Efficacy of Extracorporeal Shockwave Therapy in the Management of Chronic Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Eligibility Criteria
2.2. Selection Process, Data Collection, and Data List
2.3. Statistical Analysis
3. Results
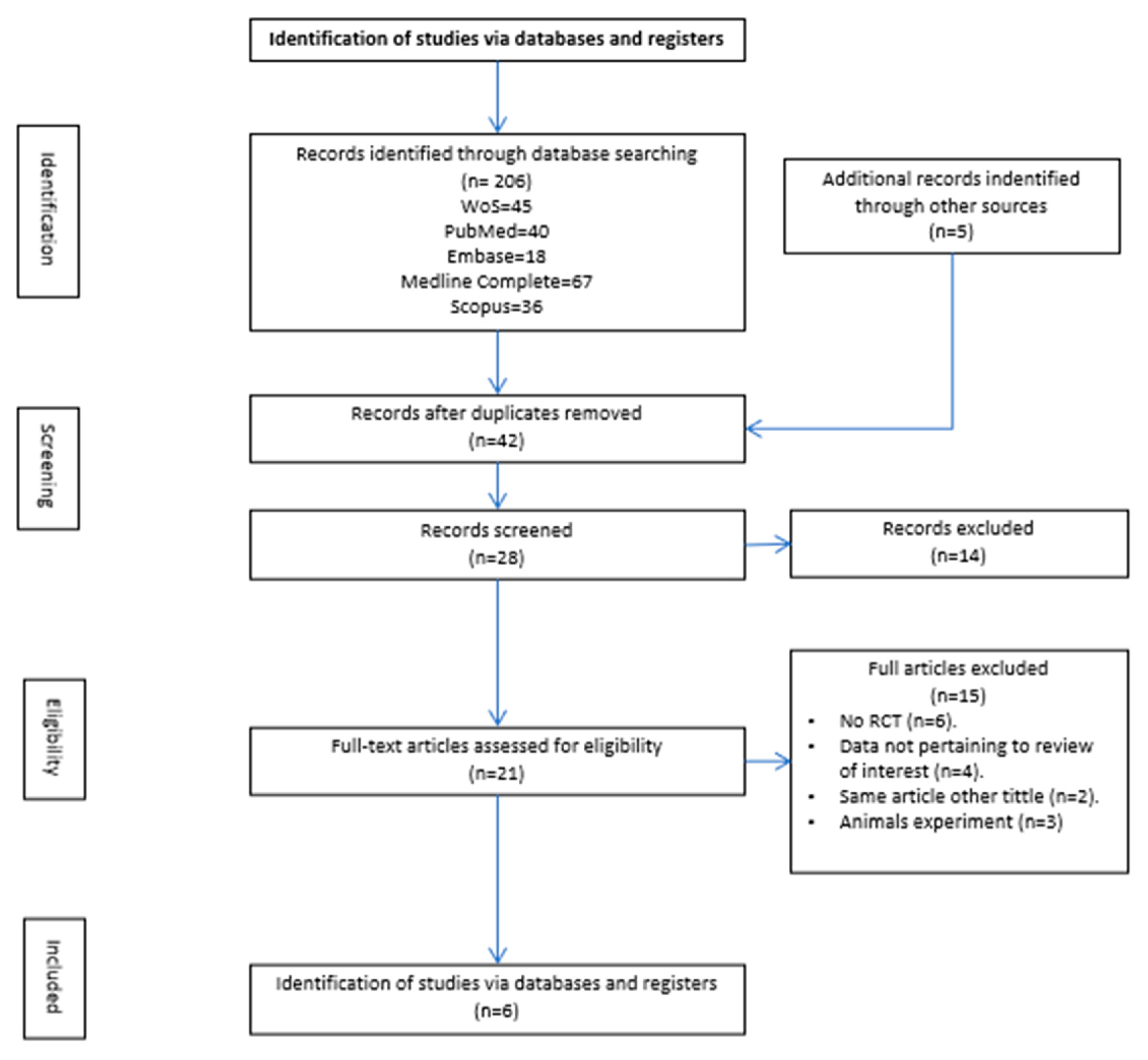
