Management of Juvenile Fibromyalgia: A Level I Evidence-Based Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
- P (Population): JFM;
- I (Intervention): Pharmacological and non-pharmacological management;
- C (Comparator): Not applicable;
- O (Outcome): PROMs, adverse events and therapy discontinuation.
- T (Timing): minimum 8 weeks of follow-up
- D (Design): RCT.
2.3. Selection and Data Collection
2.4. Data Items
2.5. Assessment of the Risk of Bias and Quality of the Recommendations
2.6. Synthesis Methods
3. Results
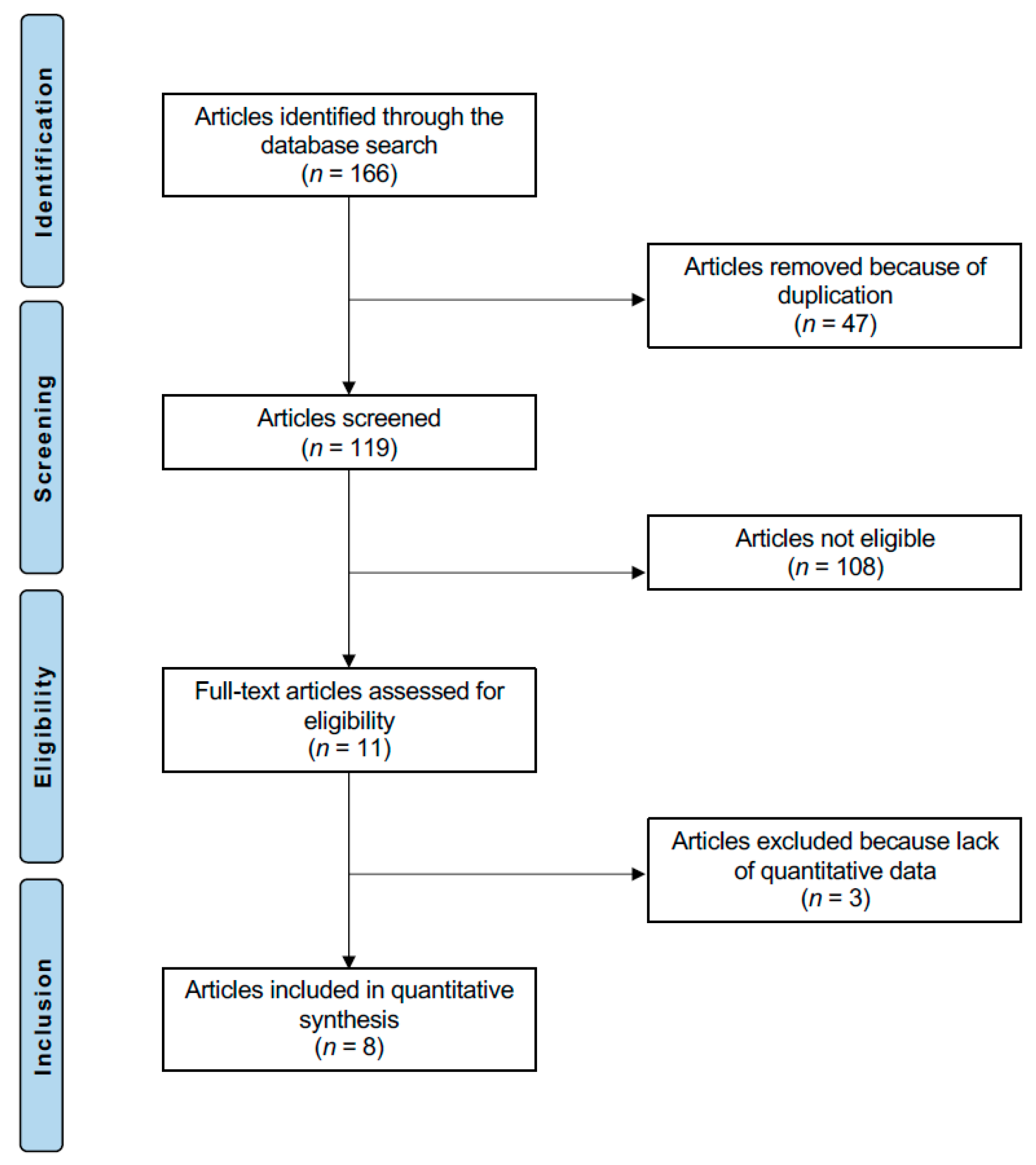
3.1. Study Selection
3.2. Risk of Bias Assessment
3.3. Study Characteristics and Results of Individual Studies
3.4. Results Syntheses
4. Discussion
4.1. Pathophysiology and Treatment Challenges in Juvenile Fibromyalgia
4.2. Pharmacological Therapies: Efficacy and Limitations
4.3. Cognitive-Behavioural Therapy and Psychological Management
4.4. Multimodal Approaches: The Role of FIT Teens
4.5. Functional Outcomes and Physical Activity Engagement
4.6. Safety Considerations and Adverse Events
4.7. Study Limitations and Methodological Heterogeneity
4.8. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yunus, M.B.; Masi, A.T. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis Rheum. 1985, 28, 138–145. [Google Scholar] [CrossRef]
- Arnold, L.M.; Bennett, R.M.; Crofford, L.J.; Dean, L.E.; Clauw, D.J.; Goldenberg, D.L.; Fitzcharles, M.A.; Paiva, E.S.; Staud, R.; Sarzi-Puttini, P.; et al. AAPT Diagnostic Criteria for Fibromyalgia. J. Pain. 2019, 20, 611–628. [Google Scholar] [CrossRef]
- Coles, M.L.; Weissmann, R.; Uziel, Y. Juvenile primary Fibromyalgia Syndrome: Epidemiology, etiology, pathogenesis, clinical manifestations and diagnosis. Pediatr. Rheumatol. Online J. 2021, 19, 22. [Google Scholar] [CrossRef]
- Matera, E.; Palumbi, R.; Peschechera, A.; Petruzzelli, M.G.; Sciruicchio, V.; de Tommaso, M.; Margari, L. Juvenile Fibromyalgia and Headache Comorbidity in Children and Adolescents: A Literature Review. Pain. Res. Manag. 2019, 2019, 3190829. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Betsch, M.; Tingart, M.; Colarossi, G. Placebo effect in pharmacological management of fibromyalgia: A meta-analysis. Br. Med. Bull. 2021, 139, 73–85. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Tingart, M.; Driessen, A.; Colarossi, G. BMI but not age and sex negatively impact on the outcome of pharmacotherapy in fibromyalgia: A systematic review. Expert. Rev. Clin. Pharmacol. 2021, 14, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Knobe, M.; Tingart, M.; Colarossi, G. Pharmacological management of fibromyalgia: A Bayesian network meta-analysis. Expert. Rev. Clin. Pharmacol. 2022, 15, 205–214. [Google Scholar] [CrossRef]
- Yokota, S.; Kikuchi, M.; Miyamae, T. Juvenile fibromyalgia: Guidance for management. Pediatr. Int. 2013, 55, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Kashikar-Zuck, S.; Johnston, M.; Ting, T.V.; Graham, B.T.; Lynch-Jordan, A.M.; Verkamp, E.; Passo, M.; Schikler, K.N.; Hashkes, P.J.; Spalding, S.; et al. Relationship between school absenteeism and depressive symptoms among adolescents with juvenile fibromyalgia. J. Pediatr. Psychol. 2010, 35, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Kashikar-Zuck, S.; Lynch, A.M.; Graham, T.B.; Swain, N.F.; Mullen, S.M.; Noll, R.B. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis Rheum. 2007, 57, 474–480. [Google Scholar] [CrossRef]
- Weiss, J.E.; Kashikar-Zuck, S. Juvenile Fibromyalgia. Rheum. Dis. Clin. North. Am. 2021, 47, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Cheng, Z.Y.; Xiao, Y.Y.; Wang, H.; Zhang, Y.F.; Zhao, Y.Y.; Jia, Y. Validation of the 2011 and 2016 American college of rheumatology diagnostic criteria for fibromyalgia in a Chinese population. Ann. Med. 2023, 55, 2249921. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Maffulli, N.; Knobe, M.; Tenze, G.; Aljalloud, A.; Colarossi, G. Pregabalin administration in patients with fibromyalgia: A Bayesian network meta-analysis. Sci. Rep. 2022, 12, 12148. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Baroncini, A.; Bell, A.; Colarossi, G. Duloxetine for fibromyalgia syndrome: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2023, 18, 504. [Google Scholar] [CrossRef]
- Lavarello, C.; Ancona, S.; Malattia, C. Juvenile Primary Fibromyalgia Syndrome: Advances in Etiopathogenesis, Clinical Assessment and Treatment: A Narrative Review. Biomedicines 2025, 13, 1168. [Google Scholar] [CrossRef]
- Coles, M.L.; Uziel, Y. Juvenile primary fibromyalgia syndrome: A Review- Treatment and Prognosis. Pediatr. Rheumatol. Online J. 2021, 19, 74. [Google Scholar] [CrossRef]
- Kashikar-Zuck, S.; King, C.; Ting, T.V.; Arnold, L.M. Juvenile Fibromyalgia: Different from the Adult Chronic Pain Syndrome? Curr. Rheumatol. Rep. 2016, 18, 19. [Google Scholar] [CrossRef]
