Immunoregulatory Imbalance in Preeclampsia: A Cross-Sectional Study of B7-H3 and Decidual NK Cell Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Subjects
2.2. Sample Collection Procedure
2.3. Expression Assessment
2.4. Data Analysis
3. Results
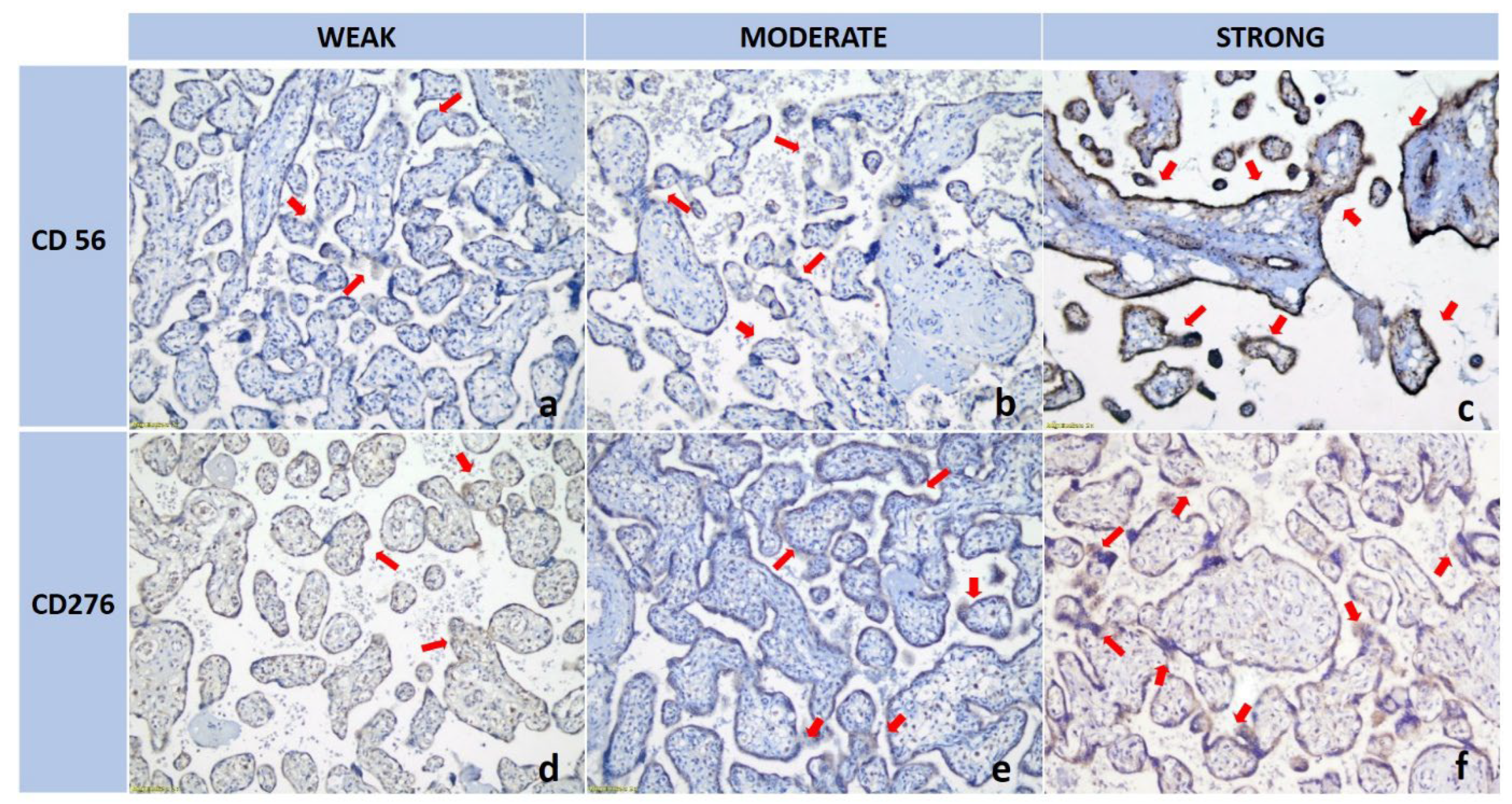
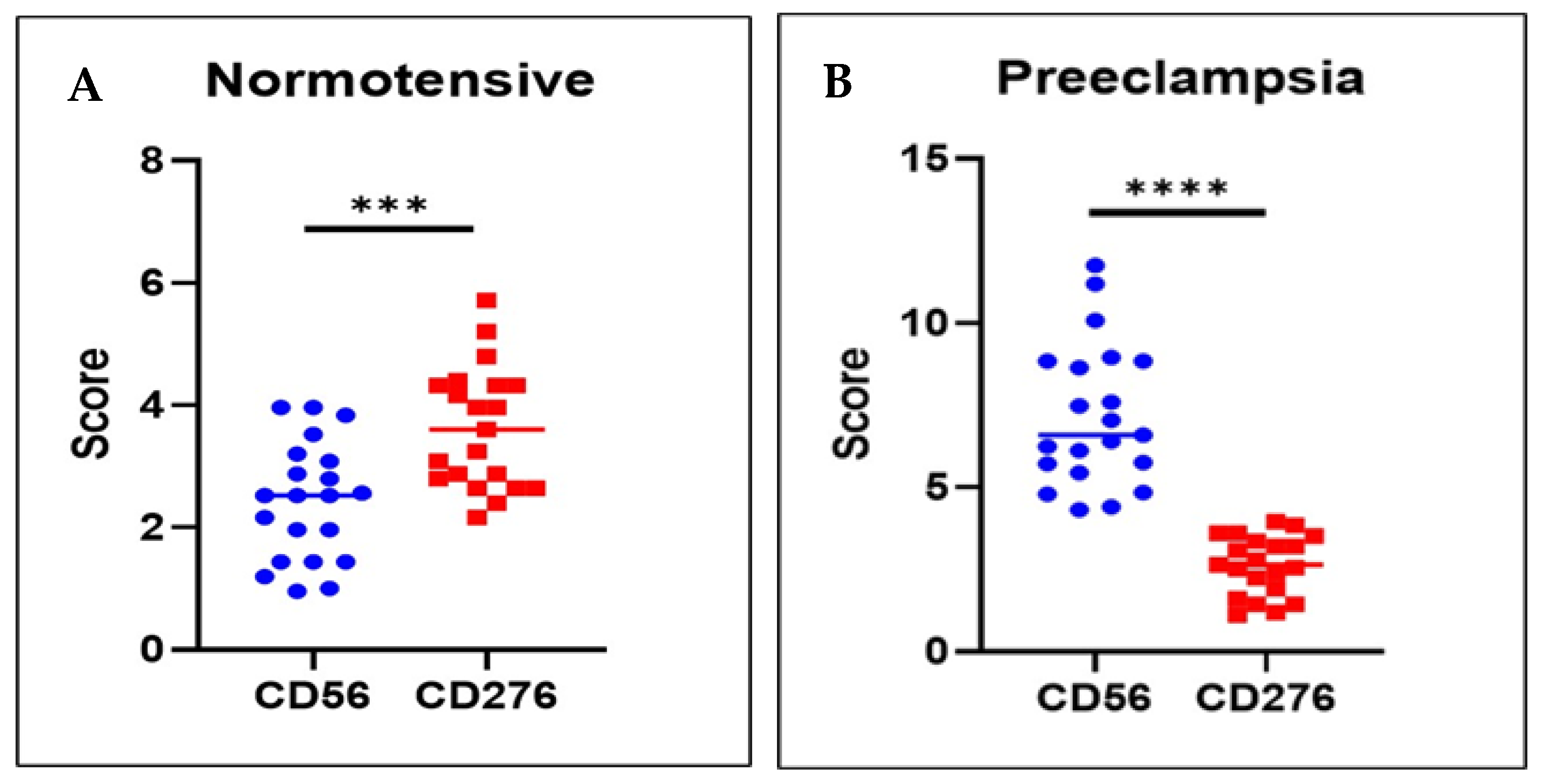
3.1. dNK Cell Expression Was Higher in the Preeclamptic Placenta than in the Normotensive Placenta
3.2. B7H3 Expression Was Lower in the Preeclamptic Placenta than in the Normotensive Placenta
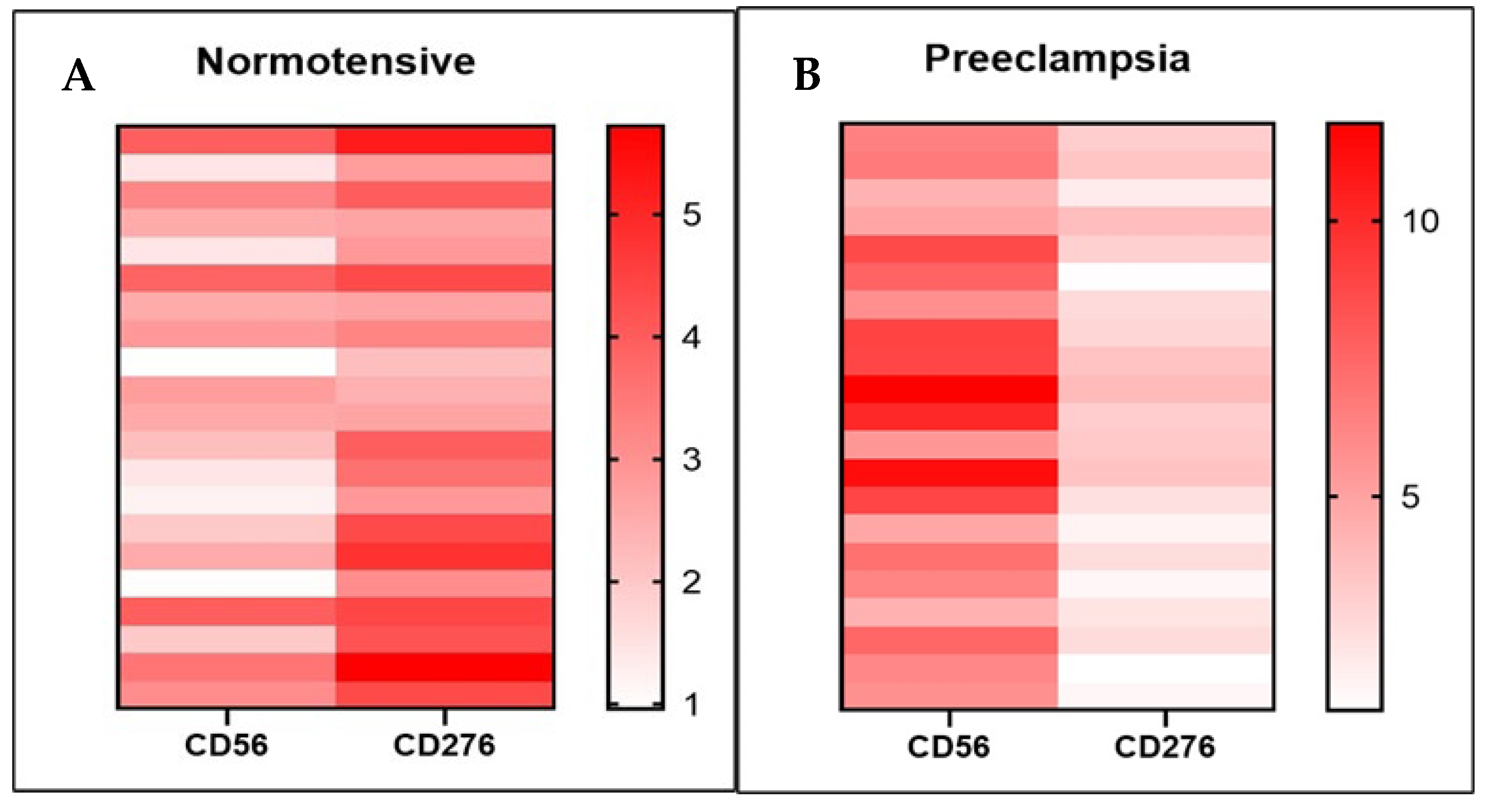
3.3. The Relationship Between B7-H3 Expression and dNK Cells
4. Discussion
4.1. Limitations of the Study
4.2. Future Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obs. 2019, 145 (Suppl. S1), 1–33. [Google Scholar] [CrossRef]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; Gotsch, F.; Erez, O. The etiology of preeclampsia. Am. J. Obs. Gynecol. 2022, 226, S844–S866. [Google Scholar] [CrossRef]
- Geldenhuys, J.; Rossouw, T.M.; Lombaard, H.A.; Ehlers, M.M.; Kock, M.M. Disruption in the Regulation of Immune Responses in the Placental Subtype of Preeclampsia. Front. Immunol. 2018, 9, 1659. [Google Scholar] [CrossRef] [PubMed]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Gumilar, K.E.; Priangga, B.; Lu, C.H.; Dachlan, E.G.; Tan, M. Iron metabolism and ferroptosis: A pathway for understanding preeclampsia. Biomed. Pharmacother. 2023, 167, 115565. [Google Scholar] [CrossRef]
- Shan, Y.; Guan, C.; Wang, J.; Qi, W.; Chen, A.; Liu, S. Impact of ferroptosis on preeclampsia: A review. Biomed. Pharmacother. 2023, 167, 115466. [Google Scholar] [CrossRef]
