Clinical, Endoscopic and Histologic Differences in Gastric Mucosa Between Younger and Older Adults: An Observational Study on the Aging Stomach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
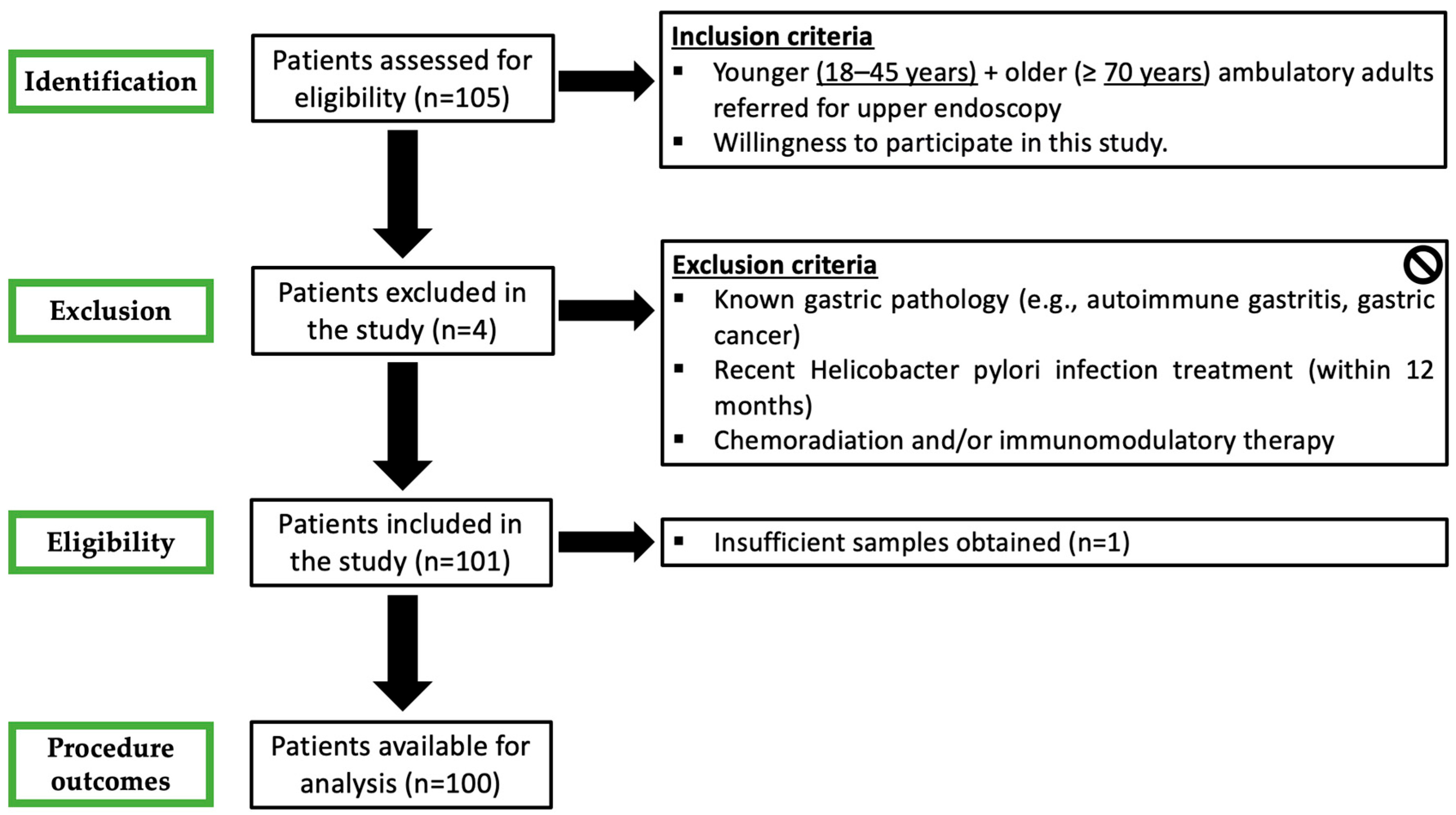
2.2. Patients
- Prospective clinical and demographic database registry including age, gender, clinical indication for upper endoscopy and ongoing therapies with a special focus on antiplatelet, anticoagulant and PPIs. PPI therapy was defined as ongoing when drugs were suspended less than 7 days before endoscopy.
- Upper endoscopy, under deep sedation, was performed according to several quality indicators, including the use of virtual chromoendoscopy, preprocedural simethicone and fundus retroflexion [29].
- Endoscopic diagnoses were coded and collected in a predefined, standardized and searchable fashion. Antithrombotic therapy was managed according to international guidelines [30].
- Two biopsy specimens were systematically obtained from the antrum, another two from the body and were compared for histological features. When endoscopic lesions (e.g., polyps, ulcer) were evident, apart from the respective lesion biopsies, random biopsies from the opposite wall of the antrum/body were collected for analysis.
- Refusal to participate in the study.
- Pregnancy.
- Previously known gastric pathology (e.g., autoimmune gastritis, GC).
- Recent H. pylori eradication (within 12 months).
- Previous gastric surgery.
- Crohn’s disease.
- Chemoradiation and/or immunomodulatory therapy.
- Technical impossibility to obtain gastric biopsies.