- Kashikar-Zuck, S.; Ting, T.V. Juvenile fibromyalgia: Current status of research and future developments. Nat. Rev. Rheumatol. 2014, 10, 89–96. [Google Scholar] [CrossRef]
- Gurel, R.; Vidra, M.; Elbaz, E.; Factor, S.; Kazum, E.; Bivas, A.; Maman, E.; Chechik, O.; Rotman, D. Arthroscopic rotator cuff repair in fibromyalgia patients had comparable outcomes to a matched control group. J. Orthop. Traumatol. 2023, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Sills, G.J. The mechanisms of action of gabapentin and pregabalin. Curr. Opin. Pharmacol. 2006, 6, 108–113. [Google Scholar] [CrossRef]
- Zareba, G. New treatment options in the management of fibromyalgia: Role of pregabalin. Neuropsychiatr. Dis. Treat. 2008, 4, 1193–1201. [Google Scholar] [CrossRef][Green Version]
- Bhusal, S.; Diomampo, S.; Magrey, M.N. Clinical utility, safety, and efficacy of pregabalin in the treatment of fibromyalgia. Drug Healthc. Patient Saf. 2016, 8, 13–23. [Google Scholar] [CrossRef]
- Faizan Ejaz, K.; Wani, R.; Akbar, A.; Umaira Khan, Q.; Ishtiaq, H.; Amir, M.; Ali, A.I.; Khan, S. Pain Management in Fibromyalgia: Evaluating the Roles of Pregabalin, Duloxetine, and Milnacipran. Cureus 2024, 16, e76631. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Vaishya, R.; Pappalardo, G.; Schneider, M.; Bell, A.; Maffulli, N. Between guidelines and clinical trials: Evidence-based advice on the pharmacological management of non-specific chronic low back pain. BMC Musculoskelet. Disord. 2023, 24, 432. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N. Choosing the appropriate pharmacotherapy for nonspecific chronic low back pain. J. Orthop. Surg. Res. 2022, 17, 556. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Betsch, M.; Catalano, G.; Driessen, A.; Tingart, M.; Baroncini, A. The pharmacological management of chronic lower back pain. Expert. Opin. Pharmacother. 2021, 22, 109–119. [Google Scholar] [CrossRef]
- Gmuca, S.; Sherry, D.D. Fibromyalgia: Treating Pain in the Juvenile Patient. Paediatr. Drugs 2017, 19, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Sil, S.; Kashikar-Zuck, S. Understanding why cognitive-behavioral therapy is an effective treatment for adolescents with juvenile fibromyalgia. Int. J. Clin. Rheumtol. 2013, 8, 213–219. [Google Scholar] [CrossRef]
- Arnold, L.M.; Bateman, L.; Palmer, R.H.; Lin, Y. Preliminary experience using milnacipran in patients with juvenile fibromyalgia: Lessons from a clinical trial program. Pediatr. Rheumatol. Online J. 2015, 13, 27. [Google Scholar] [CrossRef]
- Arnold, L.M.; Schikler, K.N.; Bateman, L.; Khan, T.; Pauer, L.; Bhadra-Brown, P.; Clair, A.; Chew, M.L.; Scavone, J. Safety and efficacy of pregabalin in adolescents with fibromyalgia: A randomized, double-blind, placebo-controlled trial and a 6-month open-label extension study. Pediatr. Rheumatol. Online J. 2016, 14, 46. [Google Scholar] [CrossRef]
- Black, W.R.; DiCesare, C.A.; Thomas, S.; Pfeiffer, M.; Williams, S.E.; Kitchen, K.; Ting, T.V.; Myer, G.D.; Kashikar-Zuck, S. Preliminary Evidence for the Fibromyalgia Integrative Training Program (FIT Teens) Improving Strength and Movement Biomechanics in Juvenile Fibromyalgia: Secondary Analysis and Results from a Pilot Randomized Clinical Trial. Clin. J. Pain. 2021, 37, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.P.; Ploderl, M. Suicidality and other severe psychiatric events with duloxetine: Re-analysis of safety data from a placebo-controlled trial for juvenile fibromyalgia. Int. J. Risk Saf. Med. 2021, 32, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Kashikar-Zuck, S.; Ting, T.V.; Arnold, L.M.; Bean, J.; Powers, S.W.; Graham, T.B.; Passo, M.H.; Schikler, K.N.; Hashkes, P.J.; Spalding, S.; et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: A multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum. 2012, 64, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kashikar-Zuck, S.; Flowers, S.R.; Strotman, D.; Sil, S.; Ting, T.V.; Schikler, K.N. Physical activity monitoring in adolescents with juvenile fibromyalgia: Findings from a clinical trial of cognitive-behavioral therapy. Arthritis Care Res. 2013, 65, 398–405. [Google Scholar] [CrossRef]
- Kashikar-Zuck, S.; Black, W.R.; Pfeiffer, M.; Peugh, J.; Williams, S.E.; Ting, T.V.; Thomas, S.; Kitchen, K.; Myer, G.D. Pilot Randomized Trial of Integrated Cognitive-Behavioral Therapy and Neuromuscular Training for Juvenile Fibromyalgia: The FIT Teens Program. J. Pain. 2018, 19, 1049–1062. [Google Scholar] [CrossRef]
- Upadhyaya, H.P.; Arnold, L.M.; Alaka, K.; Qiao, M.; Williams, D.; Mehta, R. Efficacy and safety of duloxetine versus placebo in adolescents with juvenile fibromyalgia: Results from a randomized controlled trial. Pediatr. Rheumatol. Online J. 2019, 17, 27. [Google Scholar] [CrossRef]
