Combined Effects of PFAS, Social, and Behavioral Factors on Liver Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Description of Cohort
2.1.2. Blood Measurement Sample
2.2. PFAS Extraction
2.3. Liver Markers
Fatty Liver Index for Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
2.4. Assessment of Alcohol Use and Smoking Behavior
2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics Results
3.2. Spearman Correlation Results
3.3. Linear Regression Results
3.4. Bayesian Kernel Machine Regression Results
3.4.1. Posterior Inclusion Probability (PIP)
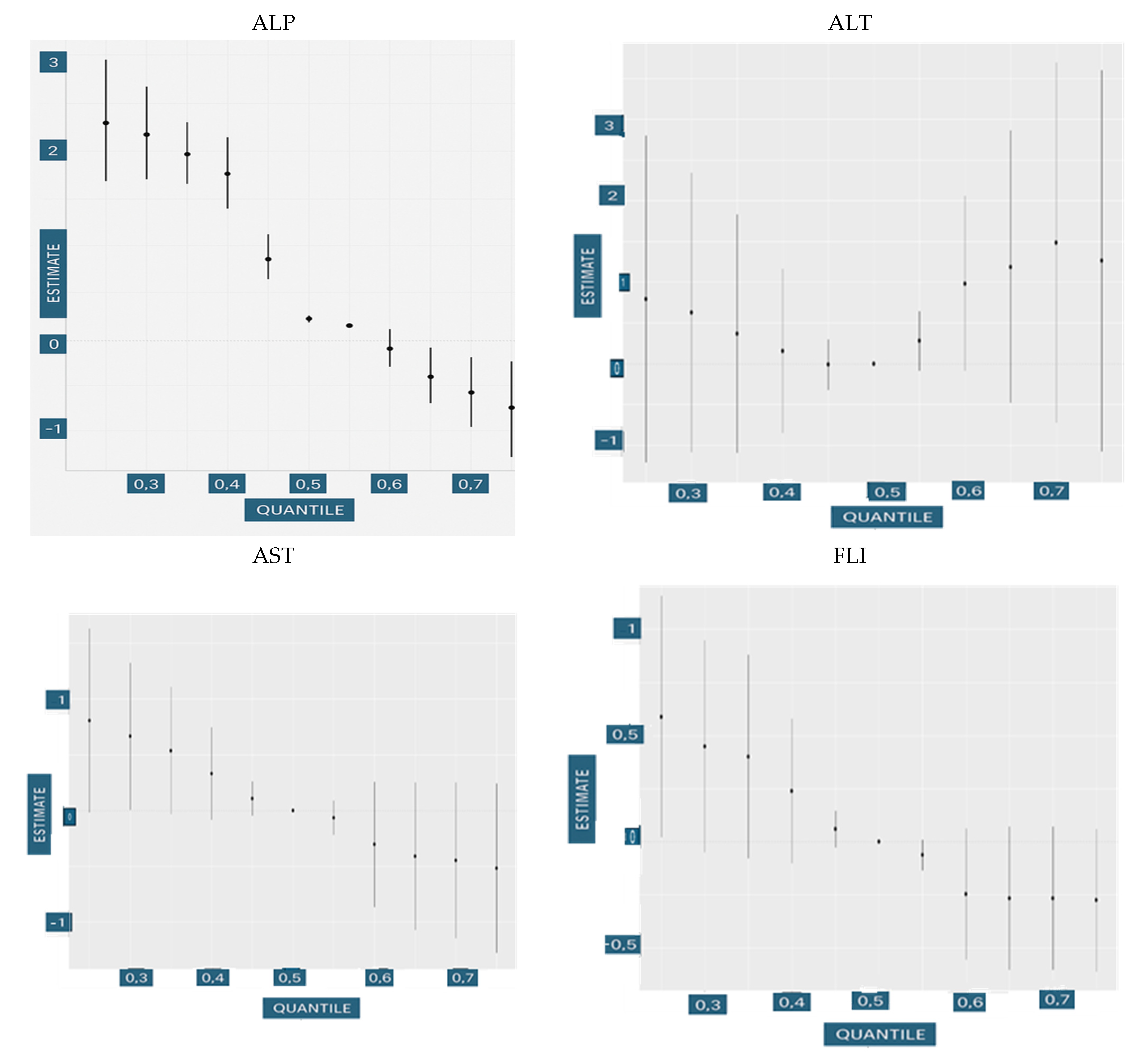
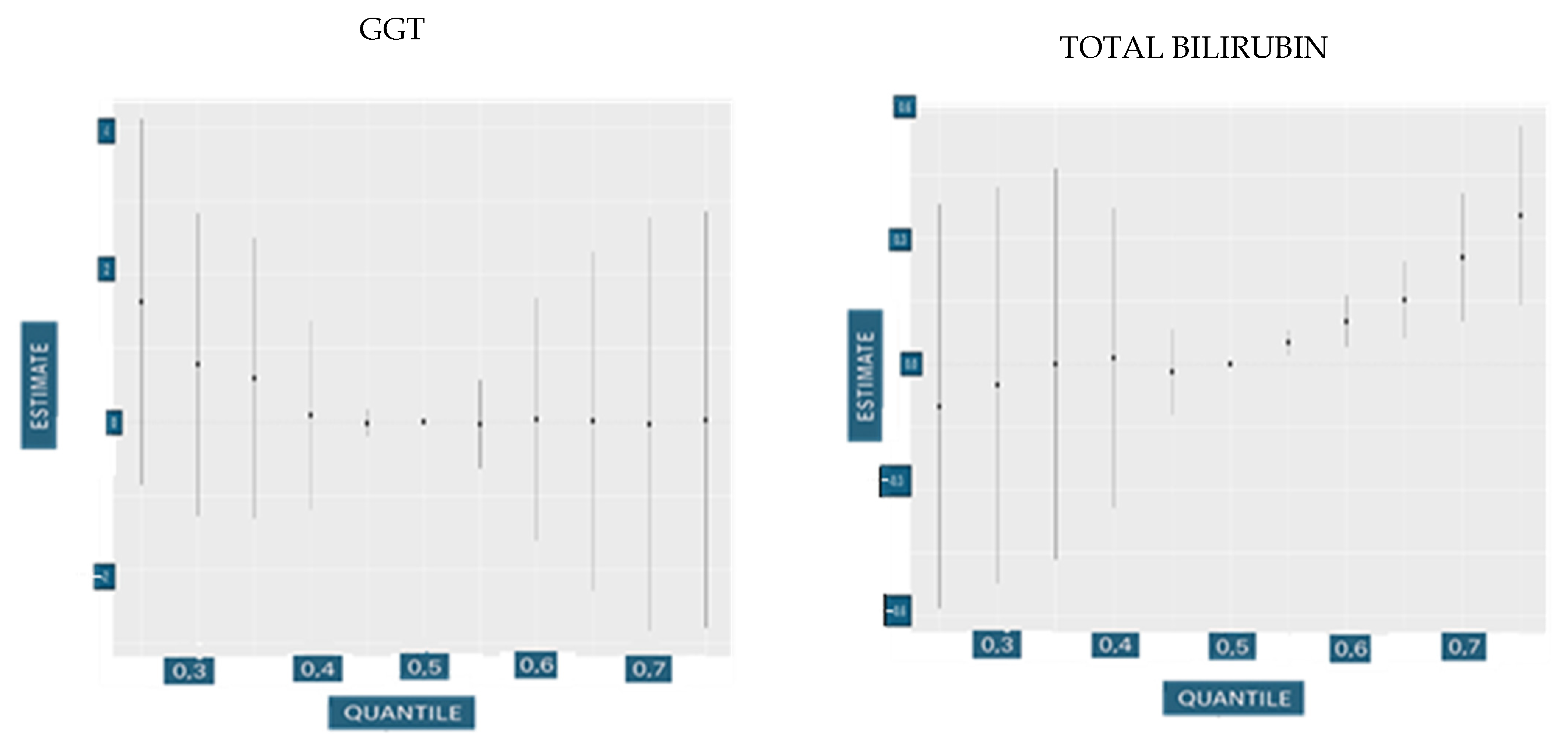
3.4.2. Univariate Dose Responses
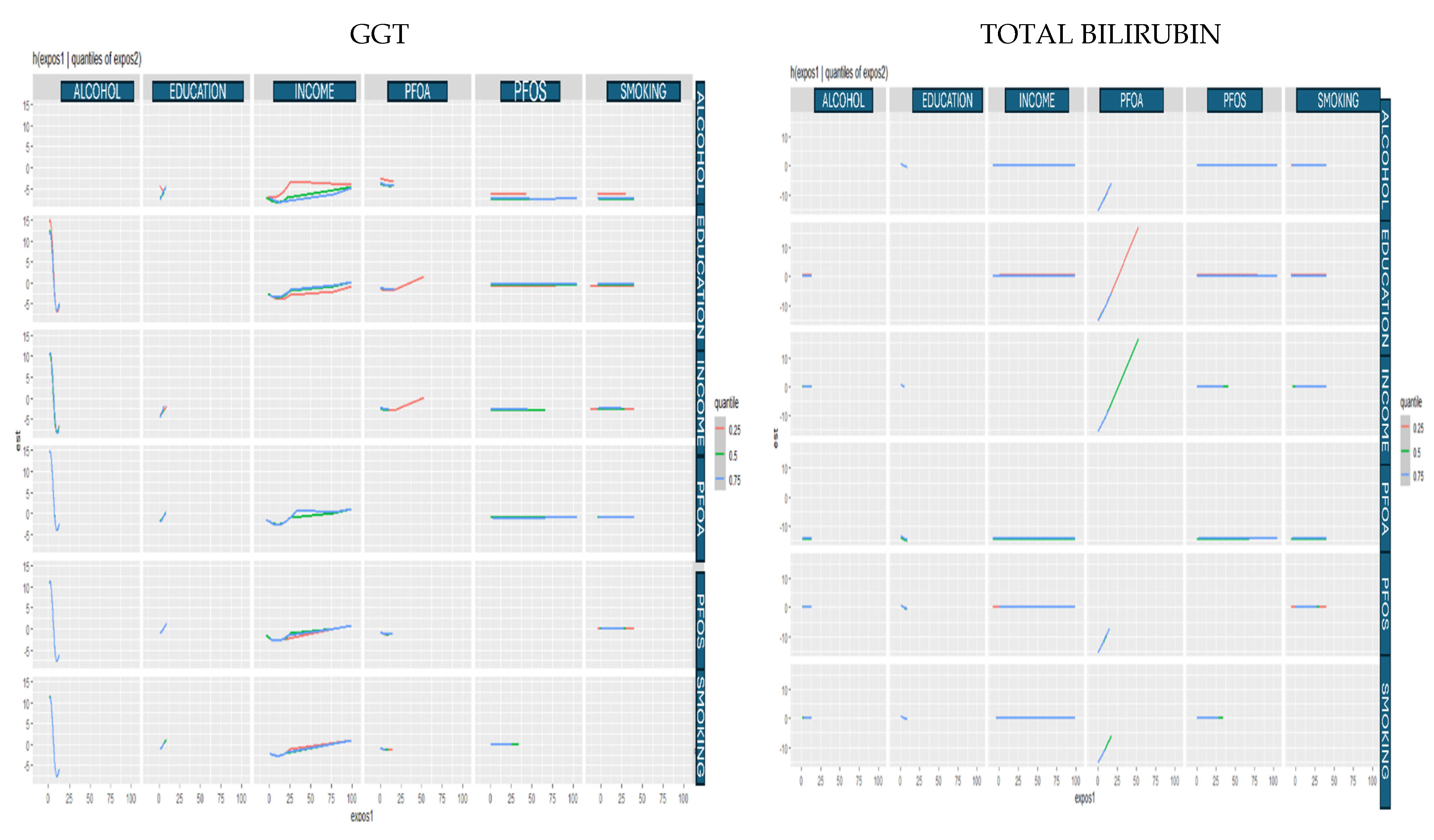
3.4.3. Bivariate Exposure Response
3.4.4. Single-Variable Risk Summary
3.4.5. Overall Exposure Effect
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; De Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- National Institute of Health. Study Links Synthetic Chemicals to Liver Damage. Available online: https://www.nih.gov/news-events/nih-research-matters/study-links-synthetic-chemicals-liver-damage (accessed on 15 November 2024).