2.3. Gastric Biopsy Analysis
2.4. Statystical Analysis
3. Results
3.1. Baseline Clinical Characteristics
3.2. Gastric Endoscopic Lesions
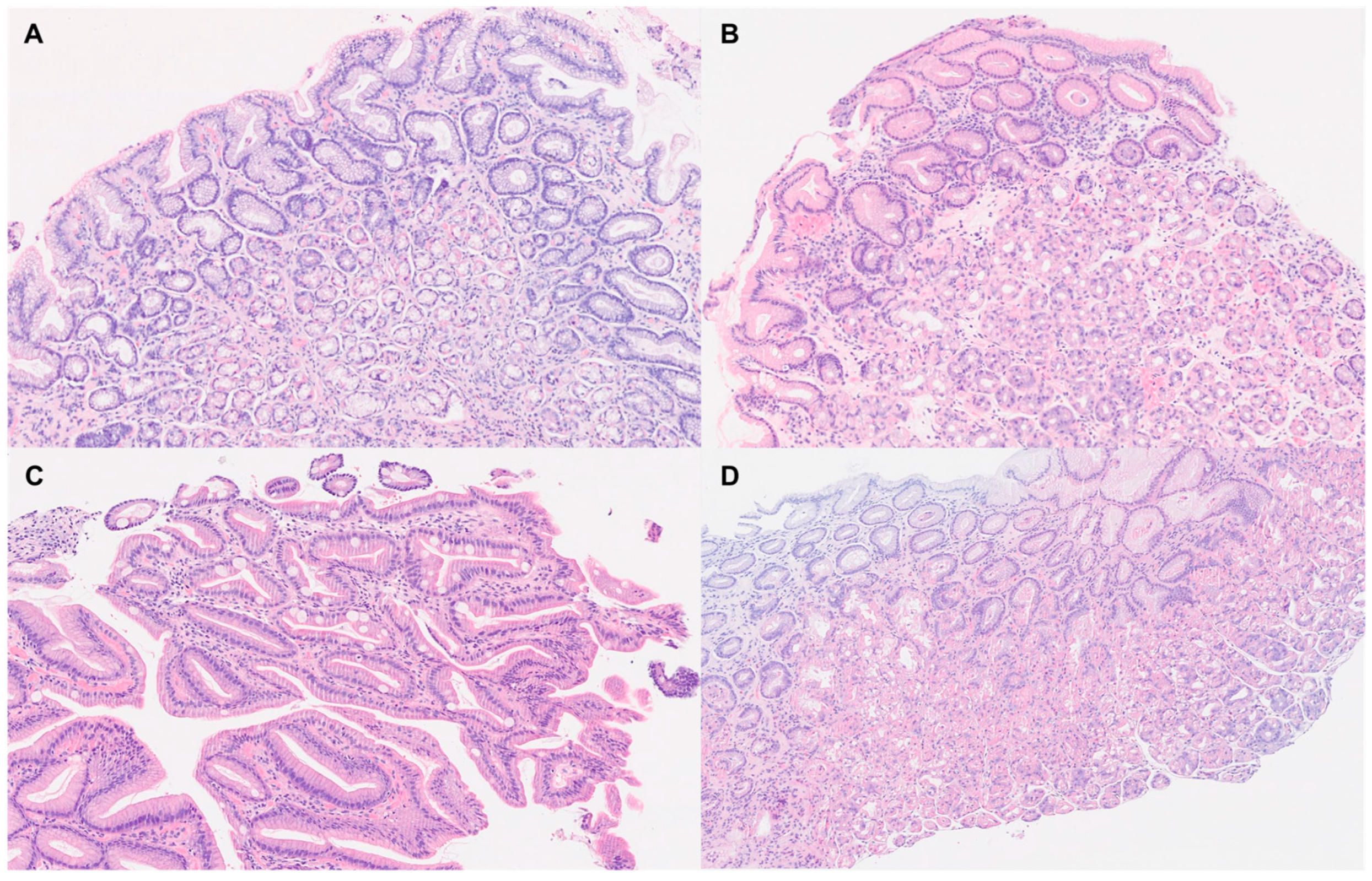
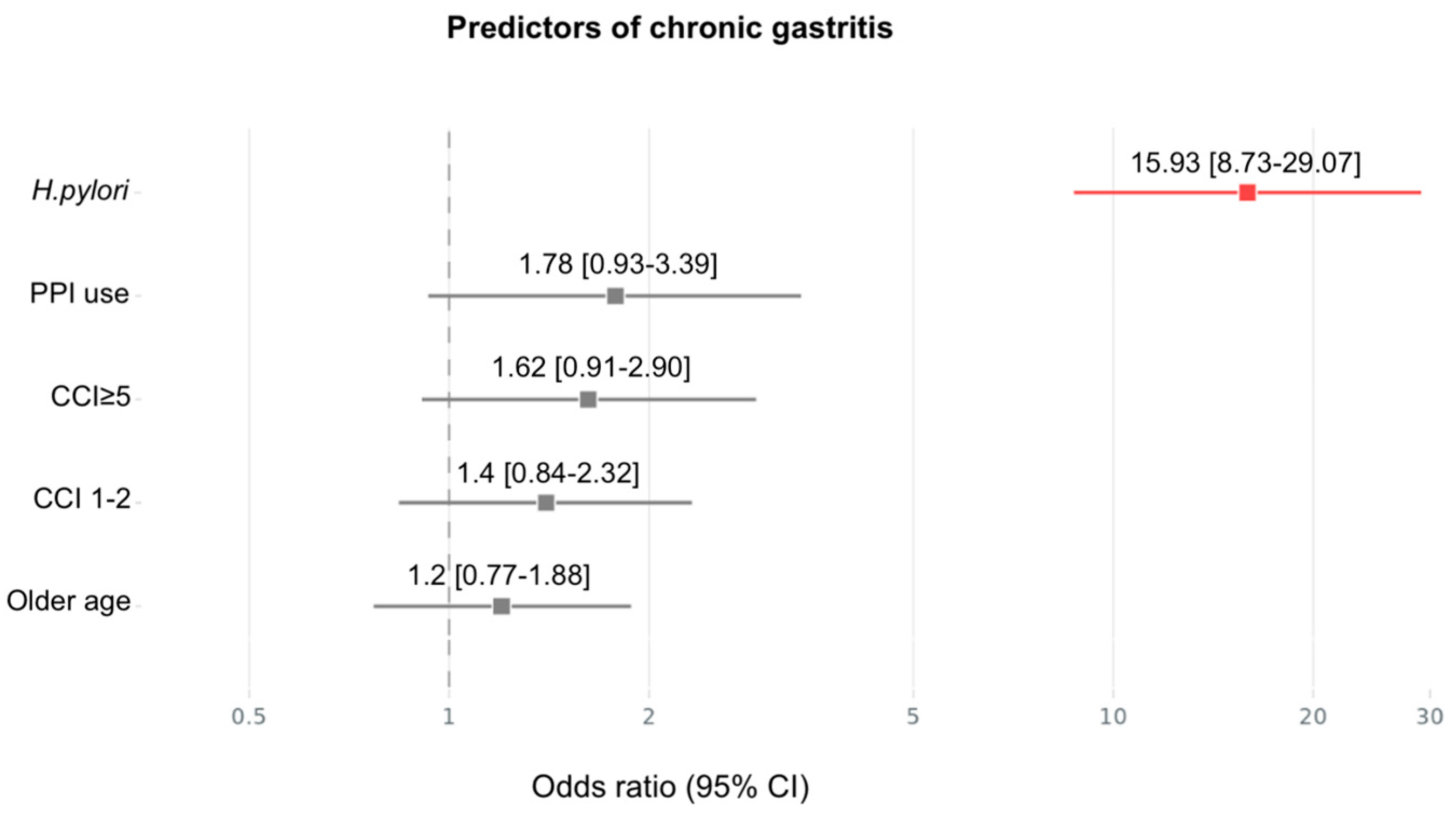
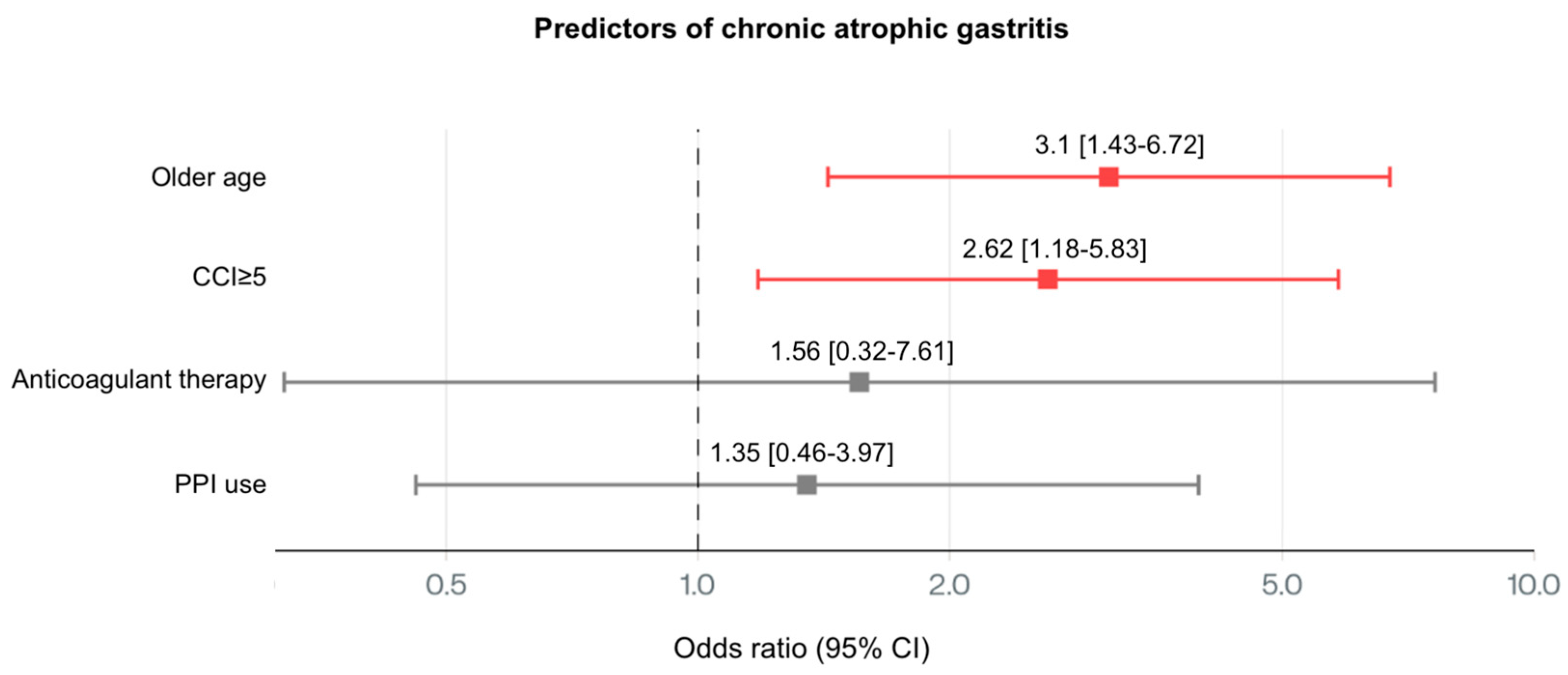
3.3. Gastric Histology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCI | Charlson comorbidity index |
| CAG | Chronic atrophic gastritis |
| GC | Gastric cancer |
| H. pylori | Helicobacter pylori |
| IM | Intestinal metaplasia |
| PPI | Proton pump inhibitor |
References
- Evert, J.; Lawler, E.; Bogan, H.; Perls, T. Morbidity profiles of centenarians: Survivors, delayers, and escapers. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 232–237. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 1 April 2024).
