Trends in MitraClip Placements and Predictors of 90-Day Heart Failure Rehospitalization: A Nationwide Analysis
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, P.T.; Mack, M.J. Secondary Mitral Regurgitation. N. Engl. J. Med. 2020, 383, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Mendelow, A.D.; Gregson, B.A.; Fernandes, H.M.; Murray, G.D.; Teasdale, G.M.; Hope, D.T.; Karimi, A.; Shaw, M.D.; Barer, D.H.; STICH Investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet 2005, 365, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Rihal, C.; Holmes, D.R., Jr. The COURAGE trial in perspective. Catheter. Cardiovasc. Interv. 2008, 72, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Camaj, A.; Kapadia, S.R.; Kar, S.; Abraham, W.T.; Lindenfeld, J.; Lim, D.S.; Grayburn, P.A.; Cohen, D.J.; Redfors, B.; et al. Hospitalizations and Mortality in Patients with Secondary Mitral Regurgitation and Heart Failure: The COAPT Trial. J. Am. Coll. Cardiol. 2022, 80, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Popolo Rubbio, A.; Testa, L.; Grasso, C.; Sisinni, A.; Tusa, M.; Agricola, E.; De Marco, F.; Petronio, A.S.; Montorfano, M.; Citro, R.; et al. Transcatheter edge-to-edge mitral valve repair in atrial functional mitral regurgitation: Insights from the multi-center MITRA-TUNE registry. Int. J. Cardiol. 