3.1. Study Selection
3.2. Results of Individual Studies
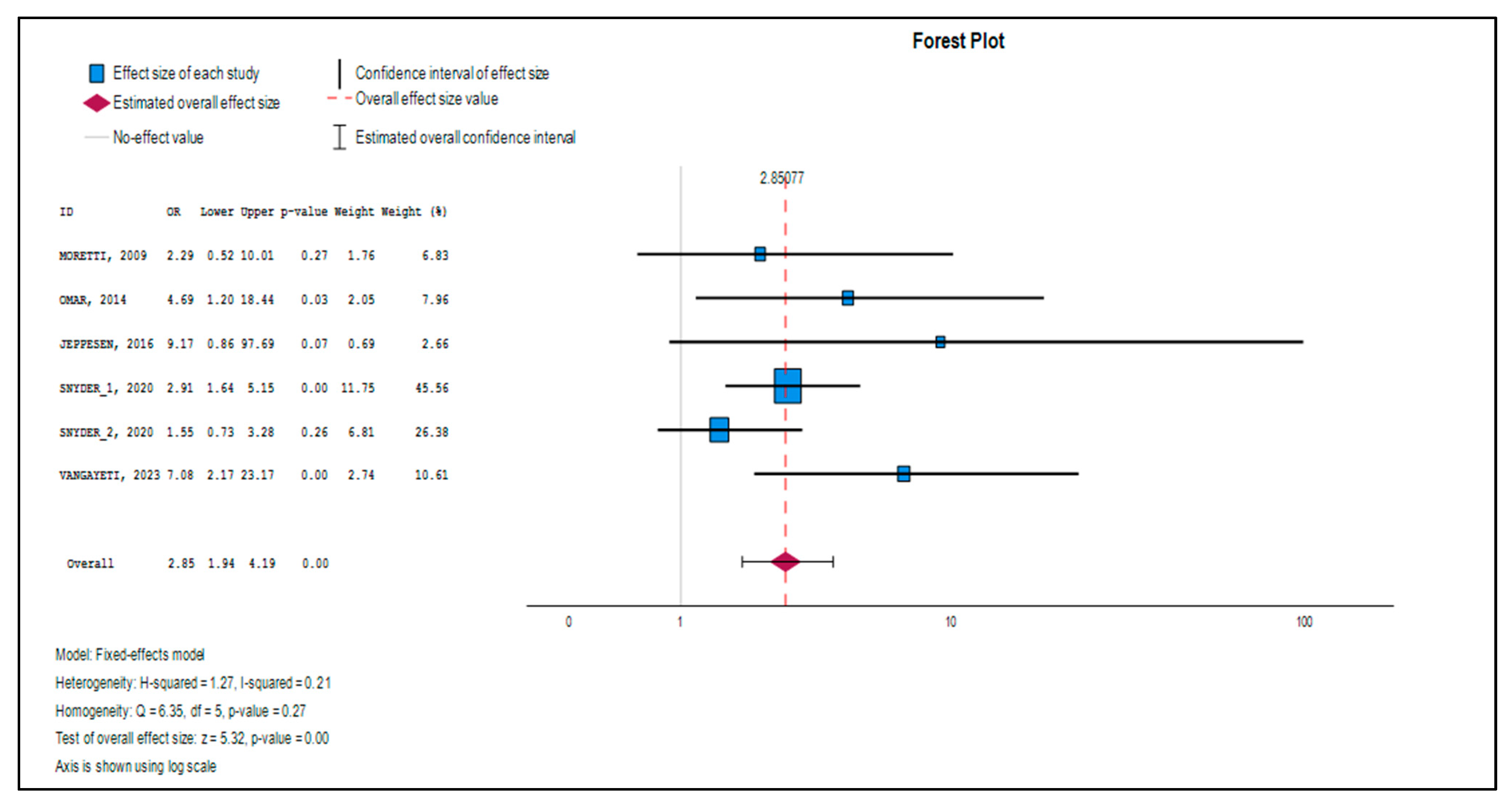
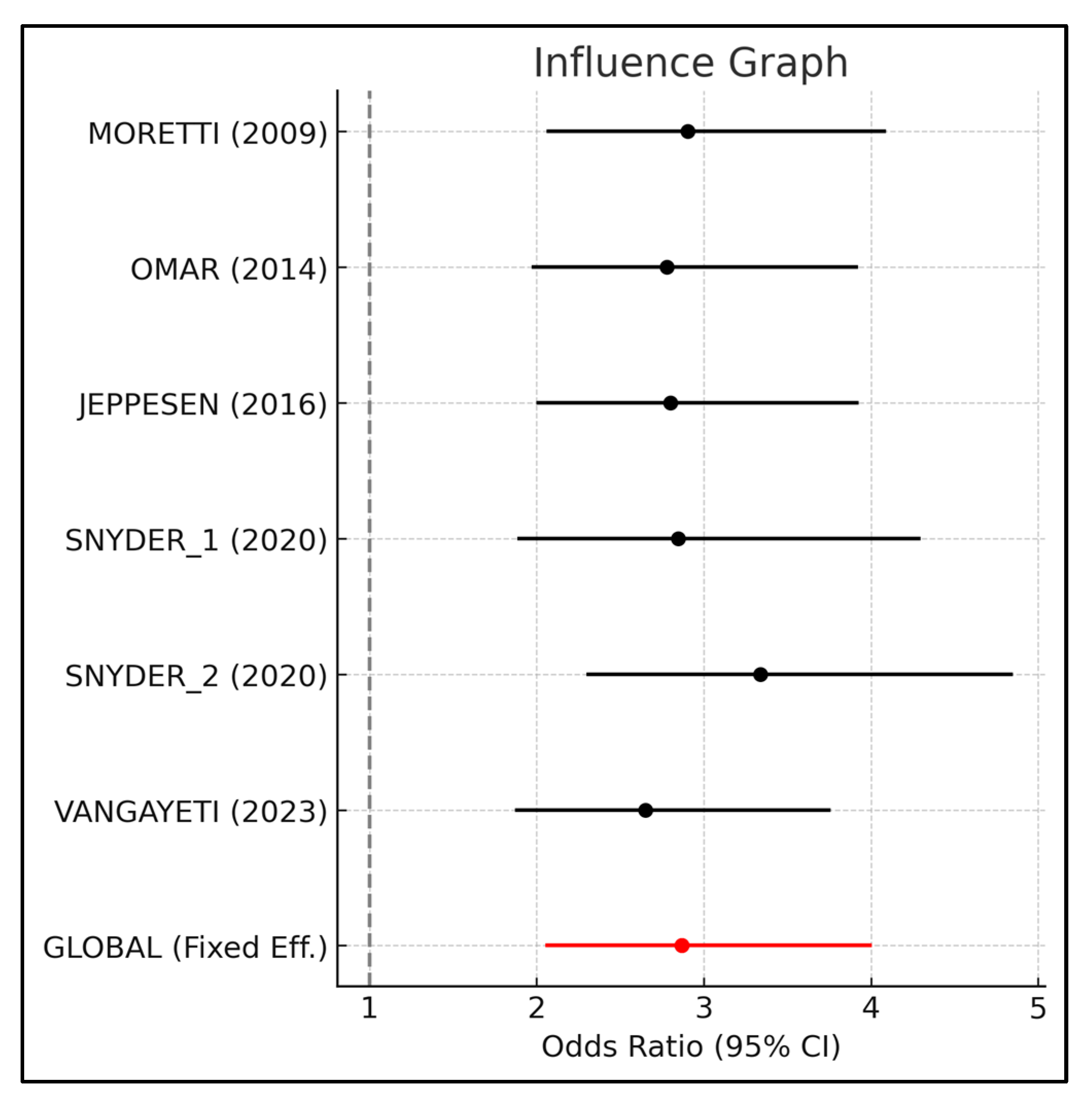
3.3. Effect Size Estimation and Primary Outcomes
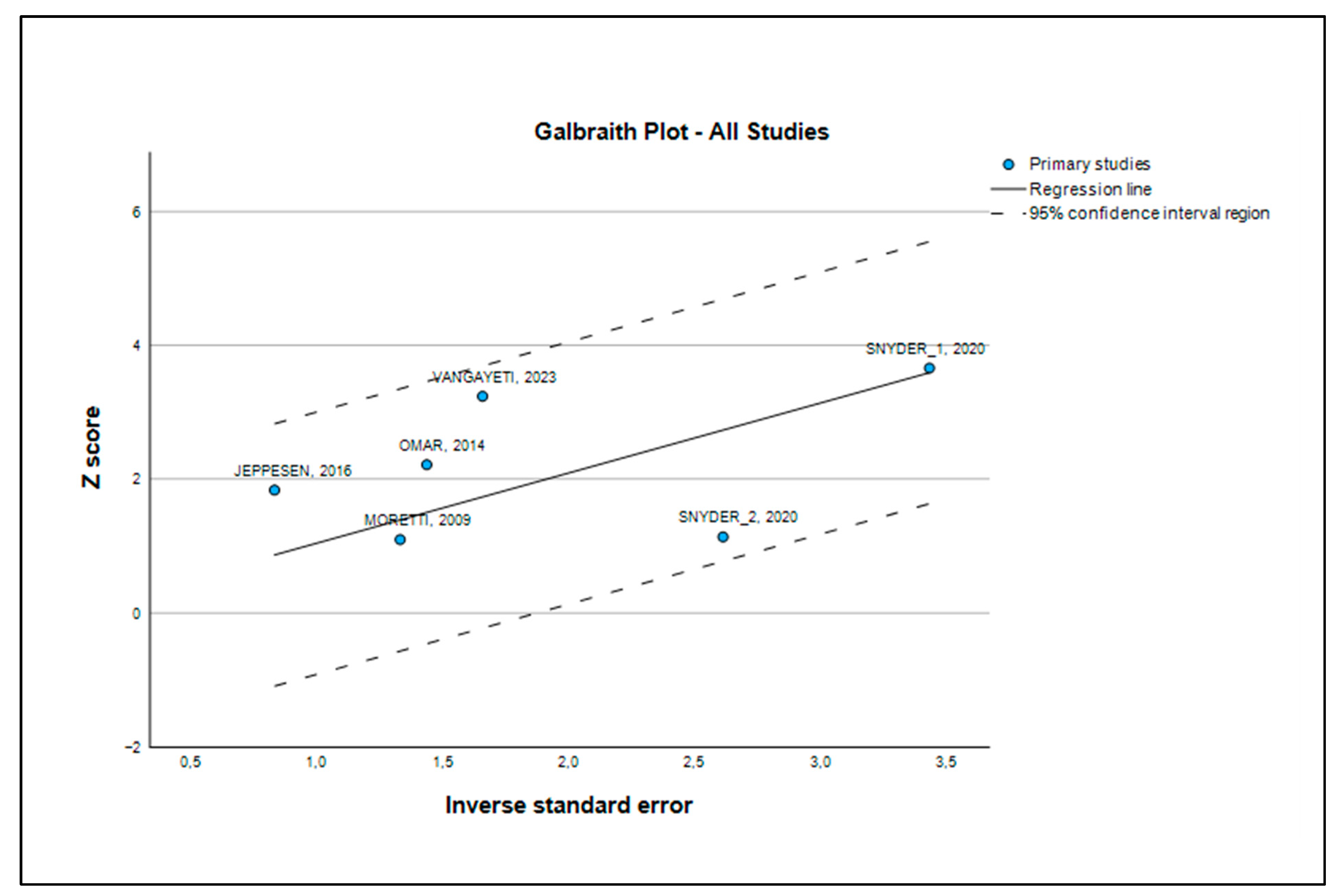
3.4. Heterogeneity Analysis