- Gaines, L.G.T. Historical and current usage of per-and polyfluoroalkyl substances (PFAS): A literature review. Am. J. Ind. Med. 2023, 66, 353–378. [Google Scholar] [CrossRef]
- Sinclair, G.M.; Long, S.M.; Jones, O.A. What are the effects of PFAS exposure at environmentally relevant concentrations? Chemosphere 2020, 258, 127340. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Costello, E.; Rock, S.; Stratakis, N.; Eckel, S.P.; Walker, D.I.; Valvi, D.; Cserbik, D.; Jenkins, T.; Xanthakos, S.A.; Kohli, R.; et al. Exposure to per-and polyfluoroalkyl substances and markers of liver injury: A systematic review and meta-analysis. Environ. Health Perspect. 2022, 130, 046001. [Google Scholar] [CrossRef]
- Marti-Aguado, D.; Clemente-Sanchez, A.; Bataller, R. Cigarette smoking and liver diseases. J. Hepatol. 2022, 77, 191–205. [Google Scholar] [CrossRef]
- Rutledge, S.M.; Asgharpour, A. Smoking and liver disease. Gastroenterol. Hepatol. 2020, 16, 617. [Google Scholar]
- Husain, K.; Scott, B.R.; Reddy, S.K.; Somani, S.M. Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol 2001, 25, 89–97. [Google Scholar] [CrossRef]
- Setshedi, M.; Wands, J.R.; de la Monte, S.M. Acetaldehyde adducts in alcoholic liver disease. Oxidative Med. Cell. Longev. 2010, 3, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Osna, N.A.; Donohue Jr, T.M.; Kharbanda, K.K. Alcoholic liver disease: Pathogenesis and current management. Alcohol Res. 2017, 38, 147–161. [Google Scholar]
- Mandrekar, P.; Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009, 50, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Kardashian, A.; Serper, M.; Terrault, N.; Nephew, L.D. Health Disparities in Chronic Liver Disease. Hepatology 2023, 77, 1382–1403. [Google Scholar] [CrossRef]
- Drewnowski, A.; Darmon, N. The economics of obesity: Dietary energy density and energy cost. Am. J. Clin. Nutr. 2005, 82, 265S–273S. [Google Scholar] [CrossRef]
- Koutny, F.; Aigner, E.; Datz, C.; Gensluckner, S.; Maieron, A.; Mega, A.; Iglseder, B.; Langthaler, P.; Frey, V.; Paulweber, B.; et al. Relationships between education and non-alcoholic fatty liver disease. Eur. J. Intern. Med. 2023, 118, 98–107. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. NHANES 2017-2018 Overview. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics&Cycle=2017-2018 (accessed on 20 February 2025).
- Johnson, N.B.; Hayes, L.D.; Brown, K.; Hoo, E.C.; Ethier, K.A. CDC National Health Report: Leading causes of morbidity and mortality and associated behavioral risk and protective factors—United States, 2005–2013. Morb. Mortal. Wkly. Rep. 2014, 63, 3–27. [Google Scholar]
