Impact of Catheter Ablation on Functional Capacity and Cardiac Stress Markers in Patients with Premature Ventricular Contractions
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Pre-Ablation Work-Up
2.3. CPET Protocol Pre Ablation
2.4. Ablation Procedure
2.5. CPET Protocol Post Ablation
2.6. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVCs | Premature Ventricular Contractions |
| CA | Catheter Ablation |
| CPET | Cardiopulmonary Exercise Testing |
| LV | Left Ventricle |
| NT-proBNP | N-terminal pro B-type Natriuretic Peptide |
| LVEF | Left Ventricular Ejection Fraction |
| METS | Metabolic Equivalents |
| AT | Anaerobic Threshold |
| VE/VO2 | Ventilatory Equivalent for Oxygen |
| VE/VCO2 | Ventilatory Equivalent for Carbon Dioxide |
| RER | Respiratory Exchange Ratio |
| GCV | Great Cardiac Vein |
| LVOT | Left Ventricular Outflow Tract |
| RVOT | Right Ventricular Outflow Tract |
| AIV | Interventricular Vein |
| SOO | Site of Origin |
| LBBB | Left Bundle Branch Block |
| RBBB | Right Bundle Branch Block |
| VA | Ventricular Arrhythmia |
| AADs | Antiarrhythmic Drugs |
| PCI | Percutaneous Coronary Intervention |
| CABG | Coronary Artery Bypass Grafting |
| OCSC | Onassis Cardiac Surgery Center |
References
- Ahn, M.-S. Current Concepts of Premature Ventricular Contractions. J. Lifestyle Med. 2013, 3, 26–33. [Google Scholar] [PubMed] [PubMed Central]
- Zang, M.; Zhang, T.; Mao, J.; Zhou, S.; He, B. Beneficial effects of catheter ablation of frequent premature ventricular complexes on left ventricular function. Heart 2014, 100, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.T.; Kay, G.N.; Kalman, J.; Borggrefe, M.; Della-Bella, P.; Dickfeld, T.; Dorian, P.; Huikuri, H.; Kim, Y.-H.; Knight, B.; et al. EHRA/HRS/APHRS Expert Consensus on Ventricular Arrhythmias. Hear. Rhythm. 2014, 11, e166–e196. [Google Scholar] [CrossRef] [PubMed]