- Vara-Luiz, F.; Mendes, I.; Palma, C.; Mascarenhas, P.; Simas, D.; Gomes, P.; Gonçalves, A.; Simão, I.; Teixeira, M.; Lopes, S.; et al. Upper gastrointestinal bleeding differences between older and younger adults: Should bleeding in non-cirrhotic patients be considered a geriatric syndrome? Ther. Adv. Gastroenterol. 2025, 18, 17562848251343416. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.A.F.; Rizk Arafat, A.A.E.; Nasralla, M.M.; Elsayed, M.M.; Shuaib, D.M. Age changes in gastric mucosa of male albino rats: Histological, immunohistochemical, histomorphometric and biochemical study. Folia Morphol. 2025, 84, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Patejdl, R. Gastrointestinal Motility Function and Dysfunction in the Elderly Patient: What Are the Effects of Aging? Visc. Med. 2024, 40, 325–330. [Google Scholar] [CrossRef]
- Löhr, J.M.; Panic, N.; Vujasinovic, M.; Verbeke, C.S. The ageing pancreas: A systematic review of the evidence and analysis of the consequences. J. Intern. Med. 2018, 283, 446–460. [Google Scholar] [CrossRef]
- Woodhouse, K.; Wynne, H.A. Age-related changes in hepatic function. Implications for drug therapy. Drugs Aging 1992, 2, 243–255. [Google Scholar] [CrossRef]
- Vara-Luiz, F.; Mendes, I.; Palma, C.; Mascarenhas, P.; Nunes, G.; Patita, M.; Fonseca, J. Age-related decline of gastric secretion: Facts and controversies. Biomedicines 2025, 13, 1546. [Google Scholar] [CrossRef]
- Gomes, A.P.; Price, N.L.; Ling, A.J.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef]
- Tarnawski, A.S.; Szabo, I. Apoptosis-programmed cell death and its relevance to gastrointestinal epithelium: Survival signal from the matrix. Gastroenterology 2001, 120, 294–299. [Google Scholar] [CrossRef]
- Salles, N. Is stomach spontaneously ageing? Pathophysiology of the ageing stomach. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 805–819. [Google Scholar] [CrossRef]
- Skokowski, J.; Vashist, Y.; Girnyi, S.; Cwalinski, T.; Mocarski, P.; Antropoli, C.; Brillantino, A.; Boccardi, V.; Goyal, A.; Ciarleglio, F.A.; et al. The Aging Stomach: Clinical Implications of H. pylori Infection in Older Adults-Challenges and Strategies for Improved Management. Int. J. Mol. Sci. 2024, 25, 12826. [Google Scholar] [CrossRef]
- Fetarayani, D.; Kahdina, M.; Waitupu, A.; Pratiwi, L.; Ningtyas, M.C.; Adytia, G.J.; Sutanto, H. Immunosenescence and the Geriatric Giants: Molecular Insights into Aging and Healthspan. Med. Sci. 2025, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; Esposito, G.; Ridola, L.; Hassan, C.; Lahner, E.; Perri, F.; Bianco, M.A.; De Francesco, V.; Buscarini, E.; Di Giulio, E.; et al. Prevalence of lesions detected at upper endoscopy: An Italian survey. Eur. J. Intern. Med. 2014, 25, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global prevalence of Helicobacter pylori infection between 1980 and 2022: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, H.A.; Talley, N.J. Gastroscopy is incomplete without biopsy: Clinical relevance of distinguishing gastropathy from gastritis. Gastroenterology 1995, 108, 917–924. [Google Scholar] [CrossRef]
- Soenen, S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. The ageing gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 12–18. [Google Scholar] [CrossRef]
- Pilotto, A.; Maggi, S.; Noale, M.; Franceschi, M.; Parisi, G.; Crepaldi, G. Association of upper gastrointestinal symptoms with functional and clinical charateristics in elderly. World J. Gastroenterol. 2011, 17, 3020–3026. [Google Scholar] [CrossRef]