2022, 349, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Friede, T.; von Bardeleben, R.S.; Butler, J.; Khan, M.S.; Diek, M.; Heinrich, J.; Geyer, M.; Placzek, M.; Ferrari, R.; et al. Percutaneous repair of moderate-to-severe or severe functional mitral regurgitation in patients with symptomatic heart failure: Baseline characteristics of patients in the RESHAPE-HF2 trial and comparison to COAPT and MITRA-FR trials. Eur. J. Heart Fail. 2024, 26, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Feldman, T.; Kar, S.; Elmariah, S.; Smart, S.C.; Trento, A.; Siegel, R.J.; Apruzzese, P.; Fail, P.; Rinaldi, M.J.; Smalling, R.W.; et al. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J. Am. Coll. Cardiol. 2015, 66, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- ICD-10-CM|Classification of Diseases, Functioning, and Disability|CDC. The Web’s Free 2025 ICD-10-CM/PCS Medical Coding Reference. Available online: https://www.icd10data.com/ (accessed on 10 June 2025).
- Grayburn, P.A.; Sannino, A.; Packer, M. Proportionate and Disproportionate Functional Mitral Regurgitation: A New Conceptual Framework That Reconciles the Results of the MITRA-FR and COAPT Trials. JACC Cardiovasc. Imaging 2019, 12, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Lindenfeld, J.; Abraham, W.T.; Grayburn, P.A.; Kar, S.; Asch, F.M.; Lim, D.S.; Nie, H.; Singhal, P.; Sundareswaran, K.S.; Weissman, N.J.; et al. Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) Investigators. Association of Effective Regurgitation Orifice Area to Left Ventricular End-Diastolic Volume Ratio with Transcatheter Mitral Valve Repair Outcomes: A Secondary Analysis of the COAPT Trial. JAMA Cardiol. 2021, 6, 427–436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avenatti, E.; Mackensen, G.; El-Tallawi, K.; Reisman, M.; Gruye, L.; Barker, C.M.; Little, S.H. Diagnostic Value of 3-Dimensional Vena Contracta Area for the Quantification of Residual Mitral Regurgitation After MitraClip Procedure. J. Am. Coll. Cardiol. Interv. 2019, 12, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Egorova, N.; Moskowitz, G.; Giustino, G.; Ailawadi, G.; Acker, M.A.; Gillinov, M.; Moskowitz, A.; Gelijns, A. Trends in MitraClip, mitral valve repair, and mitral valve replacement from 2000 to 