- Bashir, T.; Obeng-Gyasi, E. Combined Effects of Multiple Per-and Polyfluoroalkyl Substances Exposure on Allostatic Load Using Bayesian Kernel Machine Regression. Int. J. Environ. Res. Public Health 2023, 20, 5808. [Google Scholar] [CrossRef]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Suja, F.; Pramanik, B.K.; Zain, S.M. Contamination, bioaccumulation and toxic effects of perfluorinated chemicals (PFCs) in the water environment: A review paper. Water Sci. Technol. 2009, 60, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Nguyen, V.H.; Cheung, R.; Nguyen, M.H. Trends of chronic liver diseases by income level and socioeconomic factors in the United States: A population-based study. Aliment. Pharmacol. Ther. 2024, 60, 1374–1387. [Google Scholar] [CrossRef]
- Soeda, J.; Morgan, M.; McKee, C.; Mouralidarane, A.; Lin, C.; Roskams, T.; Oben, J.A. Nicotine induces fibrogenic changes in human liver via nicotinic acetylcholine receptors expressed on hepatic stellate cells. Biochem. Biophys. Res. Commun. 2012, 417, 17–22. [Google Scholar] [CrossRef]
- Stroffolini, T.; Sagnelli, E.; Sagnelli, C.; Morisco, F.; Babudieri, S.; Furlan, C.; Pirisi, M.; Russello, M.; Smedile, A.; Pisaturo, M.; et al. The association between education level and chronic liver disease of any etiology. Eur. J. Intern. Med. 2020, 75, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Färkkilä, M.; Männistö, V. Interaction between alcohol use and metabolic risk factors for liver disease: A critical review of epidemiological studies. Alcohol. Clin. Exp. Res. 2020, 44, 384–403. [Google Scholar] [CrossRef]
- Soto-Angona, Ó.; Anmella, G.; Valdés-Florido, M.J.; De Uribe-Viloria, N.; Carvalho, A.F.; Penninx, B.W.J.H.; Berk, M. Non-alcoholic fatty liver disease (NAFLD) as a neglected metabolic companion of psychiatric disorders: Common pathways and future approaches. BMC Med. 2020, 18, 261. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-L.; Yao, Z.-Y.; Zhang, Y.-F.; Cui, X.-T.; Sun, A.; Cao, J.-Y.; Wang, Z.-S. Bisphenols exposure and non-alcoholic fatty liver disease: From environmental trigger to molecular pathogenesis. Front. Endocrinol. 2025, 16, 1606654. [Google Scholar] [CrossRef] [PubMed]
- Balogun, M.; Obeng-Gyasi, E. Association of Combined PFOA, PFOS, Metals and Allostatic Load on Hepatic Disease Risk. J. Xenobiotics 2024, 14, 516–536. [Google Scholar] [CrossRef]
