A Novel Method to Determine the Respiratory Compensation Point from Percutaneous Oxygen Saturation of Healthy Adults During a Ramp-Incremental Test: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Ethics Approval, and Consent to Participate
2.2. Sample Size Calculation and Eligibility Criteria
2.3. Experimental Methods
2.4. Exercise Test Method
2.5. Expiratory Gas Analysis
2.6. Calculation of the RP Point by Doctors
2.7. Automated Calculation of the ST2 Using SpO2
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Acknowledgments
Conflicts of Interest
Abbreviations
| VT1 | ventilatory threshold 1 |
| VT2 | ventilatory threshold 2 |
| ST | SpO2 threshold |
| CPET | cardiopulmonary exercise testing |
| VT | ventilatory threshold |
| CVD | cardiovascular diseases |
| peak VO2max | peak oxygen intake |
| EMG | electromyography |
| SpO2 | oxygen saturation |
| PR | pulse rate |
| BMI | body mass index |
| VO2 | oxygen consumption |
| VCO2 | carbon dioxide production |
| VE | minute ventilation |
| VE/VO2 | ventilatory equivalents for oxygen |
| VE/VCO2 | ventilatory equivalent for carbon dioxide |
| PETO2 | end-tidal oxygen concentration |
| PETCO2 | end-tidal carbon dioxide concentration |
| HR | heart rate |
| ST2 | second SpO2 threshold |
| CI | confidence interval |
| LT | lactate threshold |
| sLT | LT in sweat |
| bLT | LT in blood |
| NIRS | near-infrared spectroscopy |
| QOL | quality of life |
| RPE | rate of perceived exercise |
References
- Levett, D.Z.H.; Jack, S.; Swart, M.; Carlisle, J.; Wilson, J.; Snowden, C.; Riley, M.; Danjoux, G.; Ward, S.A.; Older, P.; et al. Perioperative cardiopulmonary exercise testing (CPET): Consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br. J. Anaesth. 2018, 120, 484–500. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, R.M.; Craig, J.C.; Richardson, R.S. The respiratory compensation point and the deoxygenation break point are not valid surrogates for critical power and maximum lactate steady state. Med. Sci. Sports Exerc. 2018, 50, 2379–2382. [Google Scholar] [CrossRef]
- Keir, D.A.; Pogliaghi, S.; Murias, J.M. The respiratory compensation point and the deoxygenation break point are valid surrogates for critical power and maximum lactate steady state. Med. Sci. Sports Exerc. 2018, 50, 2375–2378. [Google Scholar] [CrossRef]
- Mezzani, A.; Hamm, L.F.; Jones, A.M.; McBride, P.E.; Moholdt, T.; Stone, J.A.; Urhausen, A.; Williams, M.A. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: A joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur. J. Prev. Cardiol. 2013, 20, 442–467. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Abreu, A.; Ambrosetti, M.; Cornelissen, V.; Gevaert, A.; Kemps, H.; Laukkanen, J.A.; Pedretti, R.; Simonenko, M.; Wilhelm, M.; et al. Exercise intensity assessment and prescription in cardiovascular rehabilitation and beyond: Why and how: A position statement from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2022, 29, 230–245. [Google Scholar] [CrossRef]
- Carriere, C.; Corrà, U.; Piepoli, M.; Bonomi, A.; Merlo, M.; Barbieri, S.; Salvioni, E.; Binno, S.; Mapelli, M.; Righini, F.; et al. Anaerobic threshold and respiratory compensation point identification during cardiopulmonary exercise tests in chronic heart failure. Chest 2019, 156, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Koike, A.; Nagayama, O.; Takayanagi, Y.; Wu, L.; Nishi, I.; Kato, Y.; Sato, A.; Yamashita, T.; Aonuma, K.; et al. Clinical significance of respiratory compensation during exercise testing in cardiac patients. BioSci. Trends 2018, 12, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K.; Stringer, W.W.; Casaburi, R.; Koike, A.; Cooper, C.B. Determination of the anaerobic threshold by gas exchange: Biochemical considerations, methodology and physiological effects. Z. Kardiol. 1994, 83, 1–12. [Google Scholar] [PubMed]