2016. J. Thorac. Cardiovasc. Surg. 2021, 162, 551–562.e4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Von Bardeleben, R.S.; Hobohm, L.; Kreidel, F.; Ostad, M.A.; Schulz, E.; Konstantinides, S.; Lankeit, M.; Feldman, T.; Münzel, T.; Keller, K. Incidence and in-hospital safety outcomes of patients undergoing percutaneous mitral valve edge-to-edge repair using MitraClip: Five-year German national patient sample including 13,575 implants. EuroIntervention 2019, 14, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Keßler, M.; Seeger, J.; Muche, R.; Wöhrle, J.; Rottbauer, W.; Markovic, S. Predictors of rehospitalization after percutaneous edge-to-edge mitral valve repair by MitraClip implantation. Eur. J. Heart Fail. 2019, 21, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, A.; Albanese, M.; Salerno, N.; Aquila, I.; Sabatino, J.; Sorrentino, S.; Leo, I.; Cacia, M.; Signorile, V.; Mongiardo, A.; et al. Predictors of outcomes in patients with mitral regurgitation undergoing percutaneous valve repair. Sci. Rep. 2020, 10, 17144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Sabbagh, A.; Reddy, Y.N.V.; Nishimura, R.A. Mitral Valve Regurgitation in the Contemporary Era: Insights into