Immunohistochemistry-Based Molecular Profiling of Muscle-Invasive Bladder Cancer: Analysis of 100 Consecutive Cases with Morphological Correlation
Abstract
1. Introduction
2. Materials and Methods
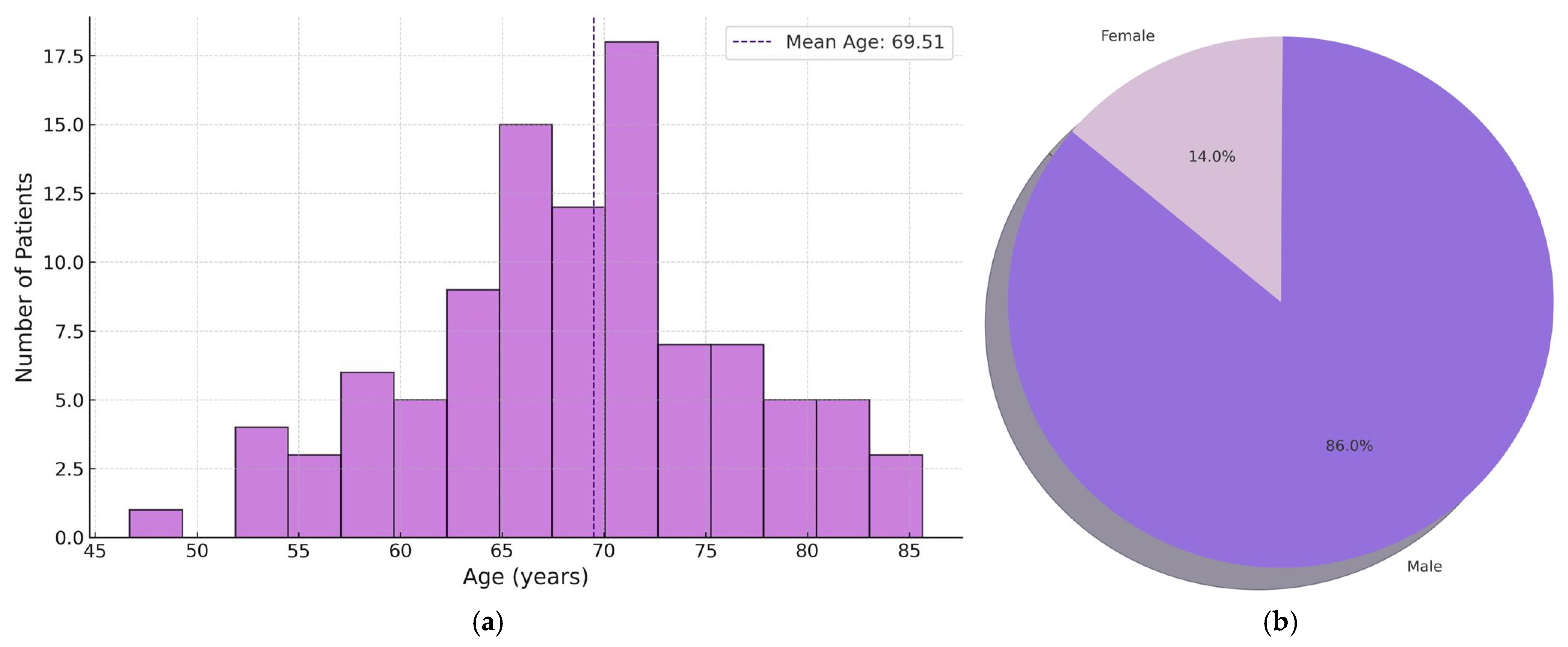
2.1. Clinical Cohort and Study Design
2.2. Immunohistochemical Analysis and Molecular Subtyping
- Basal variant: CK5/6 (+ve) and CK20 (−ve).
- Luminal variant: CK20 (+ve) and CK5/6 (−ve). The luminal tumors are further subdivided: luminal papillary if p16 is negative, and luminal unstable if p16 is positive. Luminal non-specified variant: preserved urothelial morphology, expression of either marker (CK5/6 (−ve), CK20 variable (−ve or <10%)). These cases phenotypically fall into the luminal spectrum but do not classify as luminal papillary or luminal unstable.
- Double-negative variants: CK5/6 (−ve) and CK20 (−ve). These tumors are split into two subgroups according to morphology: cases with neuroendocrine morphological characteristics are classified as neuroendocrine-like variant and cases with abundant stroma and/or sarcomatoid areas as stroma-rich variant.
- Basoluminal variant: tumors co-expressing both CK5/6 and CK20 (co-expression). In our series, such cases had mixed morphology, with different parts of the tumor showing zonal expression of different markers (e.g., one tumor component basal, another luminal).