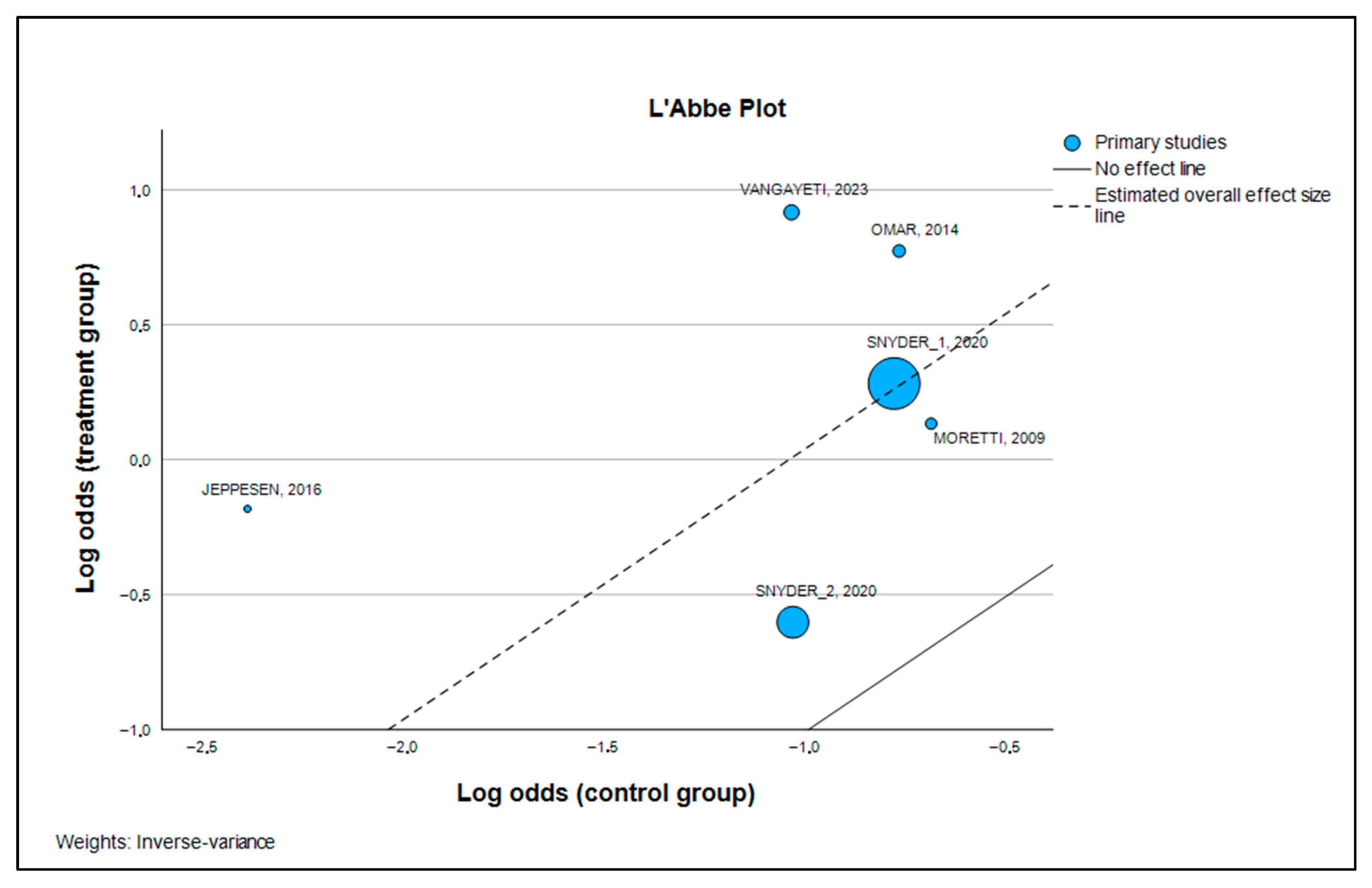
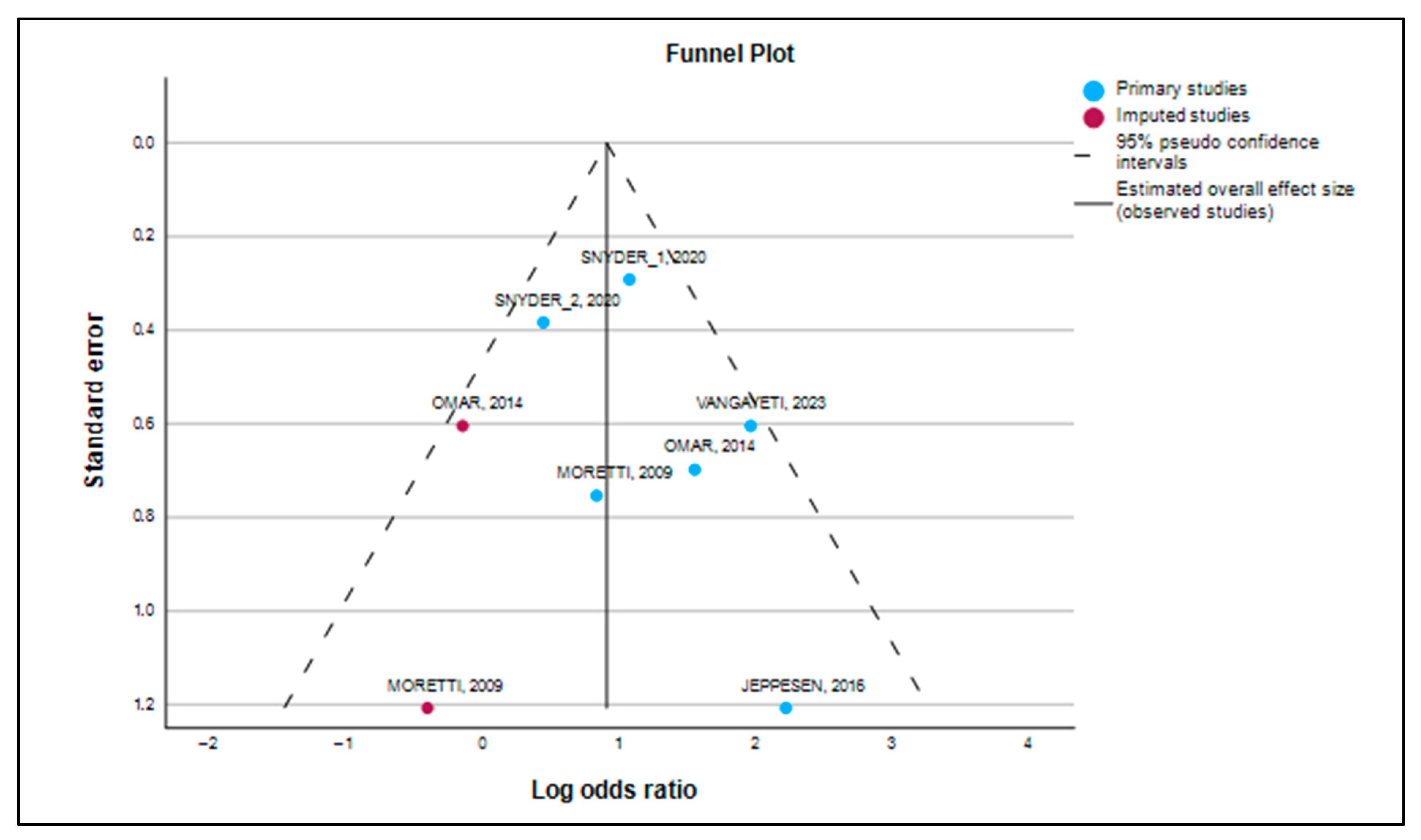
3.5. Publication Bias and Robustness Evaluation
3.6. Trim-and-Fill Analysis
4. Discussion
4.1. ESWT as an Adjunctive Therapy in the Treatment of Diabetic Foot Ulcers
4.2. Molecular and Cellular Mechanisms: A Deeper Look
4.3. ESWT in the Context of Other Adjunctive Therapies
4.4. Strengths and Robustness of the Results
4.5. Cost-Effectiveness and Clinical Considerations
4.6. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 22 July 2025).