- Howick, J.C.I.; Glasziou, P.; Greenhalgh, T.; Carl Heneghan Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H.; Goddard, O.; Hodgkinson, M. The 2011 Oxford CEBM Levels of Evidence. Oxford Centre for Evidence-Based Medicine. 2011. Available online: https://www.cebm.net/index.aspx?o=5653 (accessed on 23 January 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Saylor, C.F.; Finch, A.J., Jr.; Baskin, C.H.; Saylor, C.B.; Darnell, G.; Furey, W. Children’s Depression Inventory: Investigation of procedures and correlates. J. Am. Acad. Child. Psychiatry 1984, 23, 626–628. [Google Scholar] [CrossRef]
- Sole, E.; Galan, S.; de la Vega, R.; Castarlenas, E.; Sanchez-Rodriguez, E.; Jensen, M.P.; Miro, J. Psychometric properties of the Functional Disability Inventory for assessing Pain-related disability in children from the community. Disabil. Rehabil. 2019, 41, 2451–2458. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2; Cochrane: London, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 13 February 2022).
- Malattia, C.; Chiarella, L.; Sansone, M.; Pistorio, A.; Lavarello, C.; Carpaneto, M.; Ferri, R.; Ravelli, A.; Nobili, L. Sleep and Sleep Complaints in Juvenile Fibromyalgia Syndrome. J. Rheumatol. 2023, 50, 827–834. [Google Scholar] [CrossRef]
- Lynch-Jordan, A.M.; Connelly, M.; Guite, J.W.; King, C.; Goldstein-Leever, A.; Logan, D.E.; Nelson, S.; Stinson, J.N.; Ting, T.V.; Wakefield, E.O.; et al. Clinical Characterization of Juvenile Fibromyalgia in a Multicenter Cohort of Adolescents Enrolled in a Randomized Clinical Trial. Arthritis Care Res. 2023, 75, 1795–1803. [Google Scholar] [CrossRef]
- Dell’Erba, S.; Melissano, P.; Zegretti, A.; Paniccia, M.F.; Marri, M.; Caruso, M.; Tarantino, S.; Argento, O.; Grimaldi Capitello, T. Psychological characteristics of juvenile fibromyalgia syndrome. Pediatr. Int. 2023, 65, e15449. [Google Scholar] [CrossRef]
- Kashikar-Zuck, S.; Barnett, K.A.; Williams, S.E.; Pfeiffer, M.; Thomas, S.; Beasley, K.; Chamberlin, L.A.; Mundo, K.; Ittenbach, R.F.; Peugh, J.; et al. FIT Teens RCT for juvenile fibromyalgia: Protocol adaptations in response to the COVID-19 pandemic. Contemp. Clin. Trials Commun. 2022, 30, 101039. [Google Scholar] [CrossRef]
- Vittori, A.; Cascella, M.; Leonardi, M.; Monaco, F.; Nocerino, D.; Cuomo, A.; Ottaiano, A.; Perri, F.; Mascilini, I.; Francia, E.; et al. VOSviewer-Based Bibliometric Network Analysis for Evaluating Research on Juvenile Primary Fibromyalgia Syndrome (JPFS). Children 2022, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Gmuca, S.; Sonagra, M.; Xiao, R.; Mendoza, E.; Miller, K.S.; Thomas, N.H.; Young, J.F.; Weiss, P.F.; Sherry, D.D.; Gerber, J.S. Characterizing Neurocognitive Impairment in Juvenile Fibromyalgia Syndrome: Subjective and Objective Measures of Dyscognition. Front. Pediatr. 2022, 10, 848009. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Vigouroux, M.; Ingelmo, P. Implications of Nerve Fiber Density on the Diagnosis and Treatment of Juvenile Fibromyalgia. J. Pain. Res. 2022, 15, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Sunol, M.; Payne, M.F.; Tong, H.; Maloney, T.C.; Ting, T.V.; Kashikar-Zuck, S.; Coghill, R.C.; Lopez-Sola, M. Brain Structural Changes During Juvenile Fibromyalgia: Relationships With Pain, Fatigue, and Functional Disability. Arthritis Rheumatol. 2022, 74, 1284–1294. [Google Scholar] [CrossRef]
- Daffin, M.; Lynch-Milder, M.K.; Gibler, R.C.; Murray, C.; Green, C.M.; Kashikar-Zuck, S. A qualitative study of risk and resilience in young adult women with a history of juvenile-onset fibromyalgia. Pediatr. Rheumatol. Online J. 2021, 19, 128. [Google Scholar] [CrossRef]
- Liptan, G. The widespread myofascial pain of fibromyalgia is sympathetically maintained and immune mediated. J. Bodyw. Mov. Ther. 2023, 35, 394–399. [Google Scholar] [CrossRef]
- Tong, H.; Maloney, T.C.; Payne, M.F.; Sunol, M.; Dudley, J.A.; King, C.D.; Ting, T.V.; Kashikar-Zuck, S.; Coghill, R.C.; Lopez-Sola, M. Augmented pain-evoked primary sensorimotor cortex activation in adolescent girls with juvenile fibromyalgia. Pain 2023, 164, 2316–2326. [Google Scholar] [CrossRef]
- Ciuffini, R.; Cofini, V.; Muselli, M.; Necozione, S.; Piroli, A.; Marrelli, A. Emotional arousal and valence in patients with fibromyalgia: A pilot study. Front. Pain Res. 2023, 4, 1075722. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.; Pacheco-Barrios, K.; Slawka, E.; Camargo, L.; Castelo-Branco, L.; Cardenas-Rojas, A.; Neto, M.S.; Fregni, F. The role of symptoms severity, heart rate, and central sensitization for predicting sleep quality in patients with fibromyalgia. Pain Med. 2023, 24, 1153–1160. [Google Scholar] [CrossRef]
- Ioachim, G.; Warren, H.J.M.; Powers, J.M.; Staud, R.; Pukall, C.F.; Stroman, P.W. Distinct neural signaling characteristics between fibromyalgia and provoked vestibulodynia revealed by means of functional magnetic resonance imaging in the brainstem and spinal cord. Front. Pain Res. 2023, 4, 1171160. [Google Scholar] [CrossRef]