- Robillard, P.-Y.; Dekker, G.; Scioscia, M.; Saito, S. Progress in the understanding of the pathophysiology of immunologic maladaptation related to early-onset preeclampsia and metabolic syndrome related to late-onset preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S867–S875. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Zhao, Y.; Wang, H.; Pan, Q.; Shao, Q. Decidual Natural Killer Cells: A Good Nanny at the Maternal-Fetal Interface During Early Pregnancy. Front. Immunol. 2021, 12, 663660. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, Q.; Jin, L. Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2317. [Google Scholar] [CrossRef]
- Redman, C.W.; Staff, A.C. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am. J. Obs. Gynecol. 2015, 213, S9.e1–S9.e4. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef]
- Wei, X.W.; Zhang, Y.C.; Wu, F.; Tian, F.J.; Lin, Y. The role of extravillous trophoblasts and uterine NK cells in vascular remodeling during pregnancy. Front. Immunol. 2022, 13, 951482. [Google Scholar] [CrossRef]
- Rauf, K.A.; Dekker, G.A.; Sulistyono, A.; Aditiawarman, A.; Harsono, A.; Gumilar, K.E.; Ernawati, E.; Dachlan, E.G. 22. Decidual killer immunoglobulin-like receptor (kir)2dl1 expression and the onset of preeclampsia, birth weight and placental weight in early and late onset preeclampsia. Pregnancy Hypertens. 2018, 13, S56. [Google Scholar] [CrossRef]
- Hiby, S.E.; Regan, L.; Lo, W.; Farrell, L.; Carrington, M.; Moffett, A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum. Reprod. 