2.3. Statistical Analysis
2.4. Ethical Aspects
3. Results
3.1. Pathological Characteristics of the Patients
3.2. Molecular Classification of the Tumors
4. Discussion
4.1. Comparison with Published Data on Molecular Variant
4.2. Correlation Between Histological Subtypes and Molecular Variants
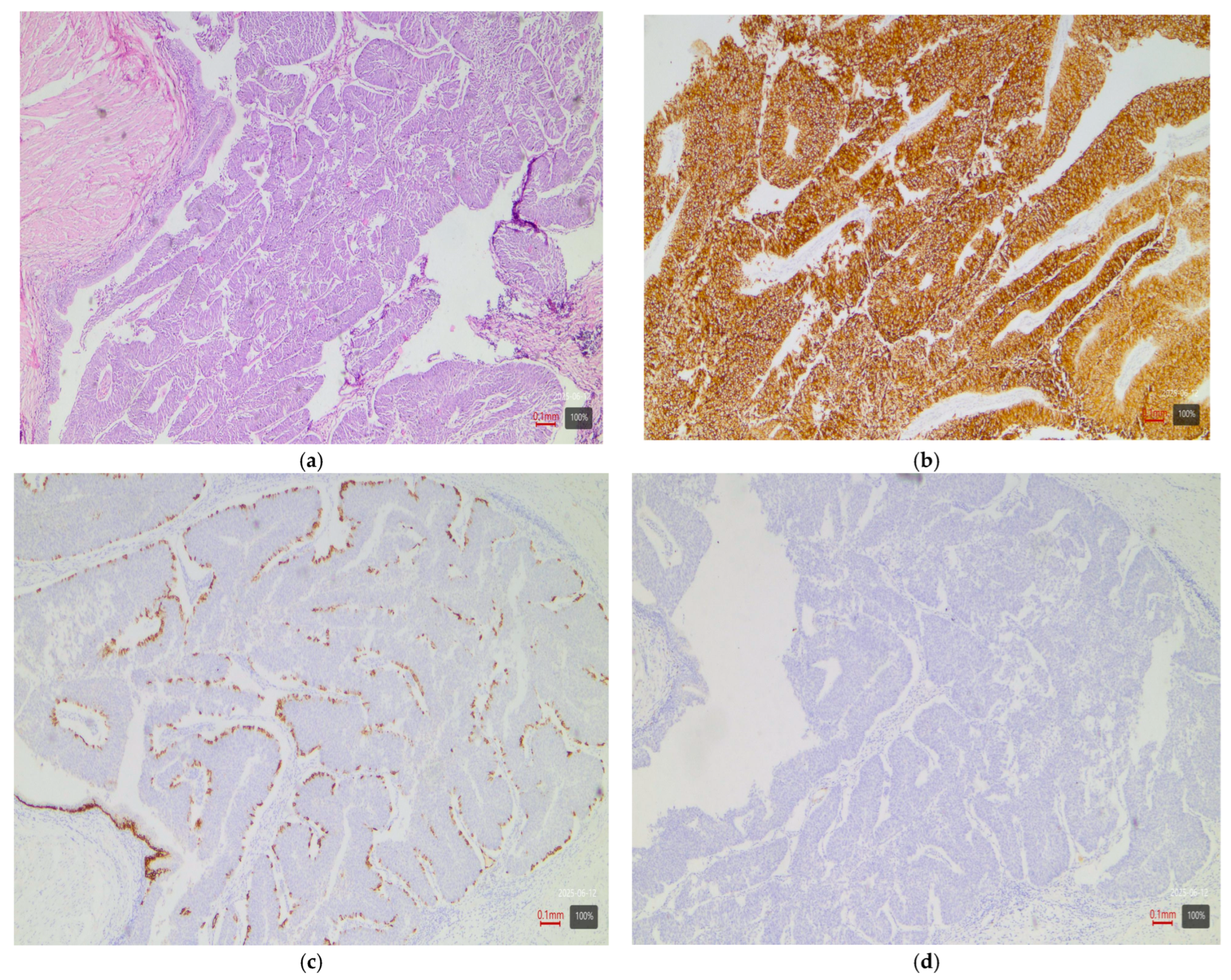
- Papillary tumors—luminal variant. Urothelial carcinomas with papillary architecture (Figure 2) almost always belong to the luminal molecular class. It is well established in the literature that luminal bladder carcinomas often arise via a so-called “papillary pathway” (from papillary precursor lesions) and carry characteristic mutations in the FGFR3 gene [6]. In agreement with this, our data show that papillary urothelial carcinomas are entirely luminal in molecular profile. In 92% of cases, they fell into one of the luminal variants, most often luminal papillary (63% of papillary tumors)—Table 3. The rarely encountered nested subtype also follows this pattern—despite its “benign” morphological appearance, its expression profile is close to luminal tumors with frequent TERT mutations [6,9]. Both cases of nested carcinoma in our series are classified as luminal papillary variant (100%)—Table 3. These results confirm that conventional papillary carcinomas (including the nested subtype) belong almost exclusively to the luminal molecular group.