- Peinado-Rubia, A.B.; Osuna-Perez, M.C.; Cortes-Perez, I.; Rojas-Navarrete, A.; Ibancos-Losada, M.D.R.; Lomas-Vega, R. Effectiveness of Vestibular Rehabilitation in Improving Health Status and Balance in Patients with Fibromyalgia Syndrome: A Single-Blind Randomized Controlled Trial. Biomedicines 2023, 11, 1297. [Google Scholar] [CrossRef]
- da Silva Almeida, D.O.; Pontes-Silva, A.; Dibai-Filho, A.V.; Costa-de-Jesus, S.F.; Avila, M.A.; Fidelis-de-Paula-Gomes, C.A. Women with fibromyalgia (ACR criteria) compared with women diagnosed by doctors and women with osteoarthritis: Cross-sectional study using functional and clinical variables. Int. J. Rheum. Dis. 2023, 26, 2278–2283. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Jordan, I.; Jones, C.; Lawson, P.; Younger, J.W. Abnormal immune system response in the brain of women with Fibromyalgia after experimental endotoxin challenge. Brain Behav. Immun. Health 2023, 30, 100624. [Google Scholar] [CrossRef] [PubMed]
- Caxaria, S.; Bharde, S.; Fuller, A.M.; Evans, R.; Thomas, B.; Celik, P.; Dell’Accio, F.; Yona, S.; Gilroy, D.; Voisin, M.B.; et al. Neutrophils infiltrate sensory ganglia and mediate chronic widespread pain in fibromyalgia. Proc. Natl. Acad. Sci. USA 2023, 120, e2211631120. [Google Scholar] [CrossRef]
- Silva, A.; Barcessat, A.R.; Goncalves, R.; Landre, C.; Brandao, L.; Nunes, L.; Feitosa, H.; Costa, L.; Silva, R.; de Lima, E.; et al. REAC Neurobiological Modulation as a Precision Medicine Treatment for Fibromyalgia. J. Pers. Med. 2023, 13, 902. [Google Scholar] [CrossRef]
- Pinto, A.M.; Luis, M.; Geenen, R.; Palavra, F.; Lumley, M.A.; Ablin, J.N.; Amris, K.; Branco, J.; Buskila, D.; Castelhano, J.; et al. Neurophysiological and psychosocial mechanisms of fibromyalgia: A comprehensive review and call for an integrative model. Neurosci. Biobehav. Rev. 2023, 151, 105235. [Google Scholar] [CrossRef]
- Leca, S.; Tavares, I. Research in Mindfulness Interventions for Patients With Fibromyalgia: A Critical Review. Front. Integr. Neurosci. 2022, 16, 920271. [Google Scholar] [CrossRef]
- Martins, C.C.; Reis, A.S.; da Motta, K.P.; Luchese, C.; Wilhelm, E.A. Mechanistic pathways of fibromyalgia induced by intermittent cold stress in mice is sex-dependently. Brain Res. Bull. 2022, 187, 11–23. [Google Scholar] [CrossRef]
- Bidari, A.; Ghavidel-Parsa, B. Nociplastic pain concept, a mechanistic basis for pragmatic approach to fibromyalgia. Clin. Rheumatol. 2022, 41, 2939–2947. [Google Scholar] [CrossRef]
- Colomer-Carbonell, A.; Sanabria-Mazo, J.P.; Hernandez-Negrin, H.; Borras, X.; Suso-Ribera, C.; Garcia-Palacios, A.; Muchart, J.; Munuera, J.; D’Amico, F.; Maes, M.; et al. Study protocol for a randomised, double-blinded, placebo-controlled phase III trial examining the add-on efficacy, cost-utility and neurobiological effects of low-dose naltrexone (LDN) in patients with fibromyalgia (INNOVA study). BMJ Open 2022, 12, e055351. [Google Scholar] [CrossRef]
- Wolfe, F.; Rasker, J.J. The Evolution of Fibromyalgia, Its Concepts, and Criteria. Cureus 2021, 13, e20010. [Google Scholar] [CrossRef]
- Arfuch, V.M.; Aguilar Martin, C.; Berenguera, A.; Caballol Angelats, R.; Goncalves, A.Q.; Carrasco-Querol, N.; Gonzalez Serra, G.; Sancho Sol, M.C.; Fuste Anguera, I.; Friberg, E.; et al. Cost-utility of a multicomponent intervention for fibromyalgia versus usual care: A pragmatic randomised controlled trial. J. Rehabil. Med. 2023, 55, jrm12361. [Google Scholar] [CrossRef]
- Kan, S.; Fujita, N.; Shibata, M.; Miki, K.; Yukioka, M.; Senba, E. Three weeks of exercise therapy altered brain functional connectivity in fibromyalgia inpatients. Neurobiol. Pain 2023, 14, 100132. [Google Scholar] [CrossRef] [PubMed]
- Beiner, E.; Brenner Miguel, S.; Friederich, H.C.; Tesarz, J.; Per, P.C. Elevated high sensitive C-reactive protein in fibromyalgia. Front. Psychiatry 2023, 14, 1237518. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.D.; Wilcox, K.T.; Moskowitz, J.T.; Wethington, E.; Addington, E.L.; Sanni, M.O.; Kim, P.; Reid, M.C. Feasibility, Acceptability, and Preliminary Efficacy of a Positive Affect Skills Intervention for Adults With Fibromyalgia. Innov. Aging 2023, 7, igad070. [Google Scholar] [CrossRef] [PubMed]
- Wasti, A.Z.; Mackawy, A.M.H.; Hussain, A.; Huq, M.; Ahmed, H.; Memon, A.G. Fibromyalgia interventions, obstacles and prospects: Narrative review. Acta Myol. 2023, 42, 71–81. [Google Scholar] [CrossRef]
- Marmann, P.; Wiatrek, W. Observational Study to Assesses the Efficacy and Safety of Microcurrent Therapy with a Portable Device in Patients Suffering from Chronic Back Pain, Skeletal System Pain, Fibromyalgia, Migraine or Depression. Med. Devices 2023, 16, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.L.; Zortea, M.; Serrano, P.V.; Brugnera Tomedi, R.; Pereira de Almeida, R.; Torres, I.L.S.; Fregni, F.; Caumo, W. High-beta oscillations at EEG resting state and hyperconnectivity of pain circuitry in fibromyalgia: An exploratory cross-sectional study. Front. Neurosci. 2023, 17, 1233979. [Google Scholar] [CrossRef] [PubMed]