Diagnosis, Management, and Future Directions. JACC Cardiovasc. Imaging 2018, 11, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Raj, V.; Abdelghany, M.; Mena-Hurtado, C.; Riaz, S.; Patel, S.; Wiener, H.; Chaudhuri, D. Impact of atrial fibrillation on the outcomes of transcatheter mitral valve repair using MitraClip: A systematic review and meta-analysis. Heart Fail. Rev. 2021, 26, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Gertz, Z.M.; Herrmann, H.C.; Lim, D.S.; Kar, S.; Kapadia, S.R.; Reed, G.W.; Puri, R.; Krishnaswamy, A.; Gersh, B.J.; Weissman, N.J.; et al. Implications of Atrial Fibrillation on the Mechanisms of Mitral Regurgitation and Response to MitraClip in the COAPT Trial. Circ. Cardiovasc. Interv. 2021, 14, e010300. [Google Scholar] [CrossRef] [PubMed]
- Saxon, J.T.; Cohen, D.J.; Chhatriwalla, A.K.; Kotinkaduwa, L.N.; Kar, S.; Lim, D.S.; Abraham, W.T.; Lindenfeld, J.; Mack, M.J.; Arnold, S.V.; et al. Impact of COPD on Outcomes After MitraClip for Secondary Mitral Regurgitation: The COAPT Trial. JACC Cardiovasc. Interv. 