2008, 23, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, D.; Sharma, N.R.; Kancharla, S.; Kolli, P.; Tripathy, A.; Sharma, A.K.; Singh, S.; Kumar, S.; Mohanty, A.K.; Jena, M.K. Role of Natural Killer Cells during Pregnancy and Related Complications. Biomolecules 2022, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Petroff, M.G.; Kharatyan, E.; Torry, D.S.; Holets, L. The immunomodulatory proteins B7-DC, B7-H2, and B7-H3 are differentially expressed across gestation in the human placenta. Am. J. Pathol. 2005, 167, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Petroff, M.G.; Perchellet, A. B7 family molecules as regulators of the maternal immune system in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 506–519. [Google Scholar] [CrossRef]
- Collins, M.; Ling, V.; Carreno, B.M. The B7 family of immune-regulatory ligands. Genome Biol. 2005, 6, 223. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, Y.; Ding, C.; Zhang, R.; Duan, T.; Zhou, Q. Decreased B7-H3 promotes unexplained recurrent miscarriage via RhoA/ROCK2 signaling pathway and regulates the secretion of decidual NK cellsdagger. Biol. Reprod. 2023, 108, 504–518. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar] [PubMed]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef]
- Marozio, L.; Nuzzo, A.M.; Gullo, E.; Moretti, L.; Canuto, E.M.; Tancredi, A.; Goia, M.; Cosma, S.; Revelli, A.; Rolfo, A.; et al. Immune Checkpoints in Recurrent Pregnancy Loss: New Insights into a Detrimental and Elusive Disorder. Int. J. Mol. Sci. 2023, 24, 13071. [Google Scholar] [CrossRef] [PubMed]
- Getu, A.A.; Tigabu, A.; Zhou, M.; Lu, J.; Fodstad, O.; Tan, M. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol. Cancer 2023, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, Q.; Jin, L. The Role of B7 Family Molecules in Maternal-Fetal Immunity. Front. Immunol. 2020, 11, 458. [Google Scholar] [CrossRef]