- Hui, W.M.; Lam, S.K. Etiology and management of chronic gastritis. Dig. Dis. 1989, 7, 51–60. [Google Scholar] [CrossRef]
- Asaka, M.; Sugiyama, T.; Nobuta, A.; Kato, M.; Takeda, H.; Graham, D.Y. Atrophic gastritis and intestinal metaplasia in Japan: Results of a large multicenter study. Helicobacter 2001, 6, 294–299. [Google Scholar] [CrossRef]
- Ito, M.; Haruma, K.; Kamada, T.; Mihara, M.; Kim, S.; Kitadai, Y.; Sumii, M.; Tanaka, S.; Yoshihara, M.; Chayama, K. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: A 5-year prospective study of patients with atrophic gastritis. Aliment. Pharmacol. Ther. 2002, 16, 1449–1456. [Google Scholar] [CrossRef]
- Kim, G.H. Proton Pump Inhibitor-Related Gastric Mucosal Changes. Gut Liver 2021, 15, 646–652. [Google Scholar] [CrossRef]
- Mathialagan, R.; Illangantilaka, A.; Radhakrishnan, H. Gastroenterology in the elderly. Medicine 2019, 47, 466–469. [Google Scholar] [CrossRef]
- Lachner, C.; Steinle, N.I.; Regenold, W.T. The neuropsychiatry of vitamin B12 deficiency in elderly patients. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 5–15. [Google Scholar] [CrossRef]
- Russell, R.M.; Krasinski, S.D.; Samloff, I.M.; Jacob, R.A.; Hartz, S.C.; Brovender, S.R. Folic acid malabsorption in atrophic gastritis. Possible compensation by bacterial folate synthesis. Gastroenterology 1986, 91, 1476–1482. [Google Scholar] [CrossRef]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021, 161, 1325–1332.e7. [Google Scholar] [CrossRef]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef]
- Karhunen, P.; Tuomisto, S.; Goebeler, S.; Martiskainen, M.; Kok, E. Common occurrence of atrophic gastritis in an ageing non-hospitalised population: An autopsy study. Age Ageing 2025, 54, afaf047. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Ribeiro, M.; Libânio, D.; Uchima, H.; Spaander, M.C.W.; Bornschein, J.; Matysiak-Budnik, T.; Tziatzios, G.; Santos-Antunes, J.; Areia, M.; Chapelle, N.; et al. Management of epithelial precancerous conditions and early neoplasia of the stomach (MAPS III): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG) and European Society of Pathology (ESP) Guideline update 2025. Endoscopy 2025, 57, 504–554. [Google Scholar] [CrossRef] [PubMed]
- Veitch, A.M.; Radaelli, F.; Alikhan, R.; Dumonceau, J.M.; Eaton, D.; Jerrome, J.; Lester, W.; Nylander, D.; Thoufeeq, M.; Vanbiervliet, G.; et al. Endoscopy in patients on antiplatelet or anticoagulant therapy: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guideline update. Endoscopy 2021, 53, 947–969. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Genta, R.M.; Malfertheiner, P.; Dinis-Ribeiro, M.; El-Serag, H.; Graham, D.Y.; Kuipers, E.J.; Leung, W.K.; Park, J.Y.; Rokkas, T.; et al. RE. GA. IN.: The Real-world Gastritis Initiative-updating the updates. Gut 2024, 73, 407–441. [Google Scholar] [CrossRef]
- Yue, H.; Shan, L.; Bin, L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2018, 21, 579–587. [Google Scholar] [CrossRef]
- Genta, R.M. Differential diagnosis of reactive gastropathy. Semin. Diagn. Pathol. 2005, 22, 273–283. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Thomopoulos, K.C.; Louvros, E.; Theocharis, G.; Giannikoulis, C.; Katsakoulis, E.; Nikolopoulou, V.N. Changes in indications for upper gastrointestinal tract endoscopy and endoscopic findings during the last fifteen years in south-western Greece. Am. J. Med. Sci. 2008, 336, 21–26. [Google Scholar] [CrossRef]