- Svedahl, K.; MacIntosh, B.R. Anaerobic threshold: The concept and methods of measurement. Can. J. Appl. Physiol. 2003, 28, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Nakashima, D.; Shiraishi, Y.; Ryuzaki, T.; Ikura, H.; Miura, K.; Suzuki, M.; Watanabe, T.; Nagura, T.; Matsumato, M.; et al. A novel device for detecting anaerobic threshold using sweat lactate during exercise. Sci. Rep. 2021, 11, 4929. [Google Scholar] [CrossRef]
- Maeda, Y.; Okawara, H.; Sawada, T.; Nakashima, D.; Nagahara, J.; Fujitsuka, H.; Ikeda, K.; Hoshino, S.; Kobari, Y.; Katsumata, Y.; et al. Implications of the onset of sweating on the sweat lactate threshold. Sensors 2023, 23, 3378. [Google Scholar] [CrossRef] [PubMed]
- Hug, F.; Laplaud, D.; Savin, B.; Grélot, L. Occurrence of electromyographic and ventilatory thresholds in professional road cyclists. Eur. J. Appl. Physiol. 2003, 90, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Lucía, A.; Vaquero, A.F.; Pérez, M.; Sánchez, O.; Sánchez, V.; Gómez, M.A.; Chicharro, J.L. Electromyographic response to exercise in cardiac transplant patients: A new method for anaerobic threshold determination? Chest 1997, 111, 1571–1576. [Google Scholar] [CrossRef][Green Version]
- Tikkanen, O.; Hu, M.; Vilavuo, T.; Tolvanen, P.; Cheng, S.; Finni, T. Ventilatory threshold during incremental running can be estimated using EMG shorts. Physiol. Meas. 2012, 33, 603–614. [Google Scholar] [CrossRef]
- Frazão, M.; Silva, P.E.; Cacau, L.A.P.; Petrucci, T.R.; Assis, M.C.; Santos, A.D.C.; Brasileiro-Santos, M.D.S. EMG breakpoints for detecting anaerobic threshold and respiratory compensation point in recovered COVID-19 patients. J. Electromyogr. Kinesiol. 2021, 59, 102567. [Google Scholar] [CrossRef]
- Abe, M.; Ushio, K.; Ishii, Y.; Nakashima, Y.; Iwaki, D.; Fukuhara, K.; Takahashi, M.; Mikami, Y. A method of determining anaerobic threshold from percutaneous oxygen saturation. Sci. Rep. 2022, 12, 20081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nikooie, R.; Gharakhanlo, R.; Rajabi, H.; Bahraminegad, M.; Ghafari, A. Noninvasive determination of anaerobic threshold by monitoring the %SpO2 changes and respiratory gas exchange. J. Strength Cond. Res. 2009, 23, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Martín-Escudero, P.; Cabanas, A.M.; Fuentes-Ferrer, M.; Galindo-Canales, M. Oxygen saturation behavior by pulse oximetry in female athletes: Breaking myths. Biosensors 2021, 11, 391. [Google Scholar] [CrossRef]
- Nielsen, H.B. Arterial desaturation during exercise in man: Implication for O2 uptake and work capacity. Scand. J. Med. Sci. Sports 2003, 13, 339–358. [Google Scholar] [CrossRef]
- González-Alonso, J.; Teller, C.; Andersen, S.L.; Jensen, F.B.; Hyldig, T.; Nielsen, B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J. Appl. Physiol. 1999, 86, 1032–1039. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Madsen, P.; Svendsen, L.B.; Roach, R.C.; Secher, N.H. The influence of PaO2, pH and SaO2 on maximal oxygen uptake. Acta Physiol. Scand. 1998, 164, 89–97. [Google Scholar] [CrossRef]
- Préfaut, C.; Durand, F.; Mucci, P.; Caillaud, C. Exercise-induced arterial hypoxaemia in athletes: A review. Sports Med. 2000, 30, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Mucci, P.; Préfaut, C. Evidence for an inadequate hyperventilation inducing arterial hypoxemia at submaximal exercise in all highly trained endurance athletes. Med. Sci. Sports Exerc. 2000, 32, 926–932. [Google Scholar] [CrossRef]