- Gilheaney, O.; Hussey, J.; McTiernan, K. The lived experiences of oropharyngeal dysphagia in adults living with fibromyalgia. Health Expect. 2024, 27, e13932. [Google Scholar] [CrossRef] [PubMed]
- Tharwat, S.; Mosad, N.R.; Abdelmessih, K.E.; Moatamed, E.; Rihan, M.; Osama, N.; Sallam, N.; Elsayed, Y. Prevalence of fibromyalgia among university students and its impact on their health-related quality of life: A survey-based study from Egypt. BMC Public Health 2023, 23, 2437. [Google Scholar] [CrossRef]
- Paschke, L.; Dreyer, N.; Worm, M.; Klinger, R. Can open label placebos improve pain and gluten tolerance via open label placebos in fibromyalgia patients? A study protocol for a randomised clinical trial in an outpatient centre. BMJ Open 2023, 13, e074957. [Google Scholar] [CrossRef]
- Pontes-Silva, A.; Dibai-Filho, A.V.; de Melo, T.S.; Santos, L.M.; de Souza, M.C.; DeSantana, J.M.; Avila, M.A. Effects of progressive intensity resistance training on the impact of fibromyalgia: Protocol for a blinded randomized controlled trial. BMC Musculoskelet. Disord. 2023, 24, 816. [Google Scholar] [CrossRef]
- Ozturk, A.; Sacaklidir, R.; Ulutatar, F. Frequency of fibromyalgia in a cohort of Turkish patients with lung cancer and its effect on pain, sleep quality, fatigue and quality of life. Medicine 2023, 102, e35586. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, R. Outpatient Ketamine Infusions for the Treatment of Fibromyalgia and Chronic Pain Syndrome: A Case Report. Cureus 2023, 15, e44909. [Google Scholar] [CrossRef]
- Du, M.; Hou, X.; Lu, S.; Kang, T.; Li, Y.; Wang, R. Effectiveness of traditional Chinese exercise in patients with fibromyalgia syndrome: A systematic review and meta-analysis of randomized clinical trials. Int. J. Rheum. Dis. 2023, 26, 2380–2389. [Google Scholar] [CrossRef]
- Flynn, D. Chronic Pain Syndromes: Fibromyalgia. FP Essent. 2023, 533, 7–15. [Google Scholar]
- Fitzmaurice, B.C.; Heneghan, N.R.; Rayen, A.T.A.; Grenfell, R.L.; Soundy, A.A. Whole-Body Photobiomodulation Therapy for Fibromyalgia: A Feasibility Trial. Behav. Sci. 2023, 13, 717. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, E.Y.; Yang, M.; Won, H.S.; Kim, Y.D. The current status of fibromyalgia in Korea: An electronic population health data study in Korea. Korean J. Pain 2023, 36, 458–464. [Google Scholar] [CrossRef]
- Byrne, A.; Jones, K.; Backhouse, M.; Rose, F.; Moatt, E.; van der Feltz-Cornelis, C. Patient and primary care practitioners’ perspectives on consultations for fibromyalgia: A qualitative evidence synthesis. Prim. Health Care Res. Dev. 2023, 24, e58. [Google Scholar] [CrossRef]
- Tesher, M.S.; Graham, T.B.; Ting, T.; Kashikar-Zuck, S.; Lynch, N.; Wroblewski, K.; Weiss, J.E. Juvenile Fibromyalgia in Patients With Juvenile Idiopathic Arthritis: Utility of the Pain and Symptom Assessment Tool. Arthritis Care Res. 2022, 74, 2085–2090. [Google Scholar] [CrossRef]
- Kashikar-Zuck, S.; Briggs, M.S.; Bout-Tabaku, S.; Connelly, M.; Daffin, M.; Guite, J.; Ittenbach, R.; Logan, D.E.; Lynch-Jordan, A.M.; Myer, G.D.; et al. Randomized clinical trial of Fibromyalgia Integrative Training (FIT teens) for adolescents with juvenile fibromyalgia—Study design and protocol. Contemp. Clin. Trials 2021, 103, 106321. [Google Scholar] [CrossRef]
- Habib, S.; Alatassi, E.U.; Mahmoud, A.; Akkad Wattar, M.R.; Almujarkesh, M.K. Cognitive Behavioral Therapy Treating Juvenile Fibromyalgia. Cureus 2021, 13, e12496. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Nozohouri, E.; Ahn, Y.; Patel, D.; Bickel, U.; Karamyan, V.T. Systemic and Brain Pharmacokinetics of Milnacipran in Mice: Comparison of Intraperitoneal and Intravenous Administration. Pharmaceutics 2023, 16, 53. [Google Scholar] [CrossRef]
- Derayea, S.M.; Madian, H.; Samir, E.; Hamad, A.A.; Badr El-Din, K.M. A feasible fluorimetric approach anchored in diaryl pyrrolone derivative for the facile analysis of milnacipran in tablets; evaluation of the method greenness. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 273, 121024. [Google Scholar] [CrossRef]
- Gupta, H.; Girma, B.; Jenkins, J.S.; Kaufman, S.E.; Lee, C.A.; Kaye, A.D. Milnacipran for the Treatment of Fibromyalgia. Health Psychol. Res. 2021, 9, 25532. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fattah, Y.H.; Elnemr, R. Efficacy of pregabalin as a monotherapy versus combined pregabalin and milnacipran in the management of fibromyalgia. Int. J. Rheum. Dis. 2020, 23, 1474–1480. [Google Scholar] [CrossRef]
- Pickering, G.; Macian, N.; Delage, N.; Picard, P.; Cardot, J.M.; Sickout-Arondo, S.; Giron, F.; Duale, C.; Pereira, B.; Marcaillou, F. Milnacipran poorly modulates pain in patients suffering from fibromyalgia: A randomized double-blind controlled study. Drug Des. Devel. Ther. 2018, 12, 2485–2496. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, M.W.; Kang, M.J.; Lee, S.G.; Lee, J.G.; Mun, C.W. Comparison Analysis between the Medication Efficacy of the Milnacipran and Functional Connectivity of Neural Networks in Fibromyalgia Patients. Brain Sci. 2020, 10, 295. [Google Scholar] [CrossRef]