- Micropapillary subtype—luminal unstable variant. Micropapillary urothelial carcinoma is an aggressive histological subtype, but it too usually falls into the luminal spectrum of the molecular classification [4,5,7,9]. Tumors with micropapillary morphology exhibit the IHC profile of a luminal tumor (CK20-positive, CK5/6-negative) and are often classified transcriptomically as luminal (lumNS) variant [4,5,7,9]. In the consensus classification, the luminal non-specified variant (LumNS) is enriched specifically with micropapillary histology and is associated with a poorer prognosis. Our data correspond to these observations—75% of micropapillary tumors in the series are classified as luminal unstable variant, and the remaining 25% as luminal papillary. Not a single micropapillary tumor showed a basal or double-negative profile. This confirms the strong link of this histological subtype to the luminal molecular lineage.
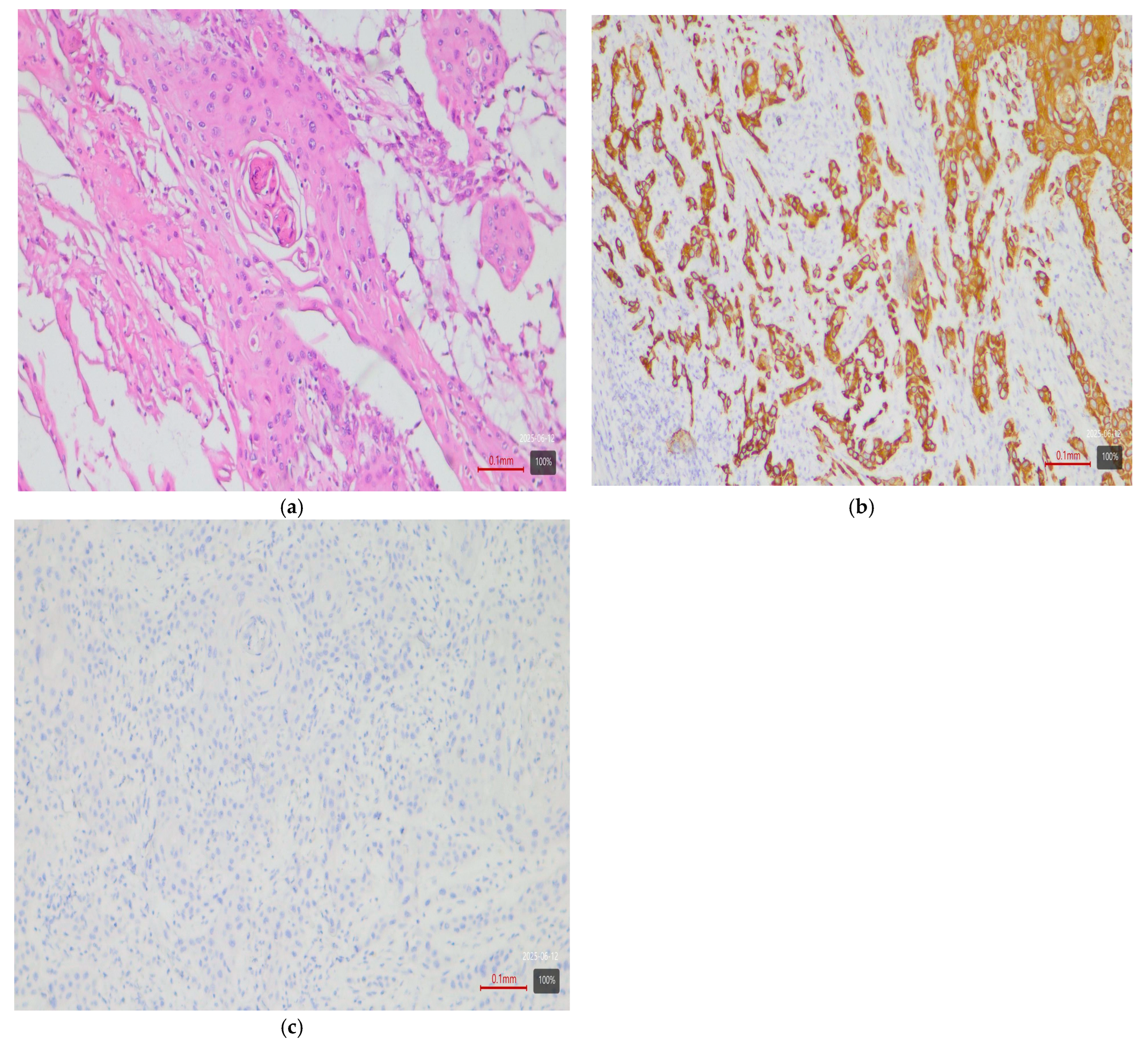
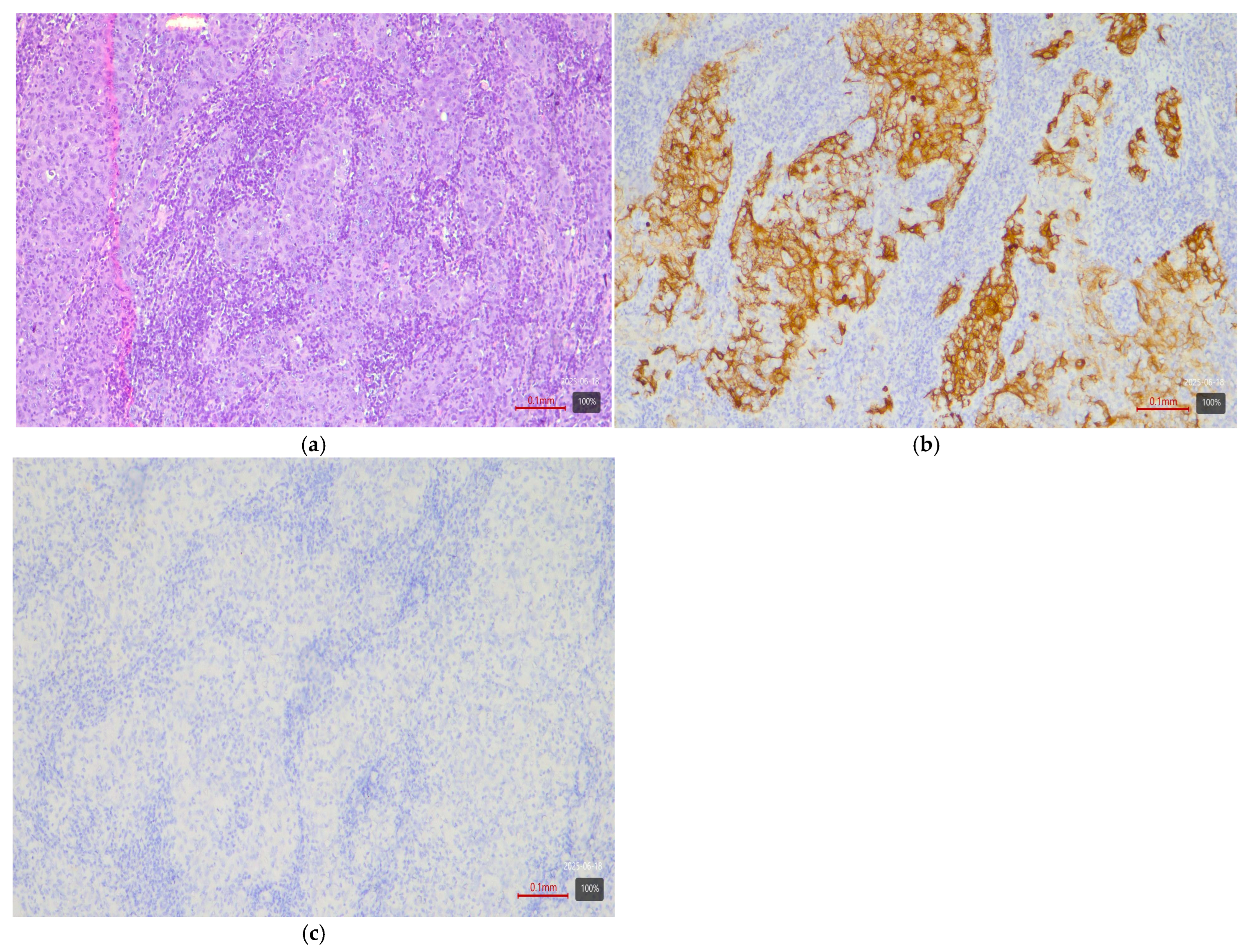
- Squamous differentiation—basal variant. There is an exceptionally strong correlation between the presence of a squamous component in a tumor and the basal molecular phenotype (Figure 3). Basal MIBCs, by definition, have a gene signature overlapping with that of normal basal cells and often demonstrate squamous morphology [5]. Various studies note that “squamous differentiation is a common feature of basal tumors” [5]. Our investigation fully corroborates this trend: all 17 tumors with squamous differentiation are of the basal molecular variant (100%) (Table 3). This finding is in complete accordance with the literature and underscores that the presence of a substantial squamous component within a urothelial carcinoma virtually guarantees membership in the basal (basal/squamous) molecular class. Clinically, identification of a basal variant may be significant, since basal tumors are associated with more aggressive behavior and better response to chemotherapy.
- Glandular differentiation—luminal variant. Urothelial carcinoma with glandular differentiation is relatively uncommon. Due to the limited number of cases, the literature does not provide a definitive classification for this variant [5]. However, the available data suggest that in mixed tumors, the glandular component is more often encountered in a luminal context [4,5,7,9]. In other words, there is a tendency toward a luminal profile in cases with glandular differentiation. Our results support these facts—although based on only three cases, the observation is that two out of three tumors with a glandular component are classified as luminal unstable variant (the remaining one is basal). This points to a predominantly luminal orientation, though a basal profile may be present in a subset of these tumors. More cases are needed for firm conclusions, but at present, glandular differentiation is most frequently associated with a luminal molecular background.