- Chiossone, L.; Vacca, P.; Orecchia, P.; Croxatto, D.; Damonte, P.; Astigiano, S.; Barbieri, O.; Bottino, C.; Moretta, L.; Mingari, M.C. In vivo generation of decidual natural killer cells from resident hematopoietic progenitors. Haematologica 2014, 99, 448–457. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, M.; Lee, S.K. Role of endometrial immune cells in implantation. Clin. Exp. Reprod. Med. 2011, 38, 119–125. [Google Scholar] [CrossRef]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; LaMarca, B. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 2023, 19, 257–270. [Google Scholar] [CrossRef]
- Yue, S.; Meng, J. Role of Decidual Natural Killer Cells in the Pathogenesis of Preeclampsia. Am. J. Reprod. Immunol. 2025, 93, e70033. [Google Scholar] [CrossRef]
- Brouwers, L.; de Gier, S.; Vogelvang, T.E.; Veerbeek, J.H.W.; Franx, A.; van Rijn, B.B.; Nikkels, P.G.J. Prevalence of placental bed spiral artery pathology in preeclampsia and fetal growth restriction: A prospective cohort study. Placenta 2024, 156, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mecacci, F.; Avagliano, L.; Lisi, F.; Clemenza, S.; Serena, C.; Vannuccini, S.; Rambaldi, M.P.; Simeone, S.; Ottanelli, S.; Petraglia, F. Fetal Growth Restriction: Does an Integrated Maternal Hemodynamic-Placental Model Fit Better? Reprod. Sci. 2021, 28, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Motomura, K.; Galaz, J.; Gershater, M.; Lee, E.D.; Romero, R.; Gomez-Lopez, N. Cellular immune responses in the pathophysiology of preeclampsia. J. Leukoc. Biol. 2022, 111, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, R.E.; Faas, M.M.; van Goor, H.; Gordijn, S.J.; Prins, J.R. Decidual macrophages and Hofbauer cells in fetal growth restriction. Front. Immunol. 2024, 15, 1379537. [Google Scholar] [CrossRef]