- Armstrong, D.G.; Tan, T.-W.; Andrew, J.M.; Boulton, S.A.B. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef]
- Crawford, F.; Mccowan, C.; Dimitrov, B.D.; Woodburn, J.; Wylie, G.H.; Booth, E.; Leese, G.P.; Bekker, H.L.; Kleijnen, J.; Fahey, T. The risk of foot ulceration in people with diabetes screened in community settings: Findings from a cohort study. QJM Int. J. Med. 2011, 104, 403–410. [Google Scholar] [CrossRef]
- Yesil, S.; Akinci, B.; Yener, S.; Bayraktar, F.; Karabay, O.; Havitcioglu, H.; Yapar, N.; Atabey, A.; Kucukyavas, Y.; Comlekci, A.; et al. Predictors of amputation in diabetics with foot ulcer: Single center experience in a large Turkish cohort. Hormones 2009, 8, 286–295. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, T.H.; Choi, J.-Y.; Kwon, Y.-J.; Choi, D.H.; Kim, K.C.; Kim, M.J.; Hwang, H.K.; Lee, K.-B. Predictors for Amputation in Patients with Diabetic Foot Wound. Vasc. Spec. Int. 2018, 34, 109–116. [Google Scholar] [CrossRef]
- Barshes, N.R.; Saedi, S.; Wrobel, J.; Kougias, P.; Kundakcioglu, O.E.; Armstrong, D.G. A model to estimate cost-savings in diabetic foot ulcer prevention efforts. J. Diabetes Complicat. 2017, 31, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.; Nobels, F.; Senneville, É.; Uckay, I.; Maas, M.; Fitridge, R. Assessment and management of diabetes-related foot infection according to the new International Working Group on the Diabetic Foot guidelines 2023—Multidisciplinary grand rounds. Diabetes Metab. Res. Rev. 2024, 40, e3737. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Tassiopoulos, A.; Kirsner, R.S. Evaluation and Management of Lower-Extremity Ulcers. N. Engl. J. Med. 2017, 377, 1559–1567. [Google Scholar] [CrossRef]
- Frairia, R.; Berta, L. Biological effects of extracorporeal shock waves on fibroblasts. A Review. Muscles Ligaments Tendons J. 2011, 1, 138–147. [Google Scholar]
- Mittermayr, R.; Antonic, V.; Hartinger, J.; Kaufmann, H.; Redl, H.; Téot, L.; Stojadinovic, A.; Schaden, W. Extracorporeal shock wave therapy (ESWT) for wound healing: Technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012, 20, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- López, J.B.C. Lectura Crítica de la Evidencia Clínica, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Rosenthal, R.; DiMatteo, M.R. Meta-Analysis: Recent Developments in Quantitative Methods for Literature Reviews. Annu. Rev. Psychol. 2001, 52, 59–82. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088. [Google Scholar] [CrossRef]
- Galbraith, R. Graphical display of estimates having differing standard errors. Technometrics 1988, 30, 271–281. [Google Scholar] [CrossRef]
- L’Abbé, K.A.; Detsky, A.S.; O’Rourke, K. Meta-analysis in clinical research. Ann. Intern. Med. 1987, 107, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Mathur, M.B.; VanderWeele, T.J. Sensitivity analysis for publication bias in meta-analyses. J. R. Stat. Soc. Ser. C Appl. Stat. 2020, 69, 1091–1119. [Google Scholar] [CrossRef]
- Shi, L.; Lin, L. The trim-and-fill method for publication bias: Practical guidelines and recommendations based on a large database of meta-analyses. Medicine 2019, 98, e15987. [Google Scholar] [CrossRef]
- Vangaveti, V.N.; Jhamb, S.; Goodall, J.; Bulbrook, J.; Biros, E.; Malabu, U.H. Extracorporeal Shockwave Therapy (ESWT) in the Management of Diabetic Foot Ulcer: A Prospective Randomized Clinical Trial. J. Foot Ankle Surg. 2023, 62, 845–849. [Google Scholar] [CrossRef]
- Snyder, R.; Galiano, R.; Mayer, P.; Rogers, L.C.; Alvarez, O. Sanuwave Trial Investigators Diabetic foot ulcer treatment with focused shockwave therapy: Two multicentre, prospective, controlled, double-blinded, randomised phase III clinical trials. J. Wound Care 2018, 27, 822–836. [Google Scholar] [CrossRef]