2020, 13, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Tigges, E.; Blankenberg, S.; von Bardeleben, R.S.; Zürn, C.; Bekeredjian, R.; Ouarrak, T.; Sievert, H.; Nickenig, G.; Boekstegers, P.; Senges, J.; et al. Implication of pulmonary hypertension in patients undergoing MitraClip therapy: Results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur. J. Heart Fail. 2018, 20, 585–594. [Google Scholar] [CrossRef] [PubMed]
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | ||
|---|---|---|---|---|---|---|---|
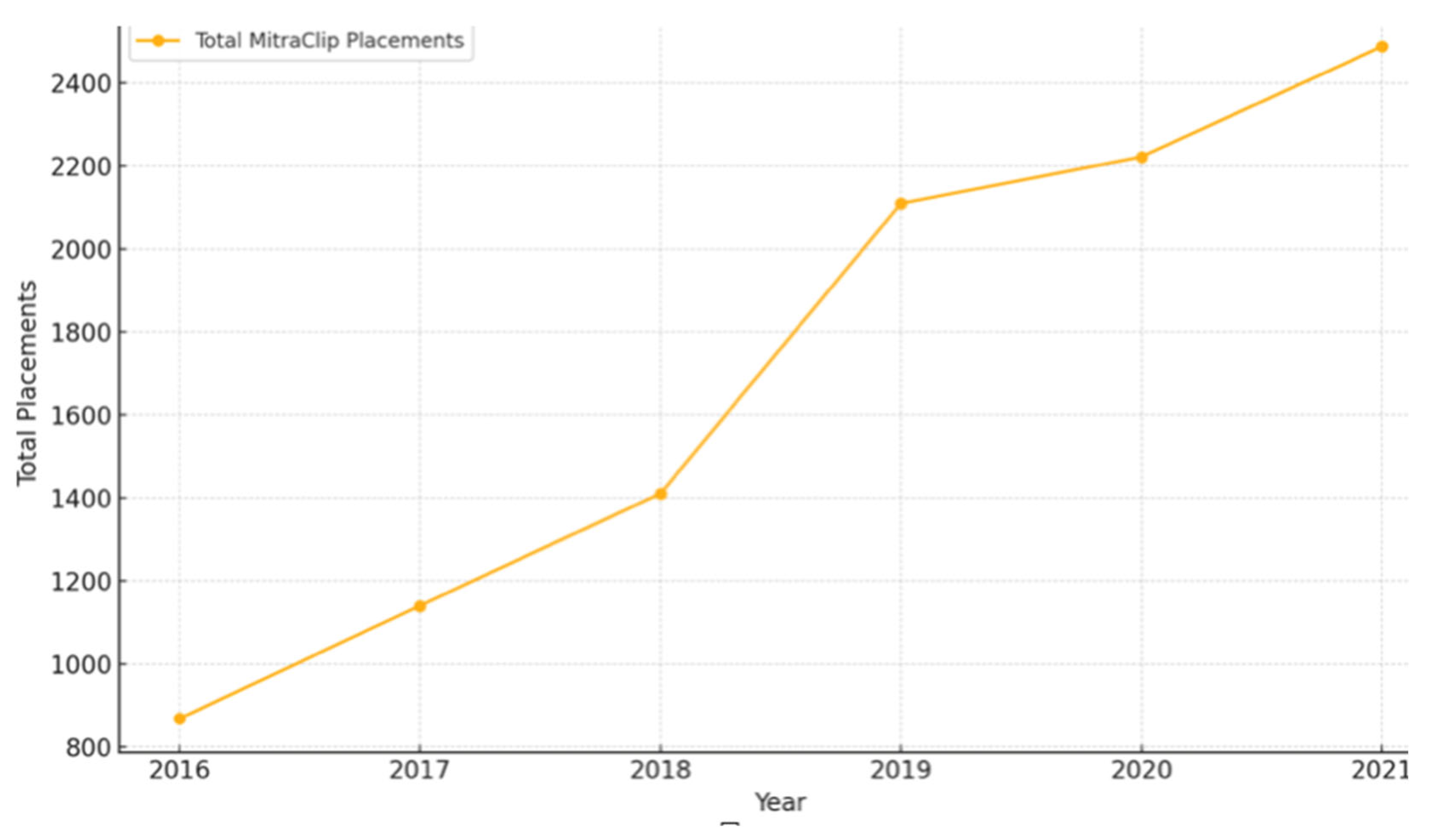
| Total Number of MitraClip placements | 869 | 1141 | 1411 | 2110 | 2222 | 2488 | |
| Mean Age | 77.71 (77.05–78.41) | 78.58 (78.02–79.14) | 77.79 (77.72–78.31) | 76.95 (76.51–77.39) | 76.13 (75.69–76.58) | 76.19 (75.78–76.59) | |
| Sex (%) | Male: | 50.06 | 52.05 | 54.07 | 54.69 | 55.65 | 54.09 |
| Female: | 49.94 | 47.94 | 45.92 | 45.38 | 44.34 | 45.90 | |
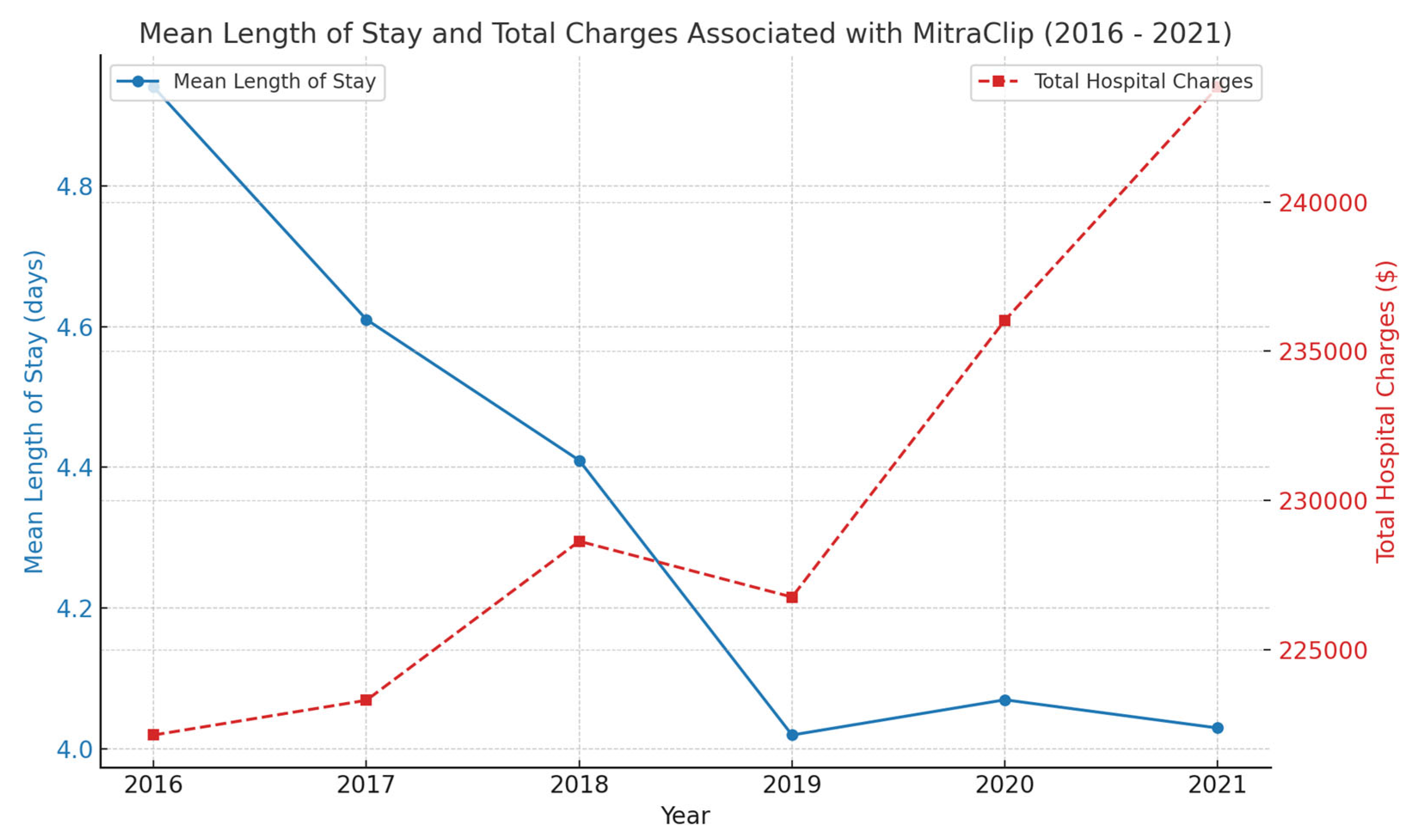
| Mean Hospital Length of Stay (days) | 4.94 (4.47–5.41) | 4.61 (3.89–5.31) | 4.41 (4.05–4.84) | 4.02 (3.73–4.32) | 4.07 (3.77–4.37) | 4.03 (3.71–4.34) | |
| Mean of Total Hospital Charges ($) | 222,130.8 | 223,295.2 | 228,626.9 | 226,755.4 | 236,031.1 | 243,849.8 | |
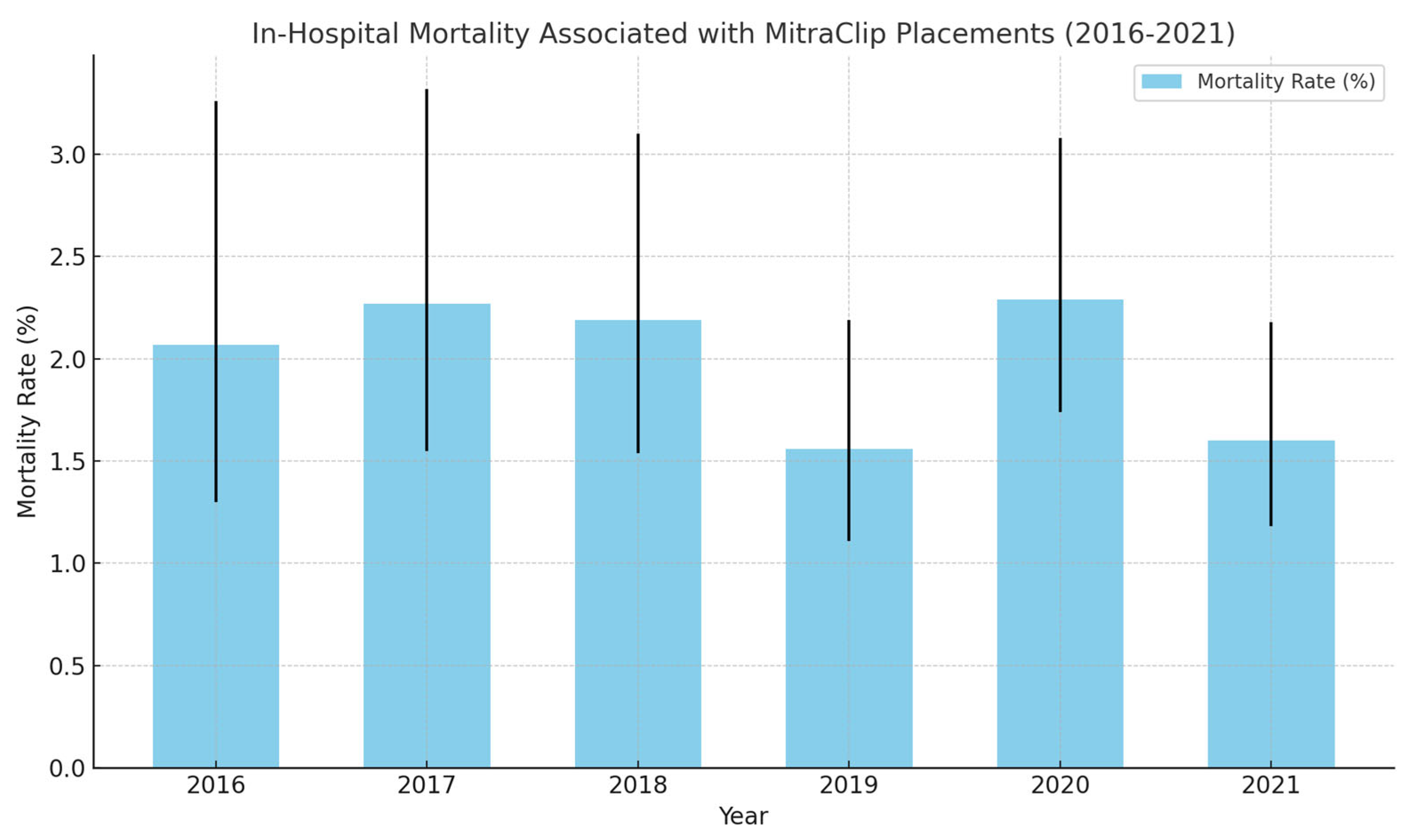
| In-Hospital Mortality (%) | 2.07 (1.30–3.26) | 2.27 (1.55–3.32) | 2.19 (1.54–3.10) | 1.56 (1.11–2.19) | 2.29 (1.74–3.08) | 1.60 (1.18–2.18) | |