- El Kadri, M.; Yokokawa, M.; Labounty, T.; Mueller, G.; Crawford, T.; Good, E.; Jongnarangsin, K.; Chugh, A.; Ghanbari, H.; Latchamsetty, R.; et al. Effect of ablation of frequent premature ventricular complexes on left ventricular function in patients with nonischemic cardiomyopathy. Hear. Rhythm. 2015, 12, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Van Herendael, H.; Zado, E.S.; Haqqani, H.; Tschabrunn, C.M.; Callans, D.J.; Frankel, D.S.; Lin, D.; Garcia, F.; Hutchinson, M.D.; Riley, M.; et al. Catheter ablation of ventricular fibrillation: Importance of left ventricular outflow tract and papillary muscle triggers. Hear. Rhythm. 2014, 11, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Latchamsetty, R.; Yokokawa, M.; Morady, F.; Kim, H.M.; Mathew, S.; Tilz, R.; Kuck, K.-H.; Nagashima, K.; Tedrow, U.; Stevenson, W.G.; et al. Multicenter Outcomes for Catheter Ablation of Idiopathic Premature Ventricular Complexes. JACC Clin. Electrophysiol. 2015, 1, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Shiraishi, H.; Shirayama, T.; Matoba, S. Improvement in Exercise Tolerance after Catheter Ablation for Premature Ventricular Complexes: A Case Report. Prog. Rehabilitation Med. 2020, 5, 20200028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary exercise testing and its application. Postgrad. Med. J. 2007, 83, 675–682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burke, M.A.; Cotts, W.G. Interpretation of B-type natriuretic peptide in cardiac disease and other comorbid conditions. Hear. Fail. Rev. 2007, 12, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.B.; Collins, S.É.; Stickland, M.K. Measurement and Interpretation of Exercise Ventilatory Efficiency. Front. Physiol. 2020, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Billet, S.; Rollin, A.; Mondoly, P.; Monteil, B.; Fournier, P.; Cariou, E.; Blaye-Felice, M.S.; Galinier, M.; Carrié, D.; Lairez, O.; et al. Hemodynamic consequences of premature ventricular contractions: Association of mechanical bradycardia and postextrasystolic potentiation with premature ventricular contraction-induced cardiomyopathy. Hear. Rhythm. 