- Lee, M.; Feldman, M. The aging stomach: Implications for NSAID gastropathy. Gut 1997, 41, 425–426. [Google Scholar] [CrossRef]
- Kim, G.H.; Park, D.Y.; Kida, M. Histological intestinal metaplasia in the stomach: Its cause, impact and management. Gut Liver 2019, 13, 603–614. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, N. Gastric cancer and family history. Korean J. Intern Med. 2016, 31, 265–273. [Google Scholar] [CrossRef]
- Correa, P. A human model of gastric carcinogenesis. Cancer Res. 1988, 48, 3554–3560. [Google Scholar] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Rugge, M.; Meggio, A.; Pravadelli, C.; Barbareschi, M.; Fassan, M.; Gentilini, M.; Zorzi, M.; De Pretis, G.; Graham, D.Y.; Genta, R.M. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer. Gut 2003, 52, 1681–1684. [Google Scholar] [CrossRef]
- Salama, N.R.; Hartung, M.L.; Müller, A. Life in the human stomach: Persistence strategies of the bacterial pathogen Helicobacter pylori. Nat. Rev. Microbiol. 2013, 11, 385–399. [Google Scholar] [CrossRef]
- Kantor, E.D.; Rehm, C.D.; Haas, J.S.; Chan, A.T.; Giovannucci, E.L. Trends in Prescription Drug Use Among Adults in the United States from 1999–2012. JAMA 2015, 314, 1818–1831. [Google Scholar] [CrossRef]
- Maes, M.L.; Fixen, D.R.; Linnebur, S.A. Adverse effects of proton-pump inhibitor use in older adults: A review of the evidence. Ther. Adv. Drug. Saf. 2017, 8, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.E.; Kim, L.S.; Yang, Y.X. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhu, J.; Lu, D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst. Rev. 2014, 2014, CD010623. [Google Scholar] [CrossRef] [PubMed]
- Abrahami, D.; McDonald, E.G.; Schnitzer, M.E.; Barkun, A.N.; Suissa, S.; Azoulay, L. Proton pump inhibitors and risk of gastric cancer: Population-based cohort study. Gut 2022, 71, 16–24. [Google Scholar] [CrossRef]
- Calabrese, F.; Pasta, A.; Bodini, G.; Furnari, M.; Grillo, F.; Mastracci, L.; Savarino, E.V.; Savarino, V.; Zentilin, P.; Giannini, E.G.; et al. Autoimmune chronic atrophic gastritis: Association between chronic proton pump inhibitors use and more severe atrophy and gastric intestinal metaplasia. Eur. J. Gastroenterol. Hepatol. 2025, 37, 905–910. [Google Scholar] [CrossRef]
- Li, Z.; Wu, C.; Li, L.; Wang, Z.; Xie, H.; He, X.; Feng, J. Effect of long-term proton pump inhibitor administration on gastric mucosal atrophy: A meta-analysis. Saudi J. Gastroenterol. 2017, 23, 222–228. [Google Scholar] [CrossRef]
- Shanika, L.G.T.; Reynolds, A.; Pattison, S.; Braund, R. Proton pump inhibitor use: Systematic review of global trends and practices. Eur. J. Clin. Pharmacol. 2023, 79, 1159–1172. [Google Scholar] [CrossRef]
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef]