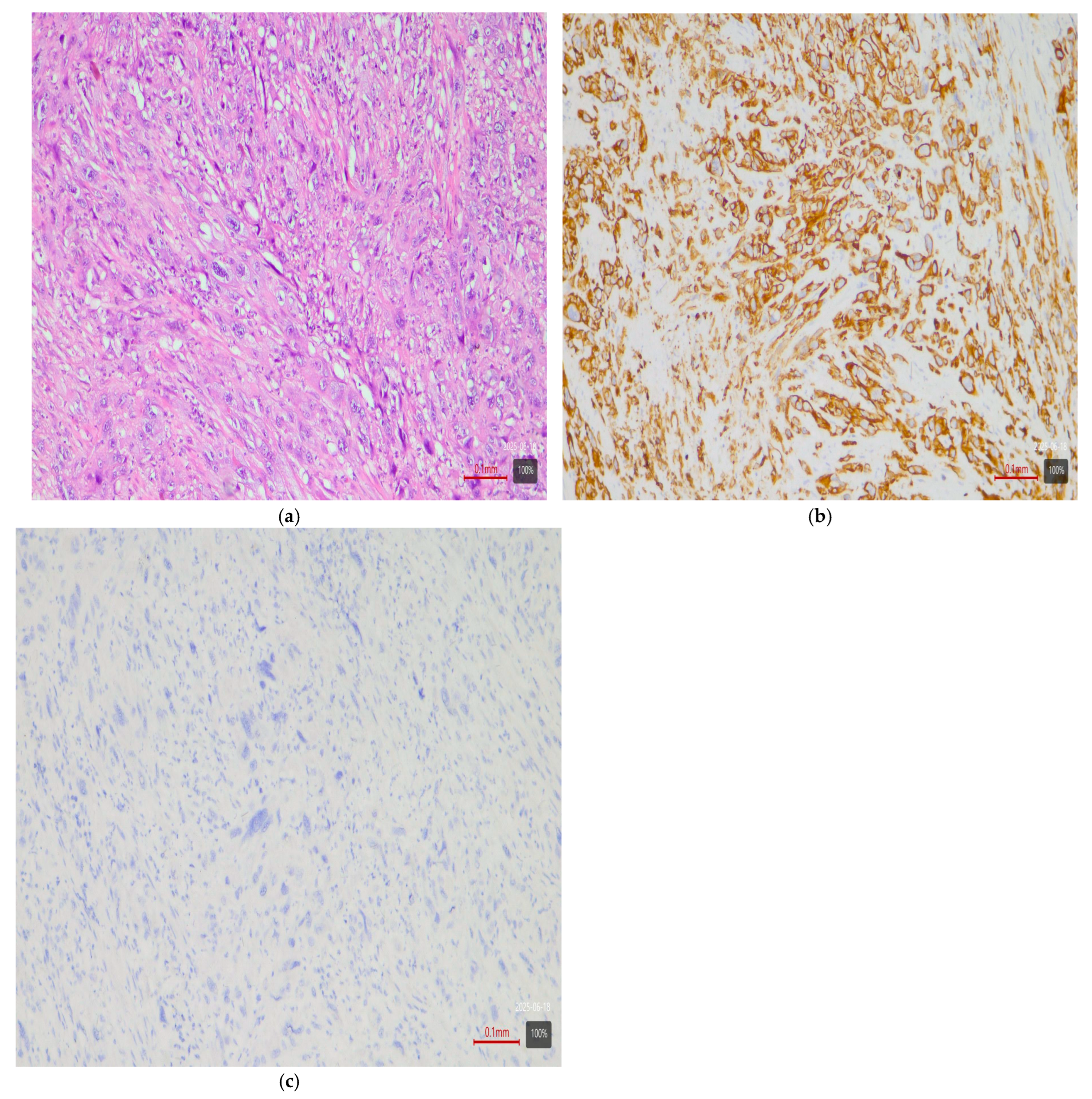
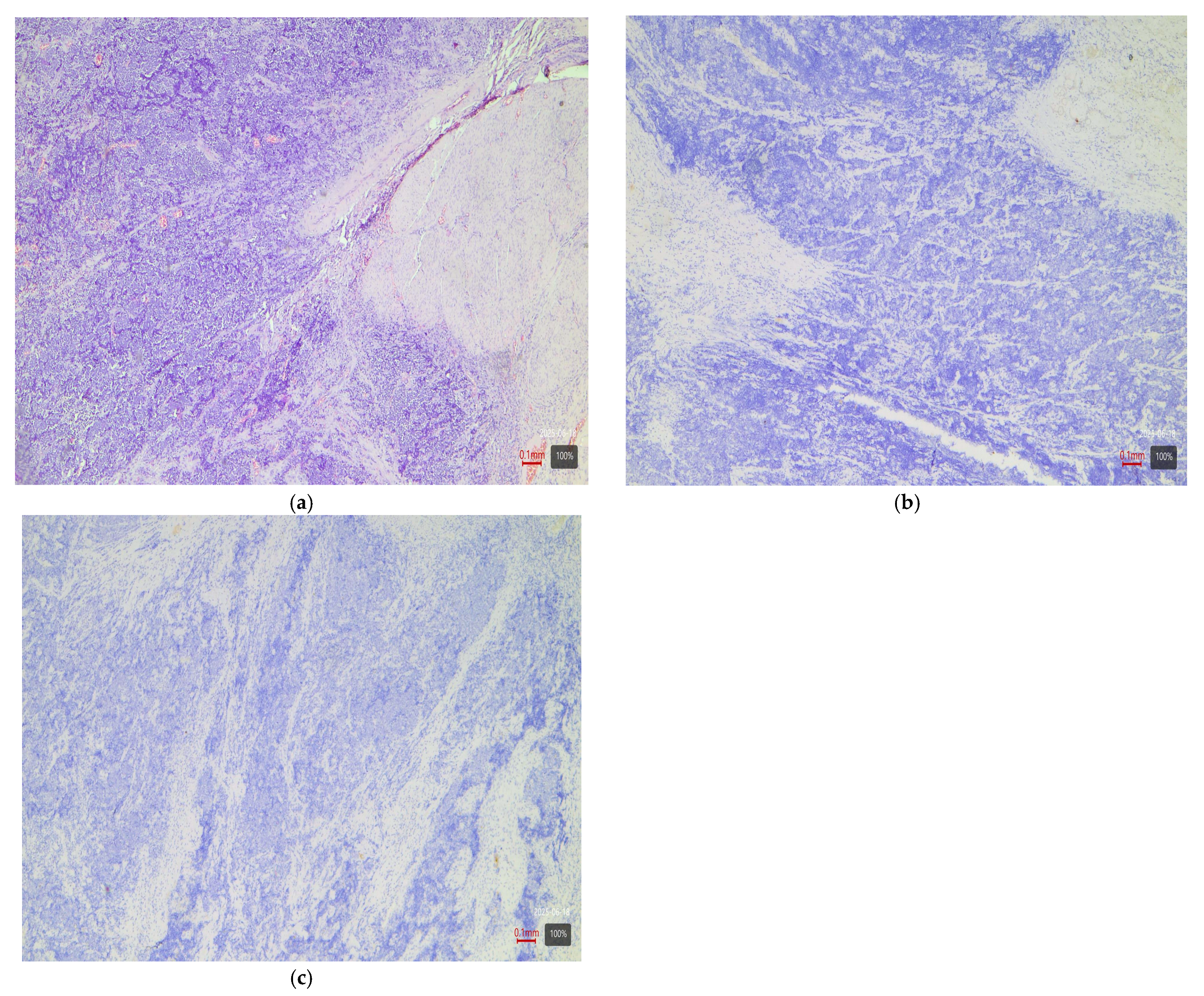
- Sarcomatoid subtype—basal or stroma-rich variant. Sarcomatoid urothelial carcinoma is an extreme manifestation of tumor dedifferentiation, in which the epithelial tumor acquires a sarcomatous appearance. Molecularly, data indicate heterogeneity: a portion of sarcomatoid tumors represent an extreme manifestation of the basal variant (Figure 4) (with a tendency toward epithelial-mesenchymal transition, EMT), while others fall into a separate stroma-rich (“mesenchymal-like”) class. Choi et al. noted that basal tumors are enriched with sarcomatoid morphological characteristics [5]. Ravanini et al., via an IHC panel (CK5/CK20), showed that 88% of sarcomatoid MIBCs have either a basal (67%) or double-negative (33%) profile [5]. Consistently, our data demonstrate 60% basal and 40% stroma-rich sarcomatoid tumors, which essentially reproduces the literature values (Table 2). This confirms that the sarcomatoid subtype does not have a single molecular analog—most cases fall in the basal spectrum, but a significant portion have a distinct mesenchymal (non-basal, non-luminal) profile. Biologically, it is hypothesized that the sarcomatoid transformation may result from the progression of a basal tumor that has undergone EMT. Interestingly, sarcomatoid cases often harbor mutations in cell adhesion genes (e.g., CDH1) and loss of epithelial markers (such as EPCAM), accompanied by expression of mesenchymal factors (e.g., SNAI2)—an observation supporting the idea of a transition from a basal to a mesenchymal profile.