| Race (%) | White | 79.47 | 81.80 | 80.46 | 79.25 | 78.25 | 78.96 |
| Black | 9.13 | 7.26 | 7.26 | 9.74 | 10.04 | 9.88 | |
| Hispanic | 6.06 | 6.62 | 7.26 | 5.60 | 5.97 | 5.89 | |
| Primary Insurance Payer (%) | Medicare | 84.60 | 88.74 | 87.45 | 84.60 | 82.65 | 84.60 |
| Median Household Income (%) | 1st quartile: | 25.55 | 21.55 | 20.79 | 22.77 | 22.76 | 22.46 |
| 2nd quartile: | 24.04 | 23.33 | 24.44 | 23.11 | 27.24 | 22.59 | |
| 3rd quartile: | 26.25 | 28.32 | 27.46 | 28.65 | 25.41 | 27.50 | |
| 4th quartile: | 24.15 | 26.80 | 27.31 | 25.47 | 24.59 | 24.25 | |
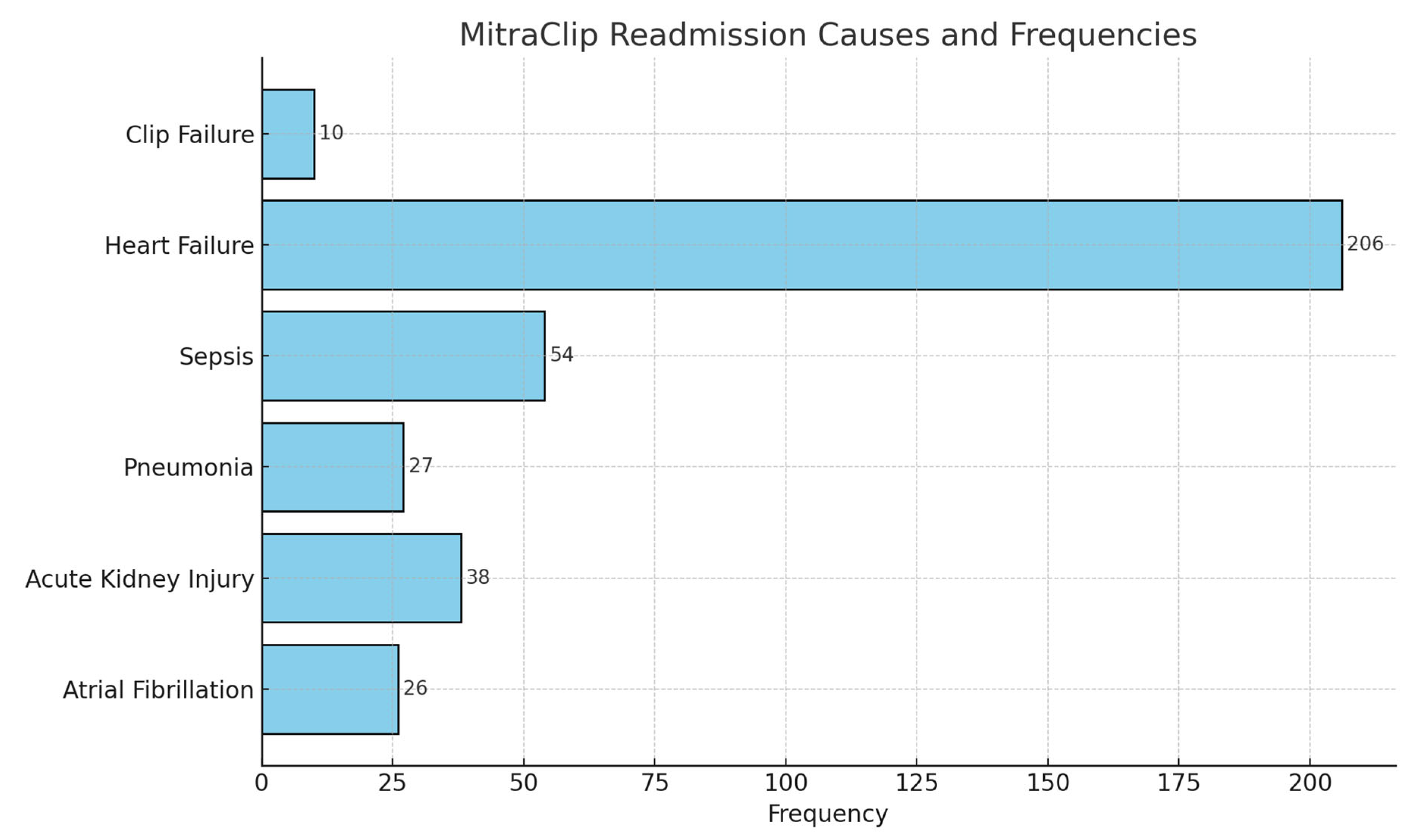
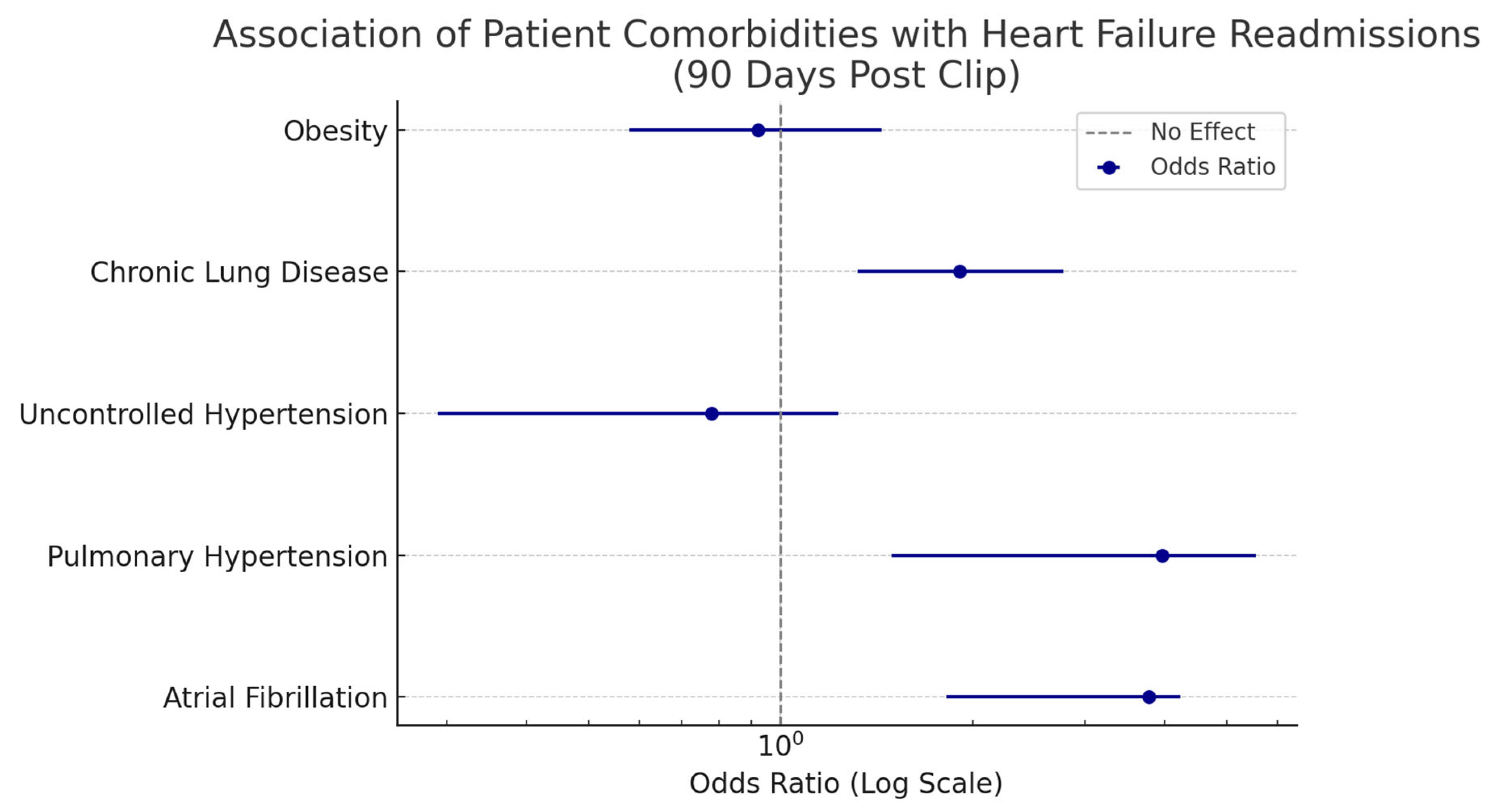
| Patient Comorbidities | Association with Heart Failure Readmissions: MV Clip Group (Odds Ratio with 95% Confidence Interval) |
|---|---|
| Atrial Fibrillation | 3.77 (1.82–4.23) |
| Pulmonary Hypertension | 3.96 (1.49–5.55) |
| Uncontrolled Hypertension | 0.78 (0.29–1.23) |
| Chronic Lung Disease | 1.91 (1.32–2.77) |