- Araújo, G.R.L.; Marques, H.S.; Santos, M.L.C.; da Silva, F.A.F.; da Brito, B.B.; Santos, G.L.C.; de Melo, F.F. Helicobacter pylori infection: How does age influence the inflammatory pattern? World J. Gastroenterol. 1998, 28, 402. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, T.B. Intrinsic ageing of gut epithelial stem cells. Mech. Ageing Dev. 2004, 125, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhu, J.; Ma, Z.; Yi, Z.; Tuo, B.; Li, T.; Liu, X. The mechanisms of gastric mucosal injury: Focus on initial chief cell loss as a key target. Cell Death Discov. 2023, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Lahner, E.; Annibale, B.; Dilaghi, E.; Luciano Millado, C.; Lenti, M.V.; Di Sabatino, A.; Miceli, E.; Massironi, S.; Zucchini, N.; Cannizzaro, R.; et al. Clinical and Endoscopic-Histological Features of Multifocal and Corpus-Restricted Atrophic Gastritis Patients With Non-Cardia Gastric Cancer or Dysplasia: A Multicenter, Cross-Sectional Study. Clin. Transl. Gastroenterol. 2025, 16, e00862. [Google Scholar] [CrossRef]
- Sonnenberg, A.; Genta, R.M. Changes in the Gastric Mucosa With Aging. Clin. Gastroenterol. Hepatol. 2015, 13, 2276–2281. [Google Scholar] [CrossRef]
- Feldman, M.; Cryer, B.; McArthur, K.E.; Huet, B.A.; Lee, E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: A prospective study. Gastroenterology 1996, 110, 1043–1052. [Google Scholar] [CrossRef]
- Francesco, V.; Zullo, A.; Amato, A.; Bergna, I.; Bendia, E.; Giorgini, G.; Buscarini, E.; Manfredi, G.; Cadoni, S.; Cannizzaro, R.; et al. Prevalence of Endoscopic and Histological Lesions at Upper Endoscopy: A Cross-Sectional, Multicentre Study in Clinical Practice. GE Port. J. Gastroenterol. 2024, 32, 1–8. [Google Scholar] [CrossRef]
- Xie, L.; Liu, G.W.; Liu, Y.N.; Li, P.Y.; Hu, X.N.; He, X.Y.; Huan, R.B.; Zhao, T.L.; Guo, H.J. Prevalence of Helicobacter pylori infection in China from 2014–2023: A systematic review and meta-analysis. World J. Gastroenterol. 2024, 30, 4636–4656. [Google Scholar] [CrossRef]
- Burucoa, C.; Axon, A. Epidemiology of Helicobacter pylori infection. Helicobacter 2017, 22 (Suppl. S1). [Google Scholar] [CrossRef]
- Pilotto, A.; Franceschi, M. Helicobacter pylori infection in older people. World J. Gastroenterol. 2014, 20, 6364–6373. [Google Scholar] [CrossRef]
- Lenti, M.V.; Joudaki, S.; Miceli, E.; Lahner, E.; Massironi, S.; Votto, M.; Licari, A.; Caimmi, S.M.E.; Bramuzzo, M.; Di Leo, G.; et al. Pediatric autoimmune gastritis: An international, multicentric study. J. Pediatr. Gastroenterol. Nutr. 2025. [Google Scholar] [CrossRef]
- Minalyan, A.; Benhammou, J.N.; Artashesyan, A.; Lewis, M.S.; Pisegna, J.R. Autoimmune atrophic gastritis: Current perspectives. Clin. Exp. Gastroenterol. 2017, 10, 19–27. [Google Scholar] [CrossRef]
- Zeng, R.; Gou, H.; Lau, H.C.H.; Yu, J. Stomach microbiota in gastric cancer development and clinical implications. Gut 2024, 73, 2062–2073. [Google Scholar] [CrossRef]
- Gyriki, D.; Nikolaidis, C.G.; Bezirtzoglou, E.; Voidarou, C.; Stavropoulou, E.; Tsigalou, C. The gut microbiota and aging: Interactions, implications, and interventions. Front. Aging 2025, 6, 1452917. [Google Scholar] [CrossRef]
| Variables | 18–45 Years (n = 50) | ≥70 Years (n = 50) | p Value | |
|---|---|---|---|---|
| Age (SD) | 33.6 ± 8.1 years | 76.2 ± 4.1 years | ||
| Gender | Male | 23 (46%) | 22 (44%) | 0.421 |
| Female | 27 (54%) | 28 (56%) | ||
| Charlson Comorbidity index | 0.2 ± 0.4 | 4.8 ± 1.6 | <0.001 | |
| Ambulatory therapy | NSAID | 1 (2%) | 8 (16%) | <0.001 |
| Antiplatelets | 3 (6%) | 16 (32%) | <0.001 | |
| Anticoagulants | 2 (4%) | 8 (16%) | 0.023 | |
| Proton pump inhibitors | 9 (18%) | 21 (42%) | <0.001 | |
| Laboratory evaluation | Hemoglobin (SD) | 13.6 ± 1.3 | 12.9 ± 1.7 | 0.044 |
| Iron (SD) | 82.3 ± 40.0 | 73.1 ± 32.6 | 0.377 | |
| Folic acid (SD) | 5.4 ± 4.3 | 5.1 ± 4.8 | 0.234 | |
| Vitamin B12 (SD) | 601 ± 87.6 | 549 ± 69.5 | 0.565 | |