- Abu-Hassan, A.A.; Ali, R.; Derayea, S.M. A new approach based on isoindole formation reaction for sensitive fluorimetric assay of milnacipran in tablets and biological fluids (plasma/urine). RSC Adv. 2020, 10, 38884–38889. [Google Scholar] [CrossRef]
- Martinez, H.R.; Figueroa-Sanchez, J.A.; Rodriguez-Gonzalez, I.; Rodriguez-Gomez, G.P. Generalized edema with pregabalin in a patient with fibromyalgia. Neurologia 2023, 38, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Abd Elmaaboud, M.A.; Awad, M.M.; El-Shaer, R.A.A.; Kabel, A.M. The immunomodulatory effects of ethosuximide and sodium butyrate on experimentally induced fibromyalgia: The interaction between IL-4, synaptophysin, and TGF-beta1/NF-kappaB signaling. Int. Immunopharmacol. 2023, 118, 110061. [Google Scholar] [CrossRef] [PubMed]
- Argenbright, C.M.; Bland, M.K.; Michener, S.L.; Wilson, J.R.; Fuchs, P.N. Pregabalin and hyperbaric oxygen therapy on pain thresholds and anxio-depressive behaviors in a preclinical fibromyalgia pain model. Front. Pain Res. 2023, 4, 1097457. [Google Scholar] [CrossRef]
- Ablin, J.N.; Lang, E.; Catalogna, M.; Aloush, V.; Hadanny, A.; Doenyas-Barak, K.; Finci, S.; Polak, N.; Fishlev, G.; Korin, C.; et al. Hyperbaric oxygen therapy compared to pharmacological intervention in fibromyalgia patients following traumatic brain injury: A randomized, controlled trial. PLoS ONE 2023, 18, e0282406. [Google Scholar] [CrossRef]
- Gilron, I.; Robb, S.; Tu, D.; Holden, R.R.; Milev, R.; Towheed, T. Combination analgesic development for enhanced clinical efficacy (the CADENCE trial): A double-blind, controlled trial of an alpha-lipoic acid-pregabalin combination for fibromyalgia pain. Pain 2023, 164, 1783–1792. [Google Scholar] [CrossRef]
- Zabihiyeganeh, M.; Afshar, S.V.; Kadijani, A.A.; Janbozorgi, M.; Akbari, A.; Yahyazadeh, H.; Mirzaei, A. How durable are the effects of cognitive-behavioural therapy in controlling fibromyalgia symptoms? A prospective cohort study. Musculoskelet. Care 2023, 21, 890–894. [Google Scholar] [CrossRef]
- McCrae, C.S.; Curtis, A.F.; Miller, M.B.; Nair, N.; Rathinakumar, H.; Davenport, M.; Berry, J.R.; McGovney, K.; Staud, R.; Berry, R.; et al. Effect of cognitive behavioural therapy on sleep and opioid medication use in adults with fibromyalgia and insomnia. J. Sleep Res. 2020, 29, e13020. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, A. The effectiveness of cognitive behavioural therapy for pain in childhood and adolescence: A meta-analytic review. Ir. J. Psychol. Med. 2016, 33, 251–264. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Beasley, M.; Prescott, G.; McNamee, P.; Keeley, P.; Artus, M.; McBeth, J.; Hannaford, P.; Jones, G.T.; Basu, N.; et al. The Maintaining Musculoskeletal Health (MAmMOTH) Study: Protocol for a randomised trial of cognitive behavioural therapy versus usual care for the prevention of chronic widespread pain. BMC Musculoskelet. Disord. 2016, 17, 179. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Migliorini, F.; Balaji, S.; Ramasubramanian, S.; Jayakumar, T.; Jeyaraman, N. Whole-body cryotherapy in orthopaedics: Current concepts. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 2245–2254. [Google Scholar] [CrossRef]
- Kulkarni, R.; Akintoye, S. A Patient with a History of Fibromyalgia Reports for an Intraoral Incisional Biopsy. Dent. Clin. N. Am. 2023, 67, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Ege, F.; Isik, R. A Comparative Assessment of the Inflammatory Markers in Patients with Fibromyalgia under Duloxetine Treatment. Front. Biosci. 2023, 28, 161. [Google Scholar] [CrossRef]
- Kamaly, N.A.; Kamel, A.S.; Sadik, N.A.; Shahin, N.N. Milnacipran and Vanillin Alleviate Fibromyalgia-Associated Depression in Reserpine-Induced Rat Model: Role of Wnt/beta-Catenin Signaling. Mol. Neurobiol. 2025, 62, 7682–7705. [Google Scholar] [CrossRef]
- Winslow, B.T.; Vandal, C.; Dang, L. Fibromyalgia: Diagnosis and Management. Am. Fam. Physician 2023, 107, 137–144. [Google Scholar] [PubMed]
- Alorfi, N.M. Pharmacological treatments of fibromyalgia in adults; overview of phase IV clinical trials. Front. Pharmacol. 2022, 13, 1017129. [Google Scholar] [CrossRef]
- Farag, H.M.; Yunusa, I.; Goswami, H.; Sultan, I.; Doucette, J.A.; Eguale, T. Comparison of Amitriptyline and US Food and Drug Administration-Approved Treatments for Fibromyalgia: A Systematic Review and Network Meta-analysis. JAMA Netw. Open 2022, 5, e2212939. [Google Scholar] [CrossRef]
- Haddad, H.W.; Jumonville, A.C.; Stark, K.J.; Temple, S.N.; Dike, C.C.; Cornett, E.M.; Kaye, A.D. The Role of Vitamin D in the Management of Chronic Pain in Fibromyalgia: A Narrative Review. Health Psychol. Res. 2021, 9, 25208. [Google Scholar] [CrossRef] [PubMed]