- Gusella, A.; Martignoni, G.; Giacometti, C. Behind the Curtain of Abnormal Placentation in Pre-Eclampsia: From Molecular Mechanisms to Histological Hallmarks. Int. J. Mol. Sci. 2024, 25, 7886. [Google Scholar] [CrossRef]
- Wallace, A.E.; Fraser, R.; Cartwright, J.E. Extravillous trophoblast and decidual natural killer cells: A remodelling partnership. Hum. Reprod. Update 2012, 18, 458–471. [Google Scholar] [CrossRef]

| Item | Preeclampsia (n = 21) | Normotensive (n = 21) | p-Value |
|---|---|---|---|
| Maternal Age (years) | |||
| ≤30 | 9 (42.9%) | 13 (61.9%) | 0.354 |
| >30 | 12 (57.1%) | 8 (38.1%) | |
| Gestational Age (weeks) | |||
| <37 | 11 (52.4%) | 2 (9.5%) | 0.006 |
| ≥37 | 10 (47.6%) | 19 (90.5%) | |
| Parity | |||
| Primigravida | 5 (23.8%) | 6 (28.6%) | 1.000 |
| Multigravida | 16 (76.2%) | 15 (71.4%) | |
| BMI | |||
| Normal | 3 (14.3%) | 9 (42.9%) | 0.160 |
| Overweight | 8 (38.1%) | 5 (23.8%) | |
| Obesity I | 5 (23.8%) | 6 (28.6%) | |
| Obesity II | 4 (19.0%) | 1 (4.8%) | |
| Obesity III | 1 (4.8%) | 0 (0.0%) | |
| Birth weight (grams) | |||
| <2000 | 7 (33.3%) | 0 (0.0%) | 0.009 |
| ≥2000 | 14 (66.7%) | 21 (100%) | |
| Percentile of birthweight | |||
| 75–90 | 7 (33.3%) | 5 (23.8%) | 0.453 |
| 50–75 | 4 (19.0%) | 8 (38.1%) | |
| 25–50 | 6 (28.6%) | 6 (28.6%) | |
| 10–25 | 2 (9.5%) | 2 (9.5%) | |
| <10 | 2 (9.5%) | 0 (0.0%) | |
| Type of Preeclampsia (n = 21) | |||
| EOP (<34 weeks) | 6 (28.6%) | ||
| LOP (≥34 weeks) | 15 (71.4%) | ||
| Gestational Age in Preeclampsia (weeks) (n = 21) | |||
| <28 | 2 (9.5%) | ||
| 28–<34 | 4 (19.1%) | ||
| ≥34 | 15 (71.4%) | ||
| Marker | Mean Expression (IRS)—Preeclampsia (n = 21) | Mean Expression (IRS)—Normotensive (n = 21) | p-Value |