- Maerten, A.; Callewaert, E.; Sanz-Serrano, J.; Devisscher, L.; Vinken, M. Effects of per-and polyfluoroalkyl substances on the liver: Human-relevant mechanisms of toxicity. Sci. Total Environ. 2024, 954, 176717. [Google Scholar] [CrossRef]
- Borghese, M.M.; Liang, C.L.; Owen, J.; Fisher, M. Individual and mixture associations of perfluoroalkyl substances on liver function biomarkers in the Canadian Health Measures Survey. Environ. Health 2022, 21, 85. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Sly, J.L.; Ruchirawat, M.; Silva, E.R.; Huo, X.; Diaz-Barriga, F.; Zar, H.J.; King, M.; Ha, E.-H.; Asante, K.A.; et al. Health consequences of environmental exposures: Changing global patterns of exposure and disease. Ann. Glob. Health 2016, 82, 10–19. [Google Scholar] [CrossRef]
- Valacchi, G.; Daiber, A. Environmental Stressors and Oxinflammatory Tissues Responses; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Calabrese, E.J.; Baldwin, L.A. Ethanol and hormesis. Crit. Rev. Toxicol. 2003, 33, 407–424. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G. Cigarette smoking and serum liver enzymes: The role of alcohol and inflammation. Ann. Clin. Biochem. 2010, 47, 321–326. [Google Scholar] [CrossRef]
- Park, E.Y.; Lim, M.K.; Oh, J.-K.; Cho, H.; Bae, M.J.; Yun, E.H.; Kim, D.-I.; Shin, H.-R. Independent and supra-additive effects of alcohol consumption, cigarette smoking, and metabolic syndrome on the elevation of serum liver enzyme levels. PLoS ONE 2013, 8, e63439. [Google Scholar] [CrossRef]
- Whitehead, T.P.; Robinson, D.; Allaway, S.L. The effects of cigarette smoking and alcohol consumption on serum liver enzyme activities: A dose-related study in men. Ann. Clin. Biochem. 1996, 33, 530–535. [Google Scholar] [CrossRef]
- Fedeli, U.; Avossa, F.; Goldoni, C.A.; Caranci, N.; Zambon, F.; Saugo, M. Education level and chronic liver disease by aetiology: A proportional mortality study. Dig. Liver Dis. 2015, 47, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.G.d.S.; Marega, M.; Carvalho, J.A.M.d.; Carmona, F.G.; Lopes, C.E.F.; Ceschini, F.L.; Bocalini, D.S.; Figueira Junior, A.J. Physical activity as a protective factor for development of non-alcoholic fatty liver in men. Einstein 2015, 13, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Mejía, M.M.; Castillo-Castañeda, S.M.; Pal, S.C.; Qi, X.; Méndez-Sánchez, N. The Multifaceted Role of Bilirubin in Liver Disease: A Literature Review. J. Clin. Transl. Hepatol. 2024, 12, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Vertuani, S.; Angusti, A.; Manfredini, S. The antioxidants and pro-antioxidants network: An overview. Curr. Pharm. Des. 2004, 10, 1677–1694. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.E.; Myers, J.P.; Vandenberg, L.N. Nonmonotonic dose–response curves occur in dose ranges that are relevant to regulatory decision-making. Dose-Response 2018, 16, 1559325818798282. [Google Scholar] [CrossRef]