2019, 16, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Stec, S.; Zaborska, B.; Pilus, A.; Lewandowski, P.; Kulakowski, P. Intermittentclaudication caused by frequent premature ventricular complexes: Resolutionafter radiofrequency ablation. Angiology 2009, 60, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Welch, W.J.; Smith, M.L.; Rea, R.F.; Bauernfeind, R.A.; Eckberg, D.L. Enhancement of sympathetic nerve activity by single premature ventricular beats in humans. Circ. 1989, 13, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Salavatian, S.; Yamaguchi, N.; Hoang, J.; Lin, N.; Patel, S.; Ardell, J.L.; Armour, J.A.; Vaseghi, M. Premature ventricular contractions activate vagal afferents and alter autonomic tone: Implications for premature ventricular contraction-induced cardiomyopathy. Am. J. Physiol. Circ. Physiol. 2019, 317, H607–H616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matteucci, A.; Russo, M.; Galeazzi, M.; Pandozi, C.; Bonanni, M.; Mariani, M.V.; Pierucci, N.; La Fazia, V.M.; Di Fusco, S.A.; Nardi, F.; et al. Impact of Ablation Energy Sources on Perceived Quality of Life and Symptom in Atrial Fibrillation Patients: A Comparative Study. J. Clin. Med. 2025, 14, 2741. [Google Scholar] [CrossRef] [PubMed]
- Daussin, F.N.; Ponsot, E.; Dufour, S.P.; Lonsdorfer-Wolf, E.; Doutreleau, S.; Geny, B.; Piquard, F.; Richard, R. Improvement of VO2 max by cardiac output and oxygen extraction adaptation during intermittent versus continuous endurance training. Eur. J. Appl. Physiol. 