- Plasmacytoid subtype—luminal variant (with heterogeneity). The plasmacytoid urothelial carcinoma (also known as the diffuse subtype) is rare, composed of discohesive tumor cells resembling plasma cells. Molecularly, the majority of studies position it in the luminal spectrum. For example, Eckstein et al. found in plasmacytoid tumors an immunophenotype of CK20+/CK5/6– and GATA3+, which is characteristic of the luminal variant [5]. In a large series of 32 patients with the plasmacytoid subtype, a significantly lower level of basal markers CK5/6 and p63 was demonstrated compared to conventional urothelial carcinoma, alongside high expression of luminal markers GATA3 and CK20 [5]. Additionally, plasmacytoid tumors often exhibit HER2 amplification/overexpression, similar to other luminal carcinomas [5]. All of this supports the notion that the plasmacytoid subtype is predominantly luminal [5,7,9]. Our results generally concur with this—none of the three plasmacytoid tumors was basal or purely double-negative. In 100% of cases, the plasmacytoid tumors fell into luminal variants, albeit distributed among different subcategories (approximately one case in lumP, one case in lumNS, and one case in basoluminal variant)—see Table 3. No broad generalizations can be made due to the small number, but the observed variability suggests that while most plasmacytoid tumors are luminal, individual cases can fall into intermediate categories (including mixed profiles). There is evidence that small series have identified individual plasmacytoid tumors with co-expression of basal markers as well—e.g., classified as genomically unstable (GU) or Lund’s Uro B variant [5]. In our cohort, the three cases illustrate precisely this heterogeneity. Nevertheless, the overall pattern—a predominantly luminal phenotype—holds, and not a single plasmacytoid carcinoma showed a purely basal or double-negative profile.
- Small-cell carcinoma—neuroendocrine-like variant. Small-cell carcinoma of the bladder is a distinct, rare histological subtype with neuroendocrine morphology. Molecularly, it corresponds to the aforementioned “neuronal” (neuroendocrine) variant, identified by TCGA as the smallest group [11]. It is characterized by frequent mutations in the tumor suppressors TP53 and RB1. In the consensus classification, these tumors fall into the neuroendocrine-like (Ne-like) class. As expected, our data show full concordance: all five cases of small-cell carcinoma and one poorly differentiated urothelial with small-cell morphology in this study are classified as double-negative variant with neuroendocrine morphology (100%)—i.e., these tumors do not express luminal or basal differentiation markers but have a distinct transcriptional signature (Figure 5). The correlation here is extremely strong, since even at the histological level, small-cell carcinomas are recognized as different from urothelial carcinoma; the molecular classification confirms this by segregating them into a separate category (“neuronal”).
- Rare variants. Some rarer histological variants of urothelial carcinoma are encountered too sporadically to make a compelling correlation, but available evidence also suggests preferred profiles. For example, the lymphoepithelioma-like subtype (urothelial carcinoma resembling lymphoepithelioma in the nasopharynx) is more associated with a basal/immune-infiltrated phenotype [5]. In our cohort, we had one case of a mixed urothelial carcinoma including a lymphoepithelioma-like component, which showed a basal immunophenotype (Figure 6). The microcystic subtype (characterized by formation of small cystic spaces) may have mixed features—some authors assign it to the luminal class, while others assign it to the basal class [11]. In our samples, we had two cases of a microcystic subtype as part of a mixed tumor; one demonstrated a luminal unstable and the other a basal immunophenotype. In our series, these rare variants are observed only as parts of mixed tumors; they are few in number, and no independent conclusions can be drawn.
- Mixed subtypes (with basoluminal profile). A significant proportion of cases—27%—have a heterogeneous morphology containing more than one of the above-mentioned histological components. In these cases, a mixed immunophenotype with co-expression of basal and luminal markers is often observed. In fact, our defined basoluminal category (9% of all tumors) reflects exactly such cases with a “double positive” profile. Similar mixed expression profiles have been described in the literature—e.g., the Lund UroB variant, or other authors who note ~5–10% of tumors with a combination of basal and luminal characteristics. Our data (9%) are in line with these observations. Mixed basoluminal tumors often correspond morphologically to mixed histological subtypes. Such tumors underscore the internal heterogeneity of some MIBCs and demonstrate that molecular variants are not always strictly demarcated—overlap within the same tumor is possible. From a practical standpoint, in these cases, the morphological and IHC assessment should account for all components in order to characterize the complete profile of the neoplasm.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Bladder carcinoma |