- Moretti, B.; Notarnicola, A.; Maggio, G.; Moretti, L.; Pascone, M.; Tafuri, S.; Patella, V. The management of neuropathic ulcers of the foot in diabetes by shock wave therapy. BMC Musculoskelet. Disord. 2009, 10, 54. [Google Scholar] [CrossRef]
- Jeppesen, S.M.; Yderstraede, K.B.; Rasmussen, B.S.B.; Hanna, M.; Lund, L. Extracorporeal shockwave therapy in the treatment of chronic diabetic foot ulcers: A prospective randomised trial. J. Wound Care 2016, 25, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.T.A.; Alghadir, A.; Al-Wahhabi, K.K.; Al-Askar, A.B. Efficacy of shock wave therapy on chronic diabetic foot ulcer: A single-blinded randomized controlled clinical trial. Diabetes Res. Clin. Pract. 2014, 106, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Weng, C.; Zhao, Z.; Fu, X. Extracorporeal Shock Wave Therapy for Chronic Wounds: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Wound Repair Regen. 2017, 25, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Qi, Z.; Pan, B.; Tao, R. Extracorporeal shock wave therapy (ESWT) favors healing of diabetic foot ulcers: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2024, 220, 111843. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, J.H.; Lee, G.B.; Shin, K.H.; Hwang, J.; Jang, S.Y.; Yoo, J.; Jang, W.Y. Application of extracorporeal shockwave therapy to improve microcirculation in diabetic foot ulcers: A prospective study. Medicine 2023, 102, e33310. [Google Scholar] [CrossRef]
- Nguyen, A.T.A.T.; Chon, J.; Galiano, R. Outcomes of Shockwave Therapy in Diabetic Foot Ulcer Treatment: An Intention-to-treat Analysis. Plast. Reconstr. Surg. Glob. Open 2025, 13, 113. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, X.B.; Chen, S.; Zhao, Z.B.; Schmitz, C.; Weng, C.S. Efficacy and safety of extracorporeal shock wave therapy for acute and chronic soft tissue wounds: A systematic review and meta-analysis. Int. Wound J. 2018, 15, 590–599. [Google Scholar] [CrossRef]
- Hitchman, L.; Totty, J.; Smith, G.E.; Carradice, D.; Twiddy, M.; Iglesias, C.; Russell, D.; Chetter, I.C. Extracorporeal shockwave therapy compared with standard care for diabetic foot ulcer healing: An updated systematic review. Int. Wound J. 2023, 20, 2303–2320. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year of Publication, Country | N, Gender (M/F) | Age (Mean ± SD) | HbA1c (Mean ± SD) | BMI (Mean ± SD) | Average Size | Years Diabetes | Ulcer Classification | Treatment Strategy | ESWT Application | Time Treatment | Healing Rate | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vangaveti et al., 2023, Australian [23] | N = 48 (34/14) E = 25 (17/8) C = 23 (17/6) | N = 62.0 (52.3–78.5) E = 62 (52–78) C = 62 (52–79) | C = 7.8 E = 8.1 | N = 35.5 (29.7–39.5) E = 36.4 ± 6.5 C = 33.4 ± 7.4 | C = 48 (20–408) E = 70 (25–226) | - | Texas Classification: A1 or higher | Effects of ESWT on wound healing in patients with DFU undergoing SOC | Sterile ultrasound gel was applied to the wound, and a sterile foil was placed on top to ensure no air bubbles were present in the treatment area. This was followed by the application of ultrasound gel followed by ESWT. The dosage regimen was administered as 0.11 mJ/mm2 at 5 Hz and an interval of 100 SW with pressure head between 6 and 10. The number of pulses was increased based on wound’s size with a minimum of 310 pulse/cm2. | 6 w | E = 5 (20%) C = 6 (26.1%) | ESWT healing rate did not reach statistical significance at 6 weeks |
| Snyder et al., 2020, US [24] | N = 206 (137/69) E = 107 (83/24) C = 99 (54/45) | E = 60.4 ± 10.4 C = 56.2 ± 9.4 | - | E = 31.8 ± 5.1 C = 31.6 ± 5.2 | E = 35 ± 32 C = 28 ± 24 | E = 18.0 ± 10.0 C = 15.7 ± 11.1 | Texas Classification:1A or 2A | Efficacy of ESWT compared with SOC. | First application of 500 impulses lasting between 2 and 5 min using an energy flux density of 0.23 mJ/mm2 at a rate of 4 impulses/seg and delivered at a power setting of E2. Hereafter, subjects received SOC and 3 additional adjunctive active or sham device applications every 3 ± 1 days over 2 w. | 12 w | E = 61 (47.7%) C = 31 (31.3%) | ESWT is an effective for neuropathic DFU that do not respond to standard care alone. |