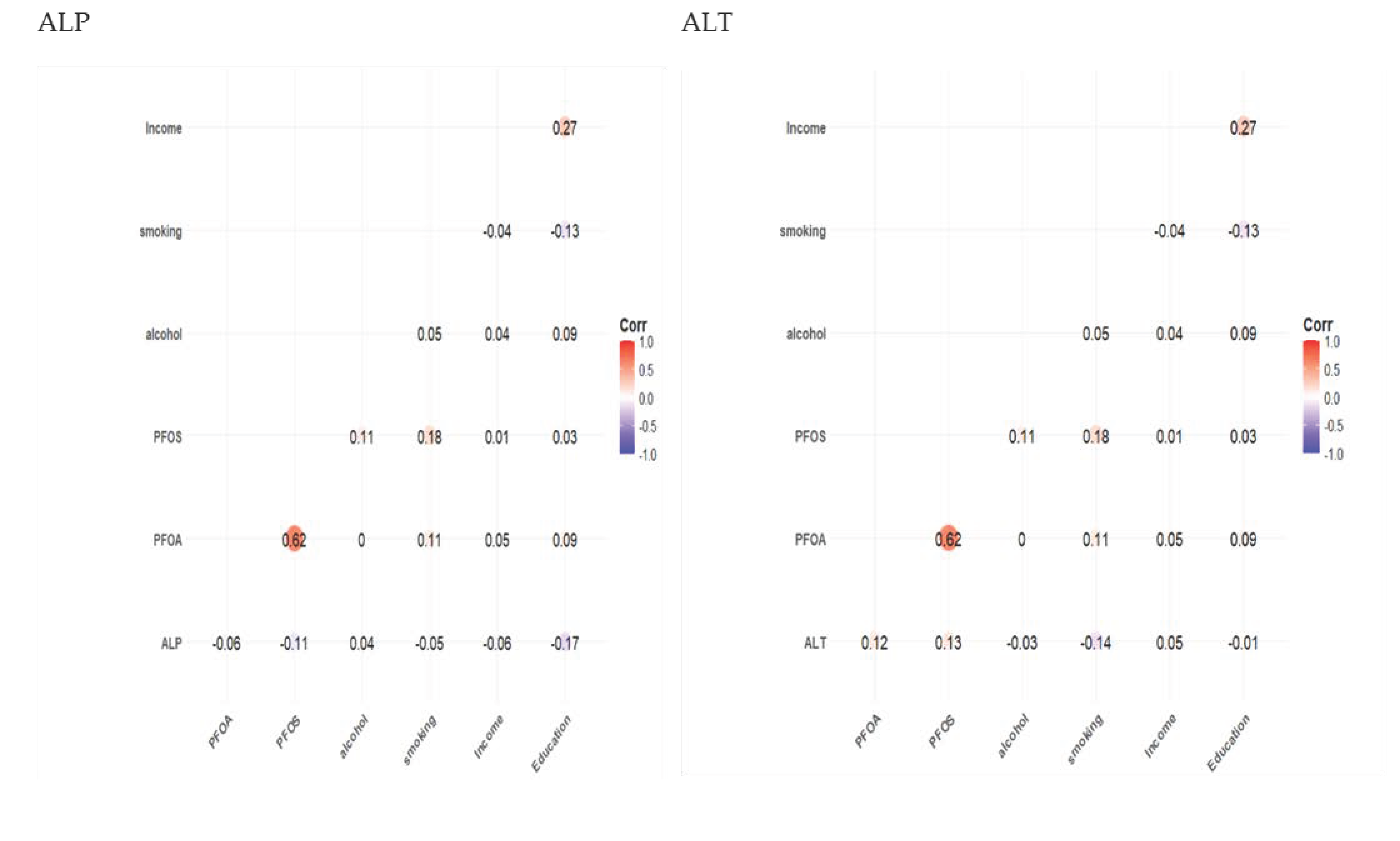
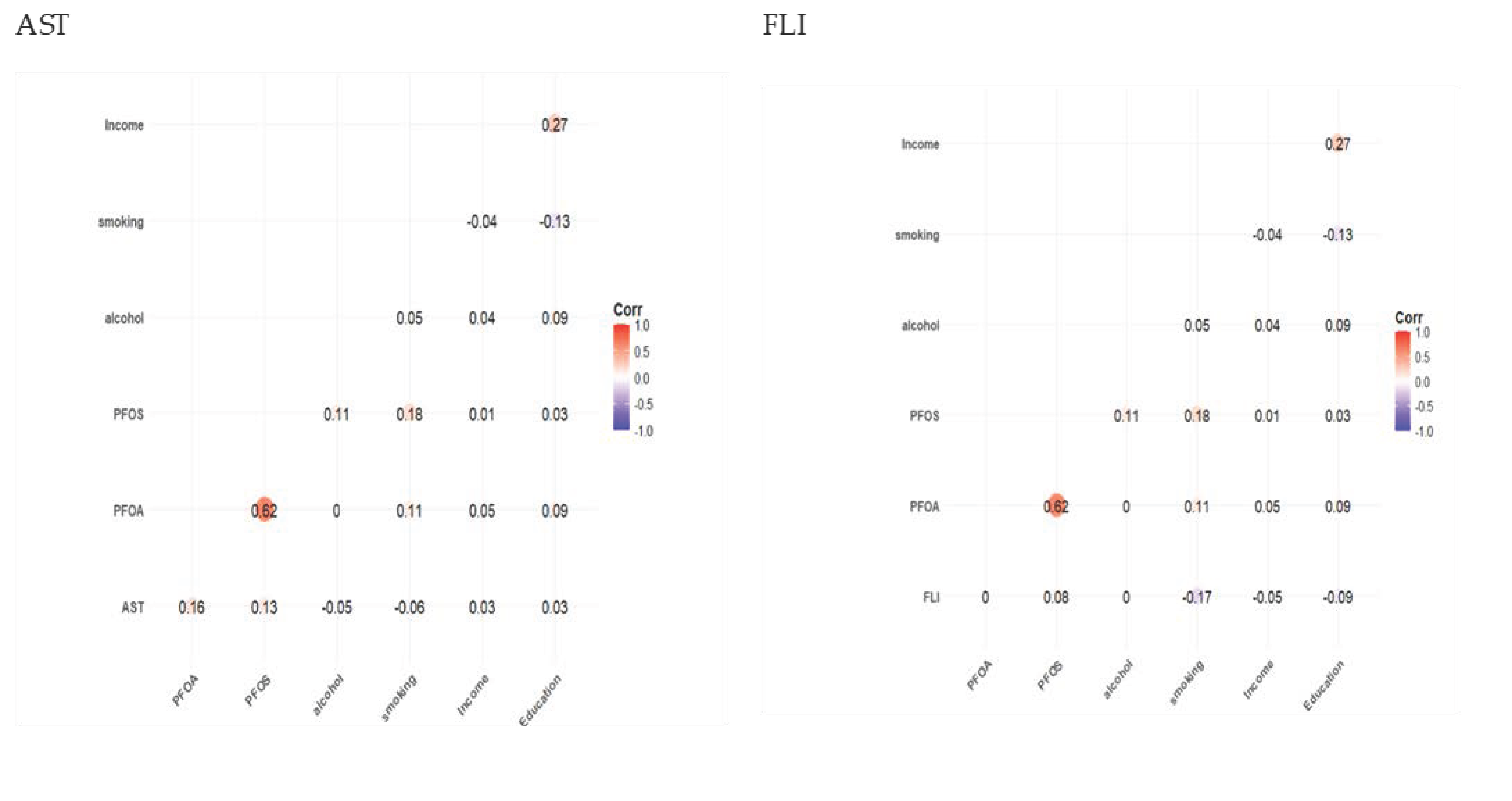

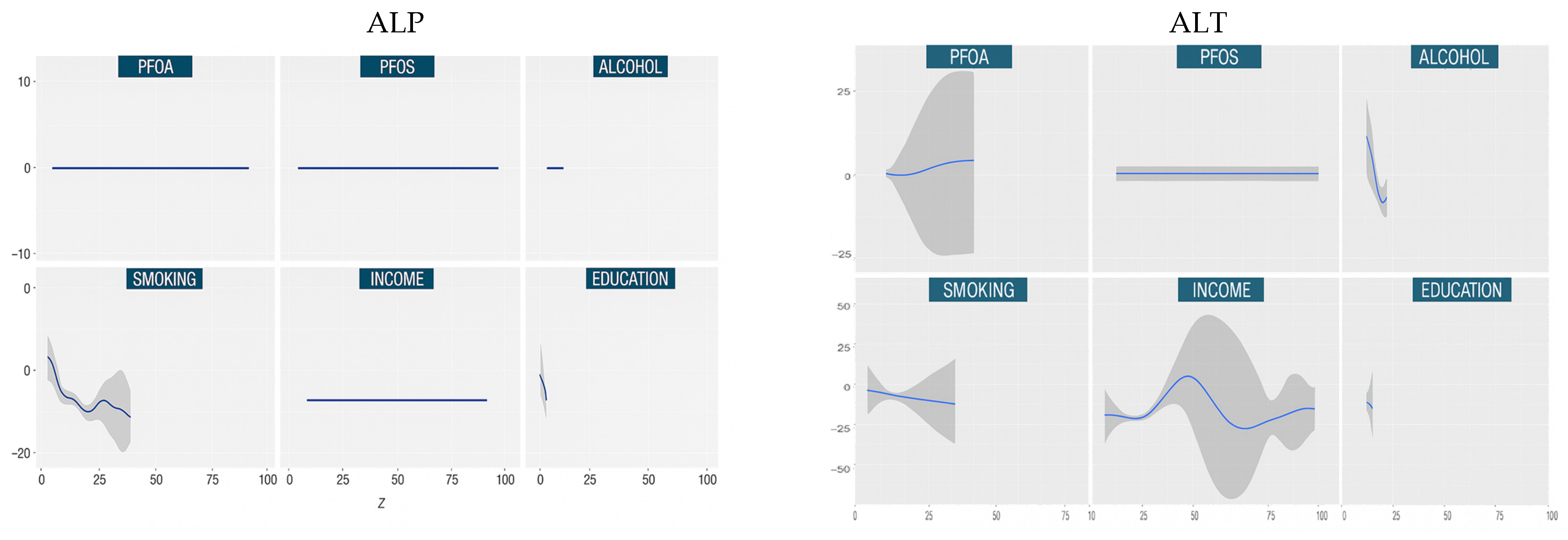
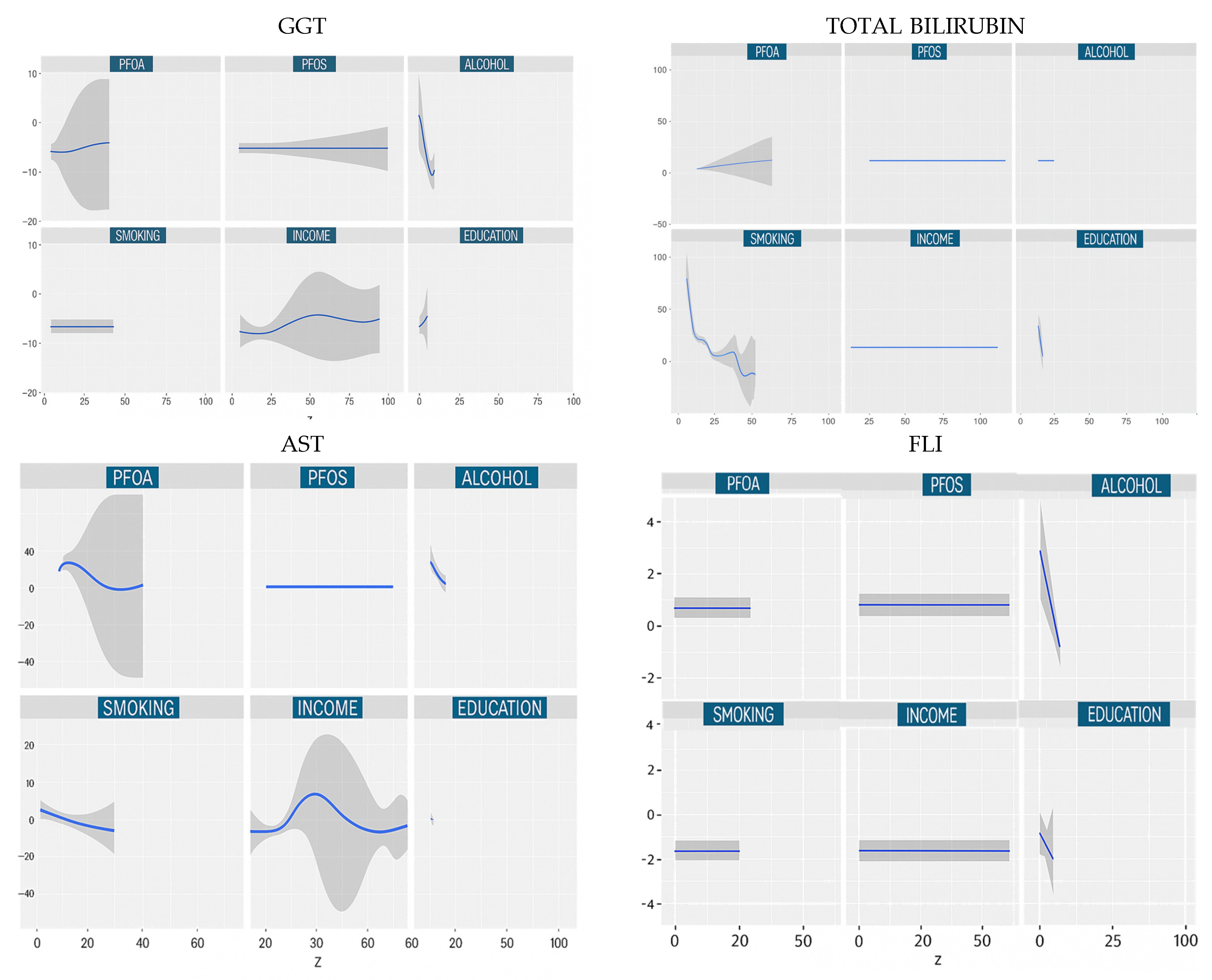


| Characteristics | Total Sample Population N = 1784 |
|---|---|
| Demographic | |
| Age: mean (SD) | 45.27 (20.85) |
| Gender—female | 50.3% |
| Gender—male | 49.3% |
| PFAS | |
| PFOA: mean (SD) | 1.71 (1.82) |
| PFOS: mean (SD) | 6.51 (7.74) |
| Liver Markers | |
| AST: mean (SD) | 21.26 (10.64) |
| ALT: mean (SD) | 21.27 (16.27) |
| ALP: mean (SD) | 87.97 (46.81) |
| GGT: mean (SD) | 28.48 (35.19) |
| Total bilirubin: mean (SD) | 0.46 (0.29) |
| US-FLI: mean (SD) | 49.14 (33.60) |
| Gender—female | 50.3% |
| Gender—male | 49.3% |
| Education | |
| Education—less than 9th grade | 9.0% |
| Education—9–11th grade (includes 12th grade with no diploma) | 11.4% |
| Education—high school graduate/GED or equivalent | 22.9% |
| Education—some college or AA degree | 33.3% |
| Education—college graduate or above | 23.4% |
| Race/Ethnicity | |
| Race/ethnicity—Black | 23.4% |
| Race/ethnicity—White | 36.6% |
| Mexican American | 16.2% |
| Race/ethnicity—other Hispanic | 9.7% |
| Race/ethnicity—Asian | 14.1% |
| Income | |
| USD 0 to USD 4999 | 2.79% |
| USD 5000 to USD 9999 | 2.97% |
| USD 10,000 to USD 14,999 | 5.75% |
| USD 15,000 to USD 19,999 | 6.48% |
| USD 20,000 to USD 24,999 | 7.57% |
| USD 25,000 to USD 34,999 | 11.27% |
| USD 35,000 to USD 44,999 | 12.48% |
| USD 45,000 to USD 54,999 | 9.21% |
| USD 55,000 to USD 64,999 | 5.81% |
| USD 65,000 to USD 74,999 | 5.94% |
| USD 75,000 to USD 99,999 | 10.66% |
| USD 100,000 and over | 19.08% |
| Behavior | Category | % of Participants |
|---|---|---|
| Alcohol use (number of days with 4 or 5 drinks in the past 12 months) | Every day | 0.81 |
| Nearly every day | 0.9 | |
| 3 to 4 times a week | 1.44 | |
| 2 times a week | 2.97 | |
| Once a week | 3.24 | |
| 2 to 3 times a month | 6.76 | |
| Once a month | 4.32 | |
| 7 to 11 times in the last year | 2.34 | |
| 3 to 6 times in the last year | 6.4 | |
| 1 to 2 times in the last year | 11.71 | |
| Never drank in past year | 59.1 | |
| Smoking (average number of cigarettes per day in the last 30 days) | 1–5 | 32.30 |
| 6–10 | 34.36 | |
| 11–15 | 7.90 | |
| 16–20 | 20.27 | |
| 21–25 | 1.03 | |
| 26–30 | 2.75 | |
| 31–35 | 0.34 | |