|---|---|---|---|
| dNK (CD56) | 7.19 ± 2.16 | 2.42 ± 0.96 | <0.001 1 |
| B7-H3 (CD276) | 2.63 ± 0.90 | 3.62 ± 0.99 | 0.002 1 |
| Subject | n | p-Value | r (Correlation Coefficient) |
|---|---|---|---|
| Normotensive | 21 | 0.004 | 0.605 |
| Preeclampsia | 21 | 0.034 | 0.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumilar, K.E.; Humala, A.I.; Wardhana, M.P.; Ernawati, E.; Sulistyono, A.; Utomo, B.; Ariani, G.; Tan, M.; Dachlan, E.G.; Dekker, G. Immunoregulatory Imbalance in Preeclampsia: A Cross-Sectional Study of B7-H3 and Decidual NK Cell Interactions. Med. Sci. 2025, 13, 239. https://doi.org/10.3390/medsci13040239
Gumilar KE, Humala AI, Wardhana MP, Ernawati E, Sulistyono A, Utomo B, Ariani G, Tan M, Dachlan EG, Dekker G. Immunoregulatory Imbalance in Preeclampsia: A Cross-Sectional Study of B7-H3 and Decidual NK Cell Interactions. Medical Sciences. 2025; 13(4):239. https://doi.org/10.3390/medsci13040239
Chicago/Turabian StyleGumilar, Khanisyah Erza, Alexander Indra Humala, Manggala Pasca Wardhana, Ernawati Ernawati, Agus Sulistyono, Budi Utomo, Grace Ariani, Ming Tan, Erry Gumilar Dachlan, and Gus Dekker. 2025. "Immunoregulatory Imbalance in Preeclampsia: A Cross-Sectional Study of B7-H3 and Decidual NK Cell Interactions" Medical Sciences 13, no. 4: 239. https://doi.org/10.3390/medsci13040239
APA StyleGumilar, K. E., Humala, A. I., Wardhana, M. P., Ernawati, E., Sulistyono, A., Utomo, B., Ariani, G., Tan, M., Dachlan, E. G., & Dekker, G. (2025). Immunoregulatory Imbalance in Preeclampsia: A Cross-Sectional Study of B7-H3 and Decidual NK Cell Interactions. Medical Sciences, 13(4), 239. https://doi.org/10.3390/medsci13040239