| Snyder et al., 2020, US [24] | N = 130 (103/27) E = 65 (54/11) C = 65 (49/16) | E = 59.1 ± 9.4 C = 56.8 ± 10.7 | - | E = 31.4 ± 5.6 C = 31.6 ± 5.5 | E = 3.71 ± 2.8 C = 3.73 ± 2.8 | E = 15.44 ± 11.4 C = 17.45 ± 13.3 | Texas Classification:1A or 2A | Efficacy of ESWT compared with SOC. | ESWT was from a dermaPACE device. Outpatient care procedure with no anesthesia. Ulcer was covered with a sterile cellulose barrier. The treatment dosage was ulcer size dependent with the numbers of impulses equal to the treatment area in cm2 × 8, with a minimum of 500 impulses at energy setting E2 at a rate of 4 shocks/seg. Area was calculated by extending the actual perimeter of the ulcer for 1.0 cm in all directions. The treatments were conducted two times/w for 3 weeks for a total of 6 treatments. | 10 w | E = 23 (35.4%) C = 17 (26.2%) | ESWT is an effective for neuropathic DFU that do not respond to standard care alone. |
| Moretti et al., 2009, Italy [25] | N = 30 (16/14) E = 15 (9/6) C = 15 (7/8) | E = 56.2 ± 4.9 C = 56.8 ± 7.5 | - | - | E = 297.8 ± 129.4 C = 245 ± 100.9 mm2 | - | Wagner 2, 3 and 4 | Evaluate if ESWT is effective in the management. of neuropathic diabetic foot ulcers to compare SOC. | Three sessions (every 72 h), with 100 pulses per 1 cm2 of wound delivered at each session at a flux density (0.03 mJ/mm2) using an electromagnetic lithotripter with a cylindrical coil, parabolic focus, and ultrasound scanning. | 20 w | E = 8 (53.3%) C = 5 (33.3%) | ESWT may be a useful adjunct in the management of diabetic foot. ulceration. |
| Jeppesen et al., 2016, Denmark [26] | N = 23 (16/7) E = 11 (5/6) C = 12 (11/1) | E = 65.3 ± 12.9 C = 67.8 ± 9.7 | E = 8.6 ± 0.8 C = 7.7 ± 1.2 | E = 27.0 ± 5.4 C = 26.3 ± 3.7 | - | E = 16.3 ± 12.2 C = 25.1 ± 15.0 | Wagner 1 and 2 | Efficacy of ESWT on healing DFU compared to SOC. | 6 ESWT treatments over 3 w. Treatments were carried out with a DUOLITH SD1 T-top shockwave device delivering focused shockwaves with energy flux density 0.2 mJ/mm2 and frequency 5 Hz. Ulcer surface and perimeter of ulcer extending 1 cm in every direction was treated with ESWT, using 250 shocks/cm2 and focal area 0–30 mm. Furthermore, 500 deep shocks focal area (15–45 mm) were applied on the anatomical location of arteries supplying ulcer location. | 3 w | E = 5 (34.5%) C = 1 (5.6%) | Potential beneficial effect of ESWT on ulcer healing as well as tissue oxygenation. |
| Omar et al., 2014, Egypt [27] | N = 38 (27/11) E = 19 (14/5) C = 19 (13/6) | E = 56.59 ± 7.35 C = 57.0 ± 5.39 | E = 8.92 ± 1.93 C = 8.22 ± 1.90 | E = 27.44 ± 2.57 C = 27.35 ± 3.94 | E = 7.89 ± 2.97 C = 8.62 ± 3.47 | E = 12.0 ± 3.66 C = 13.14 ± 3.78 | Texas Classification:1A or 2A | Evaluate the efficacy of ESWT on the healing rate. | Cleaned saline, removed necrotic tissues, and covered. ESWT at a frequency of 100 pulse/cm2, and energy flux density of 0.11 mJ/cm2. Twice a week, with one-week interval, and for a total of eight sessions. | 8 w | E = 13 (54%) C = 6 (28.5%) | ESWT significant reduction in wound size and median time ulcer healing, with no adverse reactions. |
| First Author | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
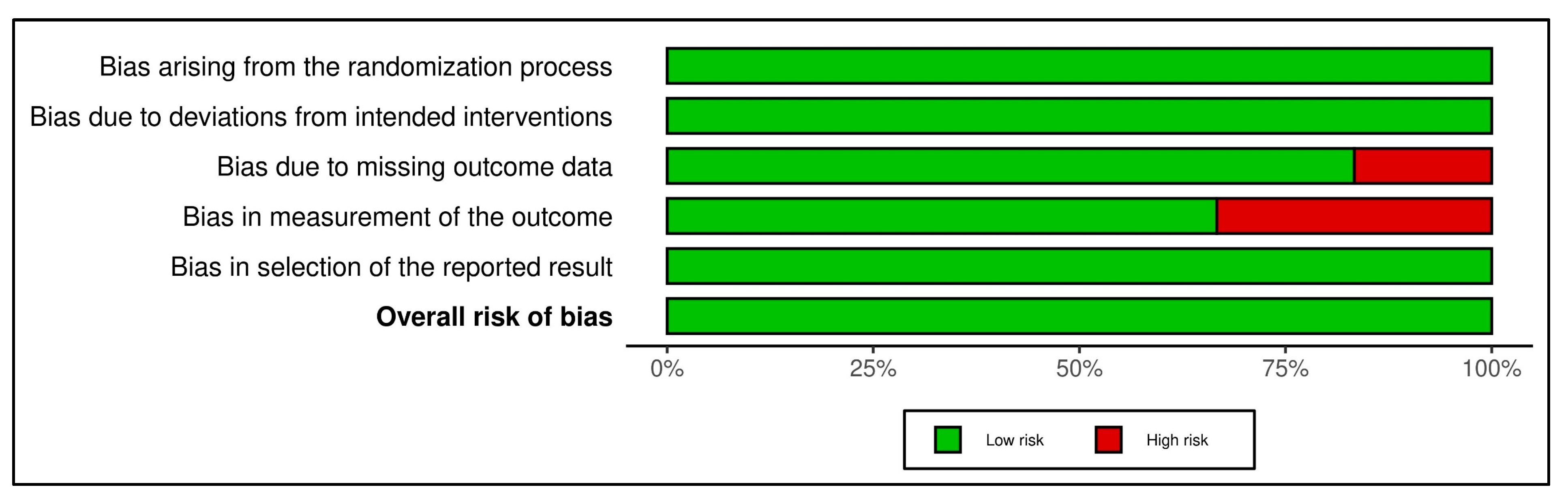
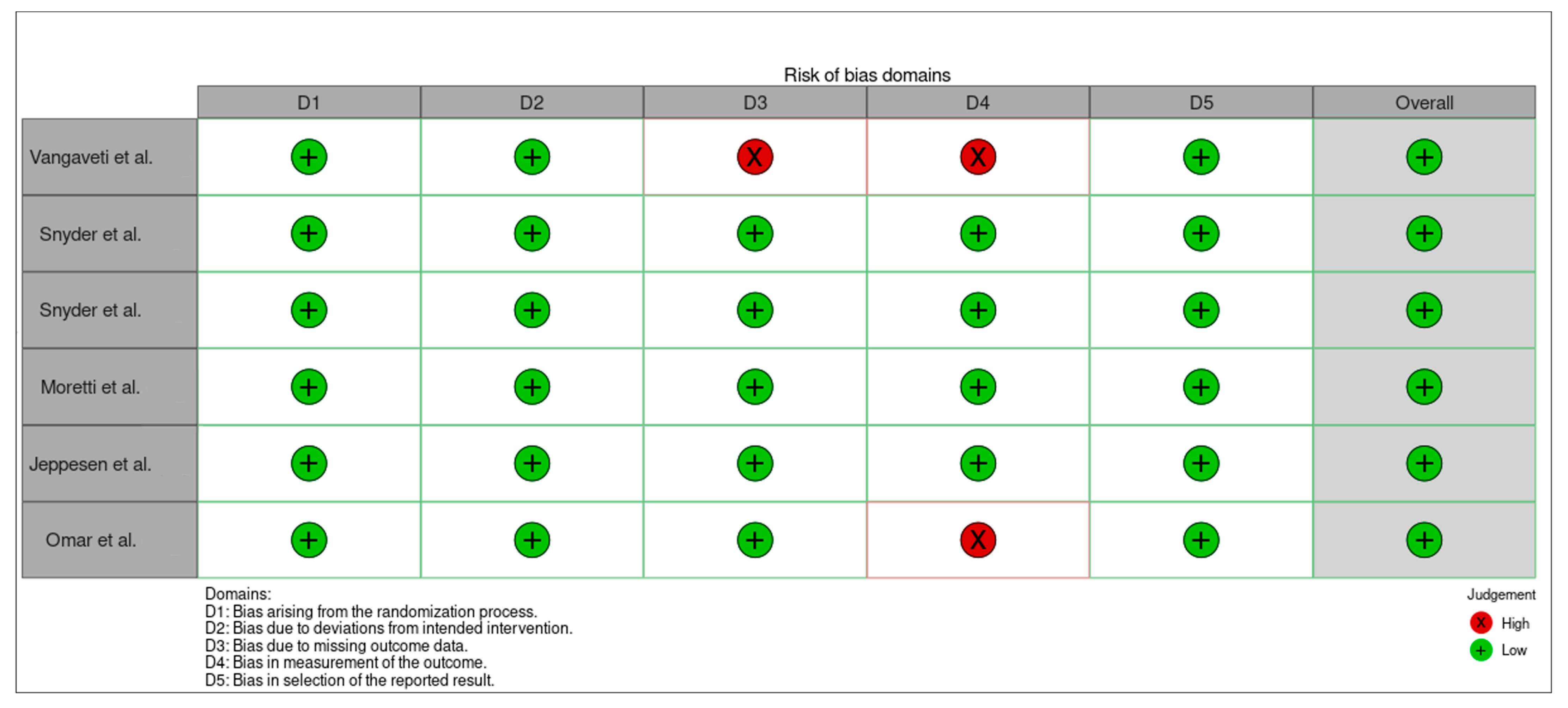
| Vangaveti et al. [23] | YES | YES | YES | NO | YES | YES | YES | YES | YES | YES | YES | 10/11 |
| Snyder et al. [24] | YES | YES | YES | NO | YES | YES | YES | YES | YES | YES | YES | 10/11 |
| Snyder et al. [24] | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | 10/11 |
| Moretti et al. [25] | YES | YES | YES | NO | YES | YES | YES | YES | YES | YES | YES | 10/11 |
| Jeppesen et al. [26] | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | 10/11 |
| Omar et al. [27] | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | 10/11 |