| 36–40 | 1.03 |
| Outcome | Variable | * Coef. Estimate | Std Deviation | t-Value | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|
| AST | PFOA | 0.1472 | 1.0602 | 0.1388 | −1.9441 | 2.2385 |
| PFOS | 0.0959 | 0.2234 | 0.4292 | −0.3447 | 0.5365 | |
| Alcohol | −0.1062 | 0.3771 | −0.2815 | −0.8501 | 0.6377 | |
| Smoking | −0.2379 | 0.1602 | −1.4854 | −0.5538 | 0.0780 | |
| Income | −0.1335 | 0.0684 | −1.9527 | −0.2684 | 0.0014 | |
| Education | 0.6615 | 1.1664 | 0.5672 | −1.6391 | 2.9621 | |
| ALT | PFOA | −1.4313 | 1.4867 | −0.9627 | −4.3636 | 1.5011 |
| PFOS | 0.5429 | 0.3134 | 1.7320 | −0.0754 | 1.1611 | |
| Alcohol | −0.2909 | 0.5296 | −0.5492 | −1.3353 | 0.7537 | |
| Smoking | −0.3477 | 0.2241 | −1.5517 | −0.7896 | 0.0943 | |
| Income | −0.1721 | 0.0960 | −1.7927 | −0.3615 | 0.0172 | |
| Education | 0.5660 | 1.6383 | 0.3454 | −2.6655 | 3.7974 | |
| ALP | PFOA | 2.5916 | 1.4739 | 1.7584 | −0.3154 | 5.4987 |
| PFOS | 0.2012 | 0.3107 | 0.6474 | −0.4117 | 0.8141 | |
| Alcohol | 1.0012 | 0.5250 | 1.9070 | −0.0343 | 2.0366 | |
| Smoking | −0.0267 | 0.2221 | −0.1203 | −0.4648 | 0.4113 | |
| Income | −0.0323 | 0.0952 | −0.3391 | −0.2200 | 0.1555 | |
| Education | −0.9675 | 1.6242 | −0.5957 | −4.1710 | 2.2361 | |
| GGT | PFOA | −1.8948 | 3.5798 | −0.5293 | −8.9556 | 5.1660 |
| PFOS | 2.0973 | 0.7548 | 2.7788 | 0.6086 | 3.5860 | |
| Alcohol | −1.9580 | 1.2751 | −1.5355 | −4.4730 | 0.5571 | |
| Smoking | −0.4319 | 0.5395 | −0.8005 | −1.4960 | 0.6322 | |
| Income | −0.4221 | 0.2312 | −1.8258 | −0.8781 | 0.0339 | |
| Education | 0.1770 | 3.9450 | 0.0449 | −7.6040 | 7.9581 | |
| Total Bilirubin | PFOA | 0.0086 | 0.0153 | 0.5602 | −0.0216 | 0.0388 |
| PFOS | 0.0014 | 0.0032 | 0.4191 | −0.0050 | 0.0077 | |
| Alcohol | −0.0060 | 0.0055 | −1.0954 | −0.0167 | 0.0048 | |
| Smoking | −0.0040 | 0.0023 | −1.7543 | −0.0086 | 0.0005 | |
| Income | −0.0005 | 0.0010 | −0.5551 | −0.0025 | 0.0014 | |
| Education | 0.0172 | 0.0169 | 1.0190 | −0.0161 | 0.0504 | |
| US FLI | PFOA | −1.9104 | 3.4856 | −0.548 | −8.8360 | 5.0151 |
| PFOS | 0.7668 | 0.6638 | 1.155 | −0.5506 | 2.0842 | |
| Alcohol | −0.6863 | 1.1666 | −0.588 | −2.0039 | 3.3762 | |
| Smoking | −0.9560 | 0.5334 | −1.792 | −2.0151 | 0.1031 | |
| Income | −0.2706 | 0.1778 | −1.522 | −0.6244 | 0.0832 | |
| Education | 4.3534 | 3.8554 | 1.129 | −3.3115 | 12.0183 |
| PIP VALUES FOR ALT | ||||
| EXPOSURE | Group | Group PIP | Conditional PIP | |
| PFOA | PFOA | 1 | 0.3312 | 0.3312 |
| PFOS | PFOS | 1 | 0.3312 | 0.0000 |
| Alcohol | Alcohol | 2 | 0.9794 | 0.5936 |
| Smoking | Smoking | 2 | 0.9794 | 0.3858 |