| MIBC | Muscle-invasive bladder carcinoma |
| TCGA | The Cancer Genome Atlas |
| IHC | Immunohistochemistry |
| TURB | Transurethral resection |
| RC | Radical cystestomy |
| FGFR | Fibroblast Growth Factor Receptor 3 |
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Doshi, B.; Athans, S.R.; Woloszynska, A. Biological differences underlying sex and gender disparities in bladder cancer: Current synopsis and future directions. Oncogenesis 2023, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Zanelli, M.; Palicelli, A.; Ascani, S.; Zizzo, M.; Cocco, G.; Björnebo, L.; Lantz, A.; Falagario, U.G.; Cormio, L.; et al. Are We Ready to Implement Molecular Subtyping of Bladder Cancer in Clinical Practice? Part 1: General Issues and Marker Expression. Int. J. Mol. Sci. 2022, 23, 7819. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, A.; De Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Zanelli, M.; Palicelli, A.; Ascani, S.; Zizzo, M.; Cocco, G.; Björnebo, L.; Lantz, A.; Landriscina, M.; Conteduca, V.; et al. Are We Ready to Implement Molecular Subtyping of Bladder Cancer in Clinical Practice? Part 2: Subtypes and Divergent Differentiation. Int. J. Mol. Sci. 2022, 23, 7844. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltran, A.; Cimadamore, A.; Montironi, R.; Cheng, L. Molecular pathology of urothelial carcinoma. Hum. Pathol. 2021, 113, 67–83. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef]
- Netto, G.J.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs-Part B: Prostate and Urinary Tract Tumors. Eur. Urol. 2022, 82, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Murase, Y.; Tsuzuki, T. Urothelial carcinoma: Variant histology, molecular subtyping, and immunophenotyping significant for treatment outcomes. Pathology 2021, 53, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Moschini, M.; D’Andrea, D.; Korn, S.; Irmak, Y.; Soria, F.; Compérat, E.; Shariat, S.F. Characteristics and clinical significance of histological variants of bladder cancer. Nat. Rev. Urol. 2017, 14, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Processali, T.; Diminutto, A.; Cerruto, M.A.; Antonelli, A. The impact of histological variants on bladder cancer outcomes. AME Med. J. 2020, 5, 4. [Google Scholar] [CrossRef]
| Subtype (WHO 2022) | Number of Cases (n) | Percentage (%) |
|---|---|---|
| UC (NOS) | 4 | 4.0% |
| Papillary UC | 27 | 27.0% |
| UC with squamous diff. | 17 | 17.0% |
| UC with glandular diff. | 3 | 3.0% |
| Sarcomatoid | 5 | 5.0% |
| Plasmacytoid | 3 | 3.0% |
| Nested | 2 | 2.0% |
| Micropapillary | 4 | 4.0% |
| Small-cell carcinoma | 5 | 5.0% |
| Poorly differentiated UC | 3 | 3.0% |
| Mixed tumor (≥2 subtype) | 27 | 27.0% |
| Total | 100 | 100% |
| Molecular Variant | Our Study (N = 100) | Number of Cases (n) | Consensus 2020 (N ≈ 1750) | TCGA 2017 (N = 408) |
|---|---|---|---|---|
| Ba/Sq | 33% | 33 | 35% | 35% (Ba/Sq) |
| LumPap | 24% | 24 | 24% | 35% (LumPap) |
| LumU | 16% | 16 | 15% | Inc. in Lum-infiltrated |
| LumNS | 10% | 10 | 8% | 6% (Luminal) |
| Stroma-rich | 2% | 2 | 15% | 19% (Lum-infiltrated) |
| NE-like | 6% | 6 | 3% | 5% (Neuronal) |
| BasoLum | 9% | 9 | No separate class | No separate class |
| Histological Subtype | Dominant Molecular Variant Literature [4,7,9] | Most Common Variant (Our Data) |
|---|---|---|
| Papillary urothelial carcinoma | Luminal (especially LumP) | LumP 63% of cases (17 cases from 27) |
| Micropapillary | Luminal (e.g., LumNS or LumU) | LumU 75% of cases (3 cases from 4) |
| Nested subtype | Luminal or Basal | LumP 100% of cases (2 cases from 2) |
| UC with squamous diff. | Basal (Ba/Sq) | Basal 100% of cases (17 cases from 17) |
| UC with glandular diff. | Luminal (limited data; likely lumU) | LumU 67% of cases (2 cases from 3) |
| Sarcomatoid | Basal | Basal (60%; 3 cases)), Stroma-rich (40%; 2 cases) total 5 cases |
| Plasmacytoid | Luminal (predominantly lumNS) Stroma rich | No dominant; ~evenly distributed among 1 LumP, 1 basolum., and 1 lumNS (~33% each/total 3 cases) |
| Small-cell carcinoma | NE-like (“neuronal”) | NE-like (100%; 5 cases from 5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraevska, E.; Popovska, S. Immunohistochemistry-Based Molecular Profiling of Muscle-Invasive Bladder Cancer: Analysis of 100 Consecutive Cases with Morphological Correlation. Med. Sci. 2025, 13, 202. https://doi.org/10.3390/medsci13030202
Kraevska E, Popovska S. Immunohistochemistry-Based Molecular Profiling of Muscle-Invasive Bladder Cancer: Analysis of 100 Consecutive Cases with Morphological Correlation. Medical Sciences. 2025; 13(3):202. https://doi.org/10.3390/medsci13030202
Chicago/Turabian StyleKraevska, Elitsa, and Savelina Popovska. 2025. "Immunohistochemistry-Based Molecular Profiling of Muscle-Invasive Bladder Cancer: Analysis of 100 Consecutive Cases with Morphological Correlation" Medical Sciences 13, no. 3: 202. https://doi.org/10.3390/medsci13030202
APA StyleKraevska, E., & Popovska, S. (2025). Immunohistochemistry-Based Molecular Profiling of Muscle-Invasive Bladder Cancer: Analysis of 100 Consecutive Cases with Morphological Correlation. Medical Sciences, 13(3), 202. https://doi.org/10.3390/medsci13030202