- Ng, H.L.; Trefz, J.; Schönfelder, M.; Wackerhage, H. Effects of a taped filter mask on peak power, perceived breathlessness, heart rate, blood lactate and oxygen saturation during a graded exercise test in young healthy adults: A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2022, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Dexter, B.; Hancock, A.; Hoffman, N.; Kerschke, S.; Hux, K.; Aggarwal, D. Implementing team-based post-stroke telerehabilitation: A case example. Int. J. Telerehabil. 2022, 14, e6438. [Google Scholar] [CrossRef]
- Cramer, S.C.; Dodakian, L.; Le, V.; See, J.; Augsburger, R.; McKenzie, A.; Zhou, R.J.; Chiu, N.L.; Heckhausen, J.; Cassidy, J.M.; et al. Efficacy of home-based telerehabilitation vs. in-clinic therapy for adults after stroke: A randomized clinical trial. JAMA Neurol. 2019, 76, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Vega, P.; Prieto-Moreno, R.; Castillo-Pérez, H.; Martínez-Ruiz, V.; Romero-Ayuso, D.; Ashe, M.C. Family caregivers’ experiences with Tele-rehabilitation for older adults with hip fracture. J. Clin. Med. 2021, 10, 5850. [Google Scholar] [CrossRef]
- Gabriel, A.S.; Parvanova, I.; Tsai, T.Y.; Finkelstein, J. Assessing patient perspectives of a cancer telerehabilitation platform using thematic analysis of semi-structured qualitative interviews. AMIA Jt. Summits Transl. Sci. Proc. 2023, 2023, 216–224. [Google Scholar]
- Brouwers, R.W.M.; Kemps, H.M.C.; Herkert, C.; Peek, N.; Kraal, J.J. A 12-week cardiac telerehabilitation programme does not prevent relapse of physical activity levels: Long-term results of the FIT@Home trial. Eur. J. Prev. Cardiol. 2022, 29, e255–e257. [Google Scholar] [CrossRef]
- Nakayama, A.; Takayama, N.; Kobayashi, M.; Hyodo, K.; Maeshima, N.; Takayuki, F.; Morita, H.; Komuro, I. Remote cardiac rehabilitation is a good alternative of outpatient cardiac rehabilitation in the COVID-19 era. Environ. Health Prev. Med. 2020, 25, 48. [Google Scholar] [CrossRef]
- Saitoh, M.; Takahashi, T.; Morisawa, T.; Honzawa, A.; Yokoyama, M.; Abulimiti, A.; Kagiyama, N.; Kasai, T.; Minamino, T.; Asai, T.; et al. Remote cardiac rehabilitation in older cardiac disease: A randomized case series feasibility study. Cardiol. Res. 2022, 13, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, S.; Halahakone, U.; Abell, B.; Kularatna, S.; McCreanor, V.; McPhail, S.M.; Redfern, J.; Briffa, T.; Parsonage, W. Hybrid cardiac telerehabilitation for coronary artery disease in Australia: A cost-effectiveness analysis. BMC Health Serv. Res. 2023, 23, 512. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, Y.; Arizono, S.; Tanaka, S.; Kawaguchi, T.; Tsugita, N.; Fuseya, T.; Magata, J.; Tawara, Y.; Segawa, T. Effects of real-time remote cardiac rehabilitation on exercise capacity and quality of life: A quasi-randomised controlled trial. BMC Geriatr. 2023, 23, 388. [Google Scholar] [CrossRef]
- Nishitani-Yokoyama, M.; Shimada, K.; Fujiwara, K.; Abulimiti, A.; Kasuya, H.; Kunimoto, M.; Yamaguchi, Y.; Tabata, M.; Saitoh, M.; Takahashi, T.; et al. Safety and feasibility of Tele-cardiac rehabilitation using remote biological signal monitoring system: A pilot study. Cardiol. Res. 2023, 14, 261–267. [Google Scholar] [CrossRef]
- Valbuena, V.S.M.; Merchant, R.M.; Hough, C.L. Racial and ethnic bias in pulse oximetry and clinical outcomes. JAMA Intern. Med. 2022, 182, 699–700. [Google Scholar] [CrossRef]
- Yoon, B.K.; Kravitz, L.; Robergs, R. VO2max, Protocol Duration, and the VO2 Plateau. Med. Sci. Sports Exerc. 2007, 39, 1189–1195. [Google Scholar] [CrossRef]
- Beltz, N.M.; Gibson, A.L.; Janot, J.M.; Kravitz, L.; Mermier, C.M.; Dalleck, L.C. Graded Exercise Testing Protocols for the Determination of VO2max: Historical Perspectives, Progress, and Future Considerations. J. Sports Med. 2016, 2016, 3968393. [Google Scholar] [CrossRef] [PubMed]