| Clinical indication for upper endoscopy | Dyspepsia | 23 (46%) | 17 (34%) | 0.079 |
| Gastroesophageal reflux disease | 5 (10%) | 10 (20%) | 0.019 | |
| Peptic ulcer disease | 10 (20%) | 9 (18%) | 0.401 | |
| Dysphagia | 4 (8%) | 3 (6%) | 0.349 | |
| Radiological findings | 3 (6%) | 2 (4%) | 0.325 | |
| Gastric polyp | 2 (4%) | 4 (8.0%) | 0.202 | |
| Diarrhea | 2 (4%) | 3 (6%) | 0.325 | |
| Iron deficiency anemia | 1 (2%) | 2 (4%) | 0.281 | |
| Endoscopic Finding | 18–45 Years (n = 50) | ≥70 Years (n = 50) | p Value | |
|---|---|---|---|---|
| Normal mucosa | 25 (50%) | 10 (20%) | <0.001 | |
| Erythema | 17 (34%) | 15 (30%) | 0.336 | |
| Erosions | 5 (10%) | 8 (16%) | 0.189 | |
| Peptic ulcer disease | Gastric ulcer | 1 (2%) | 4 (6%) | 0.004 |
| Duodenal ulcer | 0 (0%) | 6 (12%) | ||
| Gastric polyps | 2 (4%) | 7 (14%) | 0.041 | |
| Histologic Finding | 18–45 Years (n = 50) | ≥70 Years (n = 50) | p Value | |
|---|---|---|---|---|
| Normal mucosa | 22 (44%) | 2 (4%) | <0.001 | |
| Reactive gastritis | 7 (14%) | 5 (10%) | 0.271 | |
| Reactive gastritis H.pylori + | 3 (6%) | 0 (0%) | 0.0910 | |
| Chronic gastritis | 19 (38%) | 28 (56%) | 0.004 | |
| Chronic gastritis H.pylori + | 11 (22%) | 10 (20%) | 0.0681 | |
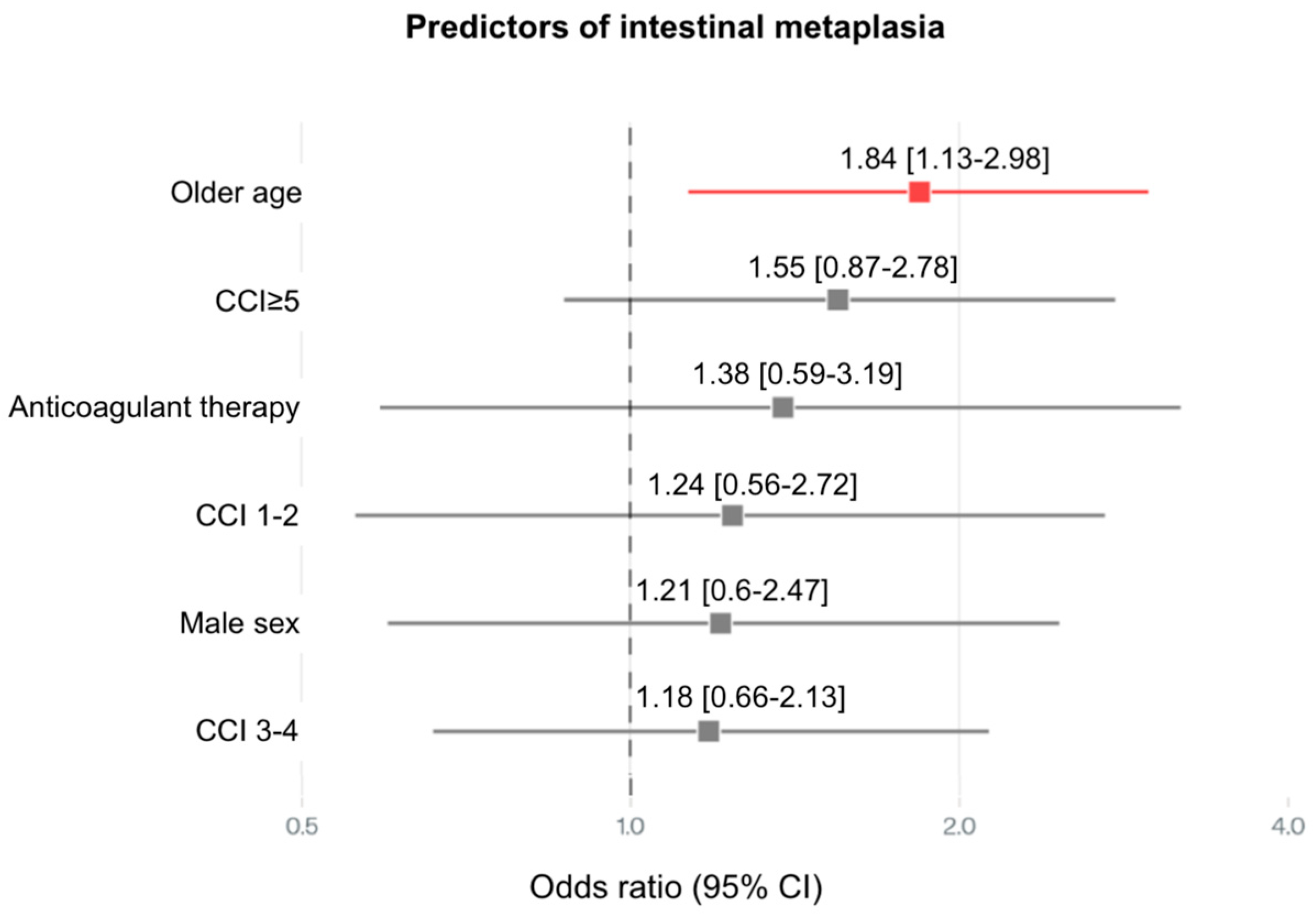
| Intestinal metaplasia | OLGIM I | 2 (4%) | 2 (4%) | <0.001 |
| OLGIM II | 0 (0%) | 3 (6%) | ||
| OLGIM III | 0 (0%) | 5 (10%) | ||
| OLGIM IV | 0 (0%) | 4 (8%) | ||
| Intestinal metaplasia H.pylori + | 1 (2%) | 2 (4%) | 0.113 | |
| Chronic atrophic gastritis | OLGA I | 2 (4%) | 3 (6%) | <0.001 |
| OLGA II | 0 (0%) | 3 (6%) | ||
| OLGA III | 0 (0%) | 4 (8%) | ||
| OLGA IV | 0 (0%) | 4 (8%) | ||
| Chronic atrophic gastritis H.pylori + | 1 (2%) | 2 (4%) | 0.113 | |
| PPI-related gastric changes | 2 (4%) | 15 (30%) | <0.001 | |
| H.pylori + | 0 (0%) | 0 (0%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vara-Luiz, F.; Mendes, I.; Palma, C.; Mascarenhas, P.; Teles, A.E.; Santos, I.C.; Nunes, G.; Patita, M.; Mocanu, I.; Pires, S.; et al. Clinical, Endoscopic and Histologic Differences in Gastric Mucosa Between Younger and Older Adults: An Observational Study on the Aging Stomach. Med. Sci. 2025, 13, 224. https://doi.org/10.3390/medsci13040224
Vara-Luiz F, Mendes I, Palma C, Mascarenhas P, Teles AE, Santos IC, Nunes G, Patita M, Mocanu I, Pires S, et al. Clinical, Endoscopic and Histologic Differences in Gastric Mucosa Between Younger and Older Adults: An Observational Study on the Aging Stomach. Medical Sciences. 2025; 13(4):224. https://doi.org/10.3390/medsci13040224
Chicago/Turabian StyleVara-Luiz, Francisco, Ivo Mendes, Carolina Palma, Paulo Mascarenhas, Ana Elisa Teles, Inês Costa Santos, Gonçalo Nunes, Marta Patita, Irina Mocanu, Sara Pires, and et al. 2025. "Clinical, Endoscopic and Histologic Differences in Gastric Mucosa Between Younger and Older Adults: An Observational Study on the Aging Stomach" Medical Sciences 13, no. 4: 224. https://doi.org/10.3390/medsci13040224
APA StyleVara-Luiz, F., Mendes, I., Palma, C., Mascarenhas, P., Teles, A. E., Santos, I. C., Nunes, G., Patita, M., Mocanu, I., Pires, S., Meira, T., Vieira, A., Pinto-Marques, P., Gomes-Pinto, D., & Fonseca, J. (2025). Clinical, Endoscopic and Histologic Differences in Gastric Mucosa Between Younger and Older Adults: An Observational Study on the Aging Stomach. Medical Sciences, 13(4), 224. https://doi.org/10.3390/medsci13040224