2007, 101, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Shirai, Y.; Lin, A.; Dixit, S. Idiopathic Outflow Tract Ventricular Arrhythmia Ablation: Pearls and Pitfalls. Arrhythmia Electrophysiol. Rev. 2019, 8, 116–121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parrini, I.; Lucà, F.; Rao, C.M.; Cacciatore, S.; Riccio, C.; Grimaldi, M.; Gulizia, M.M.; Oliva, F.; Andreotti, F. How to Manage Beta-Blockade in Older Heart Failure Patients: A Scoping Review. J. Clin. Med. 2024, 13, 2119. [Google Scholar] [CrossRef] [PubMed]

| Variable | Value |
|---|---|
| Age (years) | 54.93 ± 14.65 |
| Men | 19 (63.33%) |
| Women | 11 (36.67%) |
| Hypertension | 12 (40.0%) |
| Dyslipidemia | 12 (40.0%) |
| Diabetes | 3 (10.0%) |
| Smoking | 2 (6.67%) |
| Coronary disease | 2 (6.67%) |
| History of PCI | 1 (3.33%) |
| History of GABG | 0 (0.0%) |
| Peripheral artery disease | 1 (3.33%) |
| Chronic kidney failure | 1 (3.33%) |
| Medication before ablation | None: 8 (26.66%), beta-blocker: 19 (63.33%), and flecainide: 3 (10.0%) |
| Variable | Value |
|---|---|
| Ablation site | |
| Right ventricular outflow tract (RVOT) | 26.92% |
| Coronary cusps | 46.15% |
| Papillary muscles | 11.54% |
| Endocardial sites (RV septal, parahisian) | 7.69% |
| Coronary veins | 7.69% |
| Variable | Before Ablation (Mean ± SD) | After Ablation (Mean ± SD) | p-Value |
|---|---|---|---|
| PVC burden | 23,509.3 ± 10,700.47 | 1759 ± 1659.15 | <0.001 |
| LVEF | 54.87 ± 5.86 | 54.7 ± 3.63 | NS |
| NT-proBNP | 240.93 ± 156.54 | 138.47 ± 152.91 | 0.0065 |
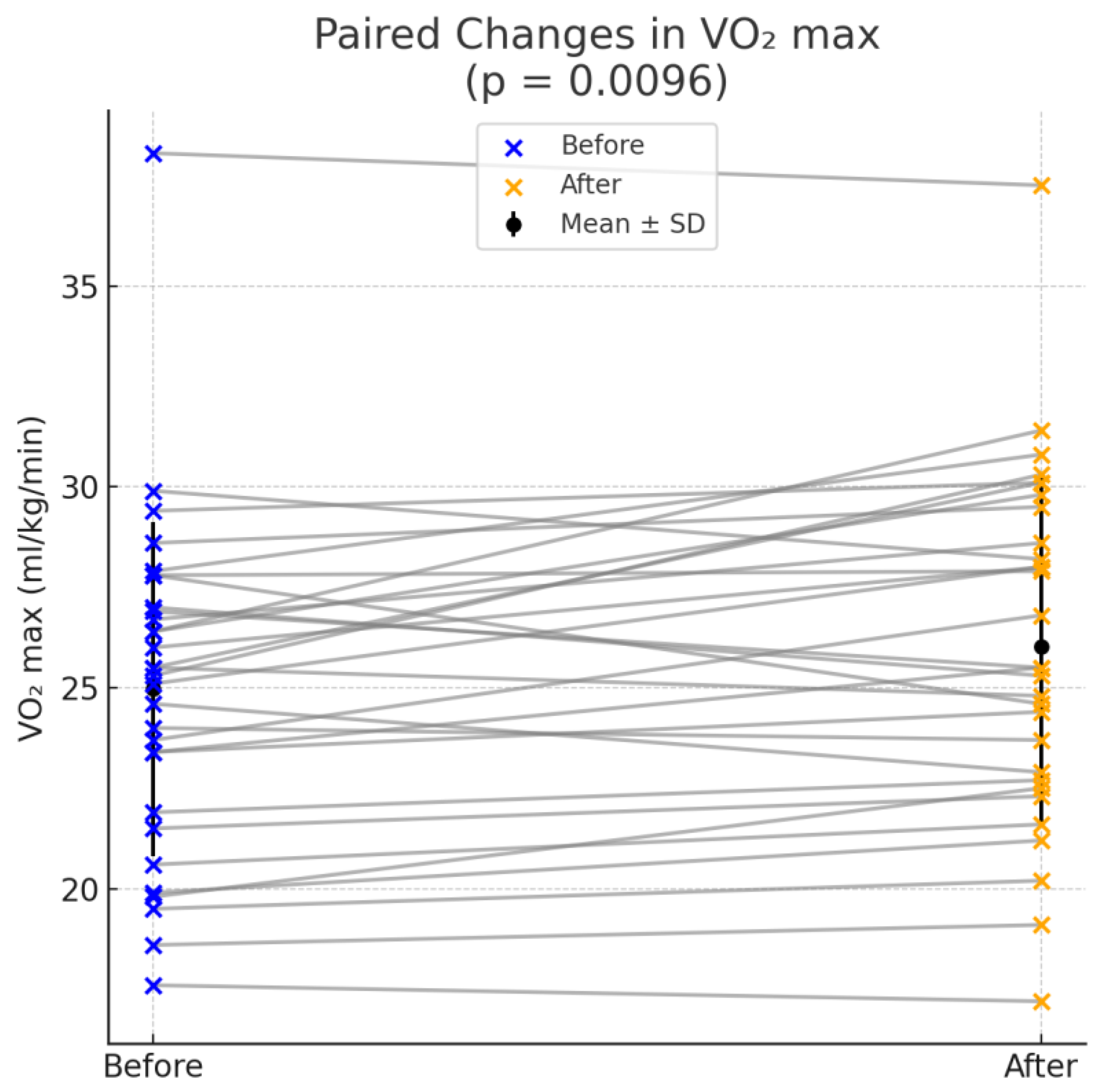
| VO2 max | 24.97 ± 4.16 | 26.02 ± 4.34 | 0.0096 |
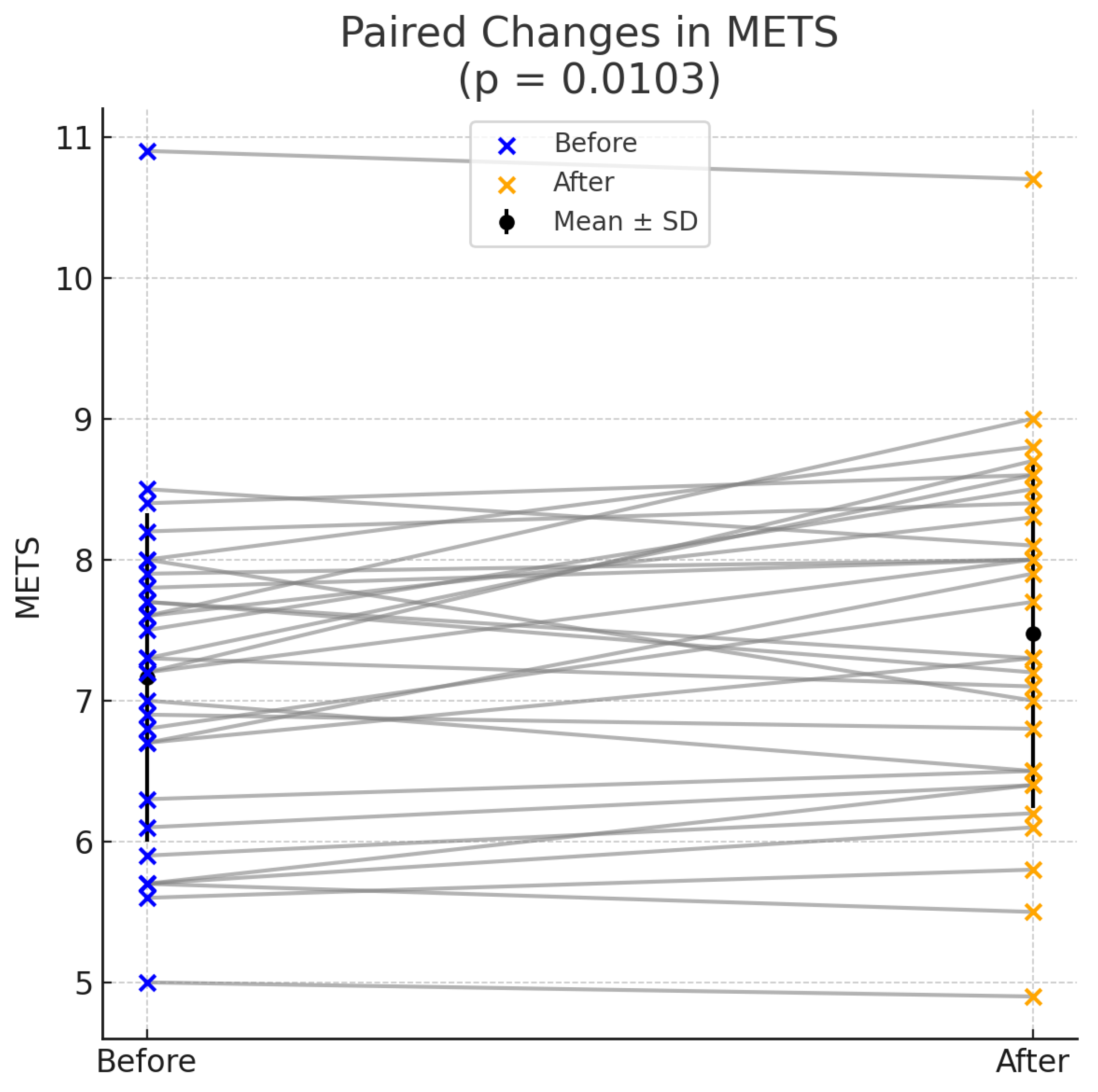
| METS | 7.16 ± 1.17 | 7.48 ± 1.24 | 0.0103 |
| % Predicted VO2 | 0.88 ± 0.16 | 0.91 ± 0.16 | 0.0793 |
| RER | 1.07 ± 0.13 | 1.08 ± 0.13 | 0.5330 |
| AT | 15.21 ± 3.25 | 16.1 ± 4.07 | 0.2368 |
| VE max (l/min) | 66.33 ± 16.48 | 69.31 ± 14.72 | NS |
| VE/VO2 | 33.07 ± 6.12 | 32.57 ± 4.83 | NS |
| VE/VCO2 | 31.37 ± 4.79 | 31.0 ± 4.57 | NS |
| Resting heart rate | 80.83 ± 16.02 | 79.4 ± 19.86 | NS |
| Max heart rate | 145.0 ± 24.07 | 149.17 ± 26.89 | NS |