| Income | Income | 3 | 0.6676 | 0.1194 |
| Education | Education | 3 | 0.6676 | 0.5482 |
| PIP VALUES FOR ALP | ||||
| EXPOSURE | Group | Group PIP | Conditional PIP | |
| PFOA | PFOA | 1 | 0.001 | 0.0002 |
| PFOS | PFOS | 1 | 0.001 | 0.0008 |
| Alcohol | Alcohol | 2 | 1.000 | 0.0016 |
| Smoking | Smoking | 2 | 1.000 | 0.9984 |
| Income | Income | 3 | 1.000 | 0.0004 |
| Education | Education | 3 | 1.000 | 0.9996 |
| PIP VALUES FOR AST | ||||
| EXPOSURE | Group | Group PIP | Conditional PIP | |
| PFOA | PFOA | 1 | 0.0480 | 0.0472 |
| PFOS | PFOS | 1 | 0.0480 | 0.0008 |
| Alcohol | Alcohol | 2 | 0.8878 | 0.8628 |
| Smoking | Smoking | 2 | 0.8878 | 0.0250 |
| Income | Income | 3 | 0.0192 | 0.0004 |
| Education | Education | 3 | 0.0192 | 0.0188 |
| PIP VALUES FOR FLI | ||||
| EXPOSURE | Group | Group PIP | Conditional PIP | |
| PFOA | PFOA | 1 | 0.0024 | 0.0004 |
| PFOS | PFOS | 1 | 0.0024 | 0.0020 |
| Alcohol | Alcohol | 2 | 0.0426 | 0.0416 |
| Smoking | Smoking | 2 | 0.0426 | 0.0010 |
| Income | Income | 3 | 0.0056 | 0.0004 |
| Education | Education | 3 | 0.0056 | 0.0052 |
| PIP VALUES FOR GGT | ||||
| EXPOSURE | Group | Group PIP | Conditional PIP | |
| PFOA | PFOA | 1 | 0.1298 | 0.1290 |
| PFOS | PFOS | 1 | 0.1298 | 0.0008 |
| Alcohol | Alcohol | 2 | 1.0000 | 1.0000 |
| Smoking | Smoking | 2 | 1.0000 | 0.0000 |
| Income | Income | 3 | 0.3976 | 0.0316 |
| Education | Education | 3 | 0.3976 | 0.3660 |
| PIP VALUES FOR TOTAL BILIRUBIN | ||||
| EXPOSURE | Group | Group PIP | Conditional PIP | |
| PFOA | PFOA | 1 | 0.0236 | 0.0222 |
| PFOS | PFOS | 1 | 0.0236 | 0.0014 |
| Alcohol | Alcohol | 2 | 0.9814 | 0.0016 |
| Smoking | Smoking | 2 | 0.9814 | 0.9798 |
| Income | Income | 3 | 0.0002 | 0.0002 |
| Education | Education | 3 | 0.0002 | 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marfo, A.; Obeng-Gyasi, E. Combined Effects of PFAS, Social, and Behavioral Factors on Liver Health. Med. Sci. 2025, 13, 99. https://doi.org/10.3390/medsci13030099
Marfo A, Obeng-Gyasi E. Combined Effects of PFAS, Social, and Behavioral Factors on Liver Health. Medical Sciences. 2025; 13(3):99. https://doi.org/10.3390/medsci13030099
Chicago/Turabian StyleMarfo, Akua, and Emmanuel Obeng-Gyasi. 2025. "Combined Effects of PFAS, Social, and Behavioral Factors on Liver Health" Medical Sciences 13, no. 3: 99. https://doi.org/10.3390/medsci13030099
APA StyleMarfo, A., & Obeng-Gyasi, E. (2025). Combined Effects of PFAS, Social, and Behavioral Factors on Liver Health. Medical Sciences, 13(3), 99. https://doi.org/10.3390/medsci13030099