| Obesity | 0.92 (0.58–1.44) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varughese, V.J.; Krishnan Nagesh, V.; Madala, S.; Bhuju, R.; Lyons, C.; Weissman, S.; Atoot, A.; Vacca, D.; Alqinai, B. Trends in MitraClip Placements and Predictors of 90-Day Heart Failure Rehospitalization: A Nationwide Analysis. Med. Sci. 2025, 13, 81. https://doi.org/10.3390/medsci13030081
Varughese VJ, Krishnan Nagesh V, Madala S, Bhuju R, Lyons C, Weissman S, Atoot A, Vacca D, Alqinai B. Trends in MitraClip Placements and Predictors of 90-Day Heart Failure Rehospitalization: A Nationwide Analysis. Medical Sciences. 2025; 13(3):81. https://doi.org/10.3390/medsci13030081
Chicago/Turabian StyleVarughese, Vivek Joseph, Vignesh Krishnan Nagesh, Seetharamaprasad Madala, Ruchi Bhuju, Carra Lyons, Simcha Weissman, Adam Atoot, Dominic Vacca, and Budoor Alqinai. 2025. "Trends in MitraClip Placements and Predictors of 90-Day Heart Failure Rehospitalization: A Nationwide Analysis" Medical Sciences 13, no. 3: 81. https://doi.org/10.3390/medsci13030081
APA StyleVarughese, V. J., Krishnan Nagesh, V., Madala, S., Bhuju, R., Lyons, C., Weissman, S., Atoot, A., Vacca, D., & Alqinai, B. (2025). Trends in MitraClip Placements and Predictors of 90-Day Heart Failure Rehospitalization: A Nationwide Analysis. Medical Sciences, 13(3), 81. https://doi.org/10.3390/medsci13030081