- Aster, H.C.; Evdokimov, D.; Braun, A.; Uceyler, N.; Sommer, C. Analgesic Medication in Fibromyalgia Patients: A Cross-Sectional Study. Pain Res. Manag. 2022, 2022, 1217717. [Google Scholar] [CrossRef]
- Romano, C.L.; Romano, D.; Bonora, C.; Mineo, G. Pregabalin, celecoxib, and their combination for treatment of chronic low-back pain. J. Orthop. Traumatol. 2009, 10, 185–191. [Google Scholar] [CrossRef]
- Cardel, M.; Kelly, A.S.; Thompson, L.A. Becoming Your Healthiest Self: An Eat-Well, Get-Fit, Feel-Great Guide for Teens. JAMA Pediatr. 2020, 174, 736. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.; Lopez-Gomez, I.; Gutierrez, L.; Ecija, C.; Catala, P.; Penacoba, C. Exploring the Preference for Fatigue-avoidance Goals as a Mediator Between Pain Catastrophizing, Functional Impairment, and Walking Behavior in Women With Fibromyalgia. Clin. J. Pain 2021, 38, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Kashikar-Zuck, S.; Tran, S.T.; Barnett, K.; Bromberg, M.H.; Strotman, D.; Sil, S.; Thomas, S.M.; Joffe, N.; Ting, T.V.; Williams, S.E.; et al. A Qualitative Examination of a New Combined Cognitive-Behavioral and Neuromuscular Training Intervention for Juvenile Fibromyalgia. Clin. J. Pain 2016, 32, 70–81. [Google Scholar] [CrossRef]
- Sil, S.; Thomas, S.; DiCesare, C.; Strotman, D.; Ting, T.V.; Myer, G.; Kashikar-Zuck, S. Preliminary evidence of altered biomechanics in adolescents with juvenile fibromyalgia. Arthritis Care Res. 2015, 67, 102–111. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Hauser, W.; Fluss, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Ariani, A.; Bazzichi, L.; Sarzi-Puttini, P.; Salaffi, F.; Manara, M.; Prevete, I.; Bortoluzzi, A.; Carrara, G.; Scire, C.A.; Ughi, N.; et al. The Italian Society for Rheumatology clinical practice guidelines for the diagnosis and management of fibromyalgia Best practices based on current scientific evidence. Reumatismo 2021, 73, 89–105. [Google Scholar] [CrossRef] [PubMed]

| Database | Search String |
|---|---|
| PubMed | (“juvenile fibromyalgia”\((MeSH Terms)) OR “juvenile fibromyalgia” OR “adolescent fibromyalgia”) AND (“drug therapy”\((MeSH Terms)) OR pharmacologial OR antidepressants OR pregabalin OR duloxetine OR milnacipran) AND (“non-pharmacological” OR “cognitive behavioral therapy” OR exercise OR physiotherapy OR “multidisciplinary approach”) AND (randomized controlled trial\((pt)) OR “RCT” OR “randomised controlled trial”) |
| Web of Science | TS = (“juvenile fibromyalgia” OR “adolescent fibromyalgia”) AND TS = (“pharmacological” OR “drug therapy” OR duloxetine OR pregabalin OR milnacipran) AND TS = (“non-pharmacological” OR “cognitive behavioral therapy” OR exercise OR physiotherapy OR “multidisciplinary approach”) AND TS = (“randomized controlled trial” OR “RCT”) |
| Embase | (‘juvenile fibromyalgia’/exp OR ‘juvenile fibromyalgia’ OR ‘adolescent fibromyalgia’) AND (‘drug therapy’/exp OR pharmacological OR duloxetine OR pregabalin OR milnacipran) AND (‘non drug therapy’/exp OR ‘cognitive behavioral therapy’ OR exercise OR physiotherapy OR ‘multidisciplinary care’) AND (‘randomized controlled trial’/exp OR ‘randomised controlled trial’ OR RCT) |
| Author, Year | Journal | Follow-Up (Weeks) | Treatment | Mean Age | Female (%) | Patients (n) |
|---|---|---|---|---|---|---|
| Arnold et al., 2015 [29] | Pediatr Rheumatol Online J | 8 | Milnacipran (50 to 100 mg/daily) | 15.7 | 90 | 96 |
| Milnacipran (50 to 100 mg/daily)/Placebo | 15.0 | 60 | 20 | |||
| Arnold et al., 2016 [30] | Pediatr Rheumatol Online J | 15 | Pregabalin (75 to 450 mg/daily) | 14.6 | 86 | 54 |
| Placebo | 14.7 | 53 | ||||
| Black et al., 2021 [31] | Clin J Pain | 8 | CBT | 15.3 | 90 | 20 |
| FIT Teens | 20 | |||||
| Hengartner et al., 2021 [32] | Int J Risk Saf Med | 13 | Duloxetine (30 to 60 mg/daily) | 15.7 | 80 | 91 |
| Placebo | 15.3 | 70 | 93 | |||
| Kashikar-Zuck et al., 2012 [33] | Arthritis Rheum | 24 | CBT | 15.2 | 95 | 57 |
| Education treatment | 14.9 | 90 | 57 | |||
| Kashikar-Zuck et al., 2013 [34] | Arthritis Care Res (Hoboken) | 9 | CBT | 15.2 | 94 | 33 |
| Education treatment | 35 | |||||
| Kashikar-Zuck et al., 2018 [35] | J Pain | 12 | CBT | 90 | 19 | |
| FIT Teens | 17 | |||||
| Upadhyaya et al., 2019 [36] | Pediatr Rheumatol Online J | 13 | Duloxetine (30 to 60 mg/daily) | 15.7 | 80 | 91 |
| Placebo | 15.3 | 70 | 93 |
| Author, Year | Treatment | Outcome of Interest | Main Results |
|---|---|---|---|
| Arnold et al., 2015 [29] | Milnacipran (50 to 100 mg/daily) | Pain, PGIS, PedsQL: Generic Core Scales, Multidimensional Fatigue Scale, MASC, CDI, AEs, vital signs (blood pressure, Heart rate) body weight, electrocardiograms, and laboratory tests, LTR | Mean improvements in pain, global disease severity, quality of life, and fatigue symptoms at the end of both open-label periods. Nausea, headache, vomiting, and dizziness as most common reported adverse events. Mean increases in heart rate and blood pressure. |
