistance and ?-Cell Functioning in a Working-Age Diabetic-Free Population in Kazakhstan. Int. J. Environ. Res. Public Health 2023, 20, 3918. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Aguilar-Salinas, C.A.; Chikowore, T.; Konradsen, F.; Ma, R.C.; Mbau, L.; Mohan, V.; Morton, R.W.; Nyirenda, M.J.; Tapela, N.; et al. The case for precision medicine in the prevention, diagnosis, and treatment of cardiometabolic diseases in low-income and middle-income countries. Lancet Diabetes Endocrinol. 2023, 11, 836–847. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Normoglycemic | Prediabetes | p | ||||
|---|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||||
| Sex | Men | 59 | 24.9 | 59 | 32.4 | NS | |
| Women | 178 | 75.1 | 123 | 67.6 | |||
| Age groups (years) | 20–29 | 8 | 3.40 | 0 | 0 | <0.000 | |
| 30–39 | 75 | 31.6 | 21 | 11.5 | |||
| 40–49 | 66 | 27.8 | 38 | 20.9 | |||
| 50–59 | 61 | 25.7 | 62 | 34.1 | |||
| 60–69 | 27 | 11.4 | 61 | 33.5 | |||
| Kazakh | 204 | 86.1 | 166 | 91.2 | NS | ||
| BMI (kg/m2) | Normal | 97 | 41.0 | 27 | 14.8 | <0.000 | |
| Overweight | 75 | 31.6 | 70 | 38.5 | |||
| Obese | 65 | 27.4 | 85 | 46.7 | |||
| Waist circumference (cm) | M: <94; F: <80 | 91 | 38.4 | 27 | 14.8 | <0.000 | |
| M: 94–102; F: 80–88 | 47 | 19.8 | 33 | 18.1 | |||
| M: 102<; F: 88< | 99 | 41.7 | 122 | 67.0 | |||
| Insulin resistance | 38 | 16.0 | 58 | 31.9 | <0.000 | ||
| Poor beta-cell function | 22 | 9.28 | 87 | 47.8 | <0.000 | ||
| Total number of participants | 237 | 56.6 | 182 | 43.4 | |||
| Characteristics | Normoglycemic | Prediabetes | p | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Age (years) | 45 | 14 | 55 | 16 | <0.000 |
| BMI | 26.30 | 7.89 | 29.38 | 7.30 | <0.000 |
| Waist circumference (cm) | 89 | 20 | 97 | 15 | <0.000 |
| Hip circumference (cm) | 101 | 14 | 108 | 13 | <0.000 |
| SBP (mmHg) | 110 | 30 | 140 | 40 | <0.000 |
| DBP (mmHg) | 80 | 20 | 82.5 | 10 | <0.000 |
| Total cholesterol (mmol/L) | 4.80 | 0.8 | 5.10 | 1.10 | <0.000 |
| LDL-cholesterol (mmol/L) | 2.10 | 0.71 | 2.36 | 0.69 | <0.000 |
| HDL-cholesterol (mmol/L) | 1.26 | 0.24 | 1.17 | 0.25 | 0.009 |
| TG (mmol/L) | 1.97 | 1.21 | 2.07 | 0.92 | 0.034 |
| Fasting glucose (mmol/L) | 5.0 | 0.54 | 6.20 | 0.7 | <0.000 |
| OGTT (mmol/L) | 5.3 | 0.8 | 5.80 | 1.85 | <0.000 |
| Fasting insulin (µU/mL) | 7.73 | 4.77 | 7.52 | 5.02 | <0.000 |
| HOMA-IR | 1.67 | 1.02 | 2.02 | 1.36 | <0.000 |
| HOMA-beta | 114.0 | 92.99 | 52.84 | 45.69 | <0.000 |
| Healthy, Low Risk | Overweight, Moderate Risk | Older, Overweight, High Risk | Obese, Middle-Aged to Older, Very High Risk | p | |
|---|---|---|---|---|---|
| % | 20.8 | 19.8 | 38.5 | 20.8 | |
| Fasting glucose | 5.99 | 6.17 | 6.28 | 6.68 | 0.001 |
| OGTT | 5.51 | 6.19 | 6.51 | 6.58 | 0.003 |
| HOMA IR | 2.35 | 2.25 | 1.96 | 2.34 | 0.194 |
| HOMA beta | 72.21 | 65.63 | 52.75 | 51.83 | 0.006 |
| IFG | 95.0% | 78.9% | 68.9% | 62.5% | 0.001 |
| IGT | 0% | 2.6% | 5.4% | 5.0% | 0.030 |
| IFG + IGT | 5.0% | 18.4% | 25.7% | 32.5% | 0.001 |
| IR | 45.0% | 36.8% | 23.0% | 35.0% | 0.097 |
| Beta-cell deficit | 30.0% | 39.5% | 60.8% | 55.0% | 0.008 |
| Model | Log-likelihood | df | AIC | BIC |
|---|---|---|---|---|
| 2 Classes | −4870.519 | 34 | 9809.039 | 9919.794 |
| 3 Classes | −4792.777 | 46 | 9677.554 | 9827.399 |
| 4 Classes | −4741.992 | 58 | 9599.985 | 9788.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuskabayeva, G.; Saruarov, Y.; Sadykova, K.; Zhunissova, M.; Nurdinov, N.; Babayeva, K.; Li, M.; Zhailkhan, A.; Kabibulatova, A.; Sarria-Santamera, A. Identifying Cardio-Metabolic Subtypes of Prediabetes Using Latent Class Analysis. Med. Sci. 2025, 13, 243. https://doi.org/10.3390/medsci13040243
Nuskabayeva G, Saruarov Y, Sadykova K, Zhunissova M, Nurdinov N, Babayeva K, Li M, Zhailkhan A, Kabibulatova A, Sarria-Santamera A. Identifying Cardio-Metabolic Subtypes of Prediabetes Using Latent Class Analysis. Medical Sciences. 2025; 13(4):243. https://doi.org/10.3390/medsci13040243
Chicago/Turabian StyleNuskabayeva, Gulnaz, Yerbolat Saruarov, Karlygash Sadykova, Mira Zhunissova, Nursultan Nurdinov, Kumissay Babayeva, Mariya Li, Akbota Zhailkhan, Aida Kabibulatova, and Antonio Sarria-Santamera. 2025. "Identifying Cardio-Metabolic Subtypes of Prediabetes Using Latent Class Analysis" Medical Sciences 13, no. 4: 243. https://doi.org/10.3390/medsci13040243
APA StyleNuskabayeva, G., Saruarov, Y., Sadykova, K., Zhunissova, M., Nurdinov, N., Babayeva, K., Li, M., Zhailkhan, A., Kabibulatova, A., & Sarria-Santamera, A. (2025). Identifying Cardio-Metabolic Subtypes of Prediabetes Using Latent Class Analysis. Medical Sciences, 13(4), 243. https://doi.org/10.3390/medsci13040243