| % Max HR predicted | 0.85 ± 0.10 | 0.89 ± 0.12 | NS |
| Exercise duration | 9.53 ± 2.03 | 10.0 ± 1.53 | NS |
| Medication after ablation | None: 8 (26.66%), beta-blocker: 19 (63.33%), and flecainide: 3 (10.0%) | None: 18 (63.3%), beta-blocker: 12 (36.6%), and flecainide: 0 (0.0%) | ↑ None, ↓ beta-blocker, and flecainide discontinued |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheilas, V.; Dritsas, A.; Martinos, A.; Gkirgkinoudi, E.; Filandrianos, G.; Chatziantoniou, A.; Kariki, O.; Mililis, P.; Saplaouras, A.; Kostopoulou, A.; et al. Impact of Catheter Ablation on Functional Capacity and Cardiac Stress Markers in Patients with Premature Ventricular Contractions. Med. Sci. 2025, 13, 95. https://doi.org/10.3390/medsci13030095
Cheilas V, Dritsas A, Martinos A, Gkirgkinoudi E, Filandrianos G, Chatziantoniou A, Kariki O, Mililis P, Saplaouras A, Kostopoulou A, et al. Impact of Catheter Ablation on Functional Capacity and Cardiac Stress Markers in Patients with Premature Ventricular Contractions. Medical Sciences. 2025; 13(3):95. https://doi.org/10.3390/medsci13030095
Chicago/Turabian StyleCheilas, Vasileios, Athanasios Dritsas, Antonios Martinos, Evangelia Gkirgkinoudi, Giorgos Filandrianos, Anastasios Chatziantoniou, Ourania Kariki, Panagiotis Mililis, Athanasios Saplaouras, Anna Kostopoulou, and et al. 2025. "Impact of Catheter Ablation on Functional Capacity and Cardiac Stress Markers in Patients with Premature Ventricular Contractions" Medical Sciences 13, no. 3: 95. https://doi.org/10.3390/medsci13030095
APA StyleCheilas, V., Dritsas, A., Martinos, A., Gkirgkinoudi, E., Filandrianos, G., Chatziantoniou, A., Kariki, O., Mililis, P., Saplaouras, A., Kostopoulou, A., Letsas, K., & Efremidis, M. (2025). Impact of Catheter Ablation on Functional Capacity and Cardiac Stress Markers in Patients with Premature Ventricular Contractions. Medical Sciences, 13(3), 95. https://doi.org/10.3390/medsci13030095