| Arnold et al., 2016 [30] | Pregabalin (75 to 450 mg/daily) vs. placebo | primary efficacy outcome: change in mean pain score based on the subject’s daily pain diaries (NRS). Secondary efficacy outcomes: mean pain score at each week, from daily pain diaries with a 24-h recall period; the change in mean pain score at week 15 with a 1-week re- call period; PGIC, change in sleep quality score at endpoint and at each week, parent GIC, FIQ-C. | Not significant improvement in mean pain score at endpoint. Significant improvements with pregabalin versus placebo in secondary outcomes of change in pain score by week and patient global impression of change. No significant improvement in other secondary outcomes measuring pain, sleep, and FM impact. Safety in line with pregabalin’ known profile in adults |
| Black et al., 2021 [31] | CBT vs. FIT Teens | Isokinetic hip and knee strength, dynamic postural stability, and 3-D motional analysis of functional tasks conducted on a standardized drop vertical jump task. | Improvements in hip abduction strength and greater external hip rotation in the FIT Teens group. Decreased hip adduction in the FIT Teens group. |
| Hengartner et al., 2021 [32] | Duloxetine (30 to 60 mg/daily) vs. placebo | Severe treatment-emergent psychiatric adverse event including all AE related to suicidality and other psychiatric disorders, treatment discontinuation due psychiatric adverse event | Significant treatment-emergent suicidal ideation and behaviour with duloxetine. The incidence of severe treatment-emergent psychiatric adverse events significantly higher in duloxetine group. |
| Kashikar-Zuck et al., 2012 [33] | CBT | Primary outcome: FDI. Secondary outcomes: CDI, VAS, tender point sensitivity, physician’s global assessment on a 0–10-cm VAS, HRQOL using the PedsQL Generic Core Scales and PedsQL Rheumatology Module, sleep quality. AEs. | CBT was superior to FM education in reducing the primary outcome of functional disability. Depression symptoms reduction was significant for both groups. Reduction in pain was not significant in either group |
| Kashikar-Zuck et al., 2013 [34] | CBT vs. Education | Actigraphy, FDI, CDI | Self-reported functioning improved in the CBT group but no significant changes were seen in either group for activity counts, sedentary, moderate, or vigorous activity. The CBT showed lower peak and light activity. |
| Kashikar-Zuck et al., 2018 [35] | CBT vs. FIT Teens | Primary outcomes: VAS, FDI. Secondary outcomes: CDI, TSK-11, PCS-C; 40, Aes | FIT Teens group had significantly greater decreases in pain. FIT Teens reported significant improvements in disability, but did not differ from CBT at the 3-month |
| Upadhyaya et al., 2019 [36] | Duloxetine (30 to 60 mg/daily) vs. placebo | Primary outcome: mean change in 24-h average pain severity of BPI. Secondary outcomes: BPI-modified short form: adolescent version severity and interference scores, PPQ (pain right now, worst pain, and average pain items), CGI-severity: overall, CGI-severity: mental illness, FDI-child, FDI-parent, CDI, Multidimensional Anxiety Scale for Children, treatment response (≥30%, ≥50% reductions on BPI average pain severity), AEs, laboratory values, height, weight, vital signs, and electrocardiograms. | Change in BPI average pain severity was not different between duloxetine and placebo. Duloxetine was better in treatment response (≥30% and ≥50% reductions on BPI average pain severity) and improvement of the general activity and relationships items on the BPI interference subscale. Onset of treatment-emergent adverse event more frequent in the duloxetine group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliorini, F.; Maffulli, N.; Memminger, M.K.; Simeone, F.; Bardazzi, T.; Vaccaro, M.G.; Colarossi, G. Management of Juvenile Fibromyalgia: A Level I Evidence-Based Systematic Review. Med. Sci. 2025, 13, 203. https://doi.org/10.3390/medsci13030203
Migliorini F, Maffulli N, Memminger MK, Simeone F, Bardazzi T, Vaccaro MG, Colarossi G. Management of Juvenile Fibromyalgia: A Level I Evidence-Based Systematic Review. Medical Sciences. 2025; 13(3):203. https://doi.org/10.3390/medsci13030203
Chicago/Turabian StyleMigliorini, Filippo, Nicola Maffulli, Michael Kurt Memminger, Francesco Simeone, Tommaso Bardazzi, Maria Grazia Vaccaro, and Giorgia Colarossi. 2025. "Management of Juvenile Fibromyalgia: A Level I Evidence-Based Systematic Review" Medical Sciences 13, no. 3: 203. https://doi.org/10.3390/medsci13030203
APA StyleMigliorini, F., Maffulli, N., Memminger, M. K., Simeone, F., Bardazzi, T., Vaccaro, M. G., & Colarossi, G. (2025). Management of Juvenile Fibromyalgia: A Level I Evidence-Based Systematic Review. Medical Sciences, 13(3), 203. https://doi.org/10.3390/medsci13030203