| ID | Effect Size | Std. Error | Z | Sig. (2-Tailed) | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Moretti et al. [25] | 0.827 | 0.7536 | 1.097 | 0.273 | −0.650 | 2.304 |
| Omar et al. [27] | 1.546 | 0.6980 | 2.215 | 0.027 | 0.178 | 2.914 |
| Jeppesen et al. [26] | 2.216 | 1.2073 | 1.835 | 0.066 | −0.151 | 4.582 |
| Snyder et al. [24] | 1.068 | 0.2917 | 3.660 | <0.001 | 0.496 | 1.640 |
| Snyder et al. [24] | 0.436 | 0.3833 | 1.137 | 0.256 | −0.316 | 1.187 |
| Vangaveti et al. [23] | 1.958 | 0.6046 | 3.238 | 0.001 | 0.773 | 3.143 |
| Effect Size | Std. Error | Z | Sig. (2-Tailed) | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Overall | 0.048 | 0.1969 | 5.320 | <0.001 | 0.662 | 1.434 |
| Chi-Square (Q Statistic) | df | Sig. | |
|---|---|---|---|
| Overall | 6.351 | 5 | 0.274 |
| Index | 95% Confidence Interval | |||
|---|---|---|---|---|
| Lower | Upper | |||
| Overall | H-squared | 1.270 | 0.553 | 2.916 |
| I-squared (%) | 21.3 | 0.0 | 65.7 | |
| Kendall’s Tau | p |
|---|---|
| −0.333 | 0.200 |
| Egger’s Regression-Based Test a | ||||||
|---|---|---|---|---|---|---|
| Parameter. | Coefficient | Std. Error | t | Sig. (2-Tailed) | 95% Confidence Interval | |
| Lower | Upper | |||||
| (Intercept) | 0.488 | 0.5005 | 0.974 | 0.385 | −0.902 | 1.877 |
| SE b | 1.281 | 1.0377 | 1.234 | 0.285 | −1.600 | 4.162 |
| CI (95.0%) | ||||||
|---|---|---|---|---|---|---|
| Omitted Study | Year | n | OR | Lower Limit | Upper Limit | Relative Change (%) |
| Moretti et al. [25] | 2009 | 642 | 2.9031 | 2.0601 | 4.0911 | 1.23 |
| Omar et al. [27] | 2014 | 634 | 2.7795 | 1.9694 | 3.923 | −3.08 |
| Jeppesen et al. [26] | 2016 | 649 | 2.8008 | 1.9985 | 3.9252 | −2.34 |
| Snyder et al. [24] | 2020 | 466 | 2.8467 | 1.8859 | 4.2971 | −0.73 |
| Snyder et al. [24] | 2020 | 542 | 3.3396 | 2.2997 | 4.8496 | 16.45 |
| Vangaveti et al. [23] | 2023 | 614 | 2.6523 | 1.8723 | 3.7573 | −7.51 |
| GLOBAL | 672 | 2.8678 | 2.0532 | 4.0055 | ||
| Number | Effect Size | Std. Error | Z | Sig. (2-Tailed) | 95% Confidence Interval | 95% Confidence Interval | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Exp. Effect Size | Lower | Upper | ||||||
| Observed | 6 | 1.048 | 0.1969 | 5.320 | <0.001 | 0.662 | 1.434 | 2.851 | 1.938 | 4.193 |
| Observed + Imputed a | 8 | 0.900 | 0.1850 | 4.867 | <0.001 | 0.538 | 1.263 | 2.461 | 1.712 | 3.536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Muñoz, M.; Rueda-Zapata, L.; Martinez-Barrios, F.-J.; Nováková, T.; Lopezosa-Reca, E.; Gonzalez-Sanchez, M.; Fernandez-Torres, R.; Galan-Mercant, A. Efficacy of Extracorporeal Shockwave Therapy in the Management of Chronic Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis. Med. Sci. 2025, 13, 219. https://doi.org/10.3390/medsci13040219
Ruiz-Muñoz M, Rueda-Zapata L, Martinez-Barrios F-J, Nováková T, Lopezosa-Reca E, Gonzalez-Sanchez M, Fernandez-Torres R, Galan-Mercant A. Efficacy of Extracorporeal Shockwave Therapy in the Management of Chronic Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis. Medical Sciences. 2025; 13(4):219. https://doi.org/10.3390/medsci13040219
Chicago/Turabian StyleRuiz-Muñoz, Maria, Lidia Rueda-Zapata, Francisco-Javier Martinez-Barrios, Tereza Nováková, Eva Lopezosa-Reca, Manuel Gonzalez-Sanchez, Raul Fernandez-Torres, and Alejandro Galan-Mercant. 2025. "Efficacy of Extracorporeal Shockwave Therapy in the Management of Chronic Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis" Medical Sciences 13, no. 4: 219. https://doi.org/10.3390/medsci13040219
APA StyleRuiz-Muñoz, M., Rueda-Zapata, L., Martinez-Barrios, F.-J., Nováková, T., Lopezosa-Reca, E., Gonzalez-Sanchez, M., Fernandez-Torres, R., & Galan-Mercant, A. (2025). Efficacy of Extracorporeal Shockwave Therapy in the Management of Chronic Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis. Medical Sciences, 13(4), 219. https://doi.org/10.3390/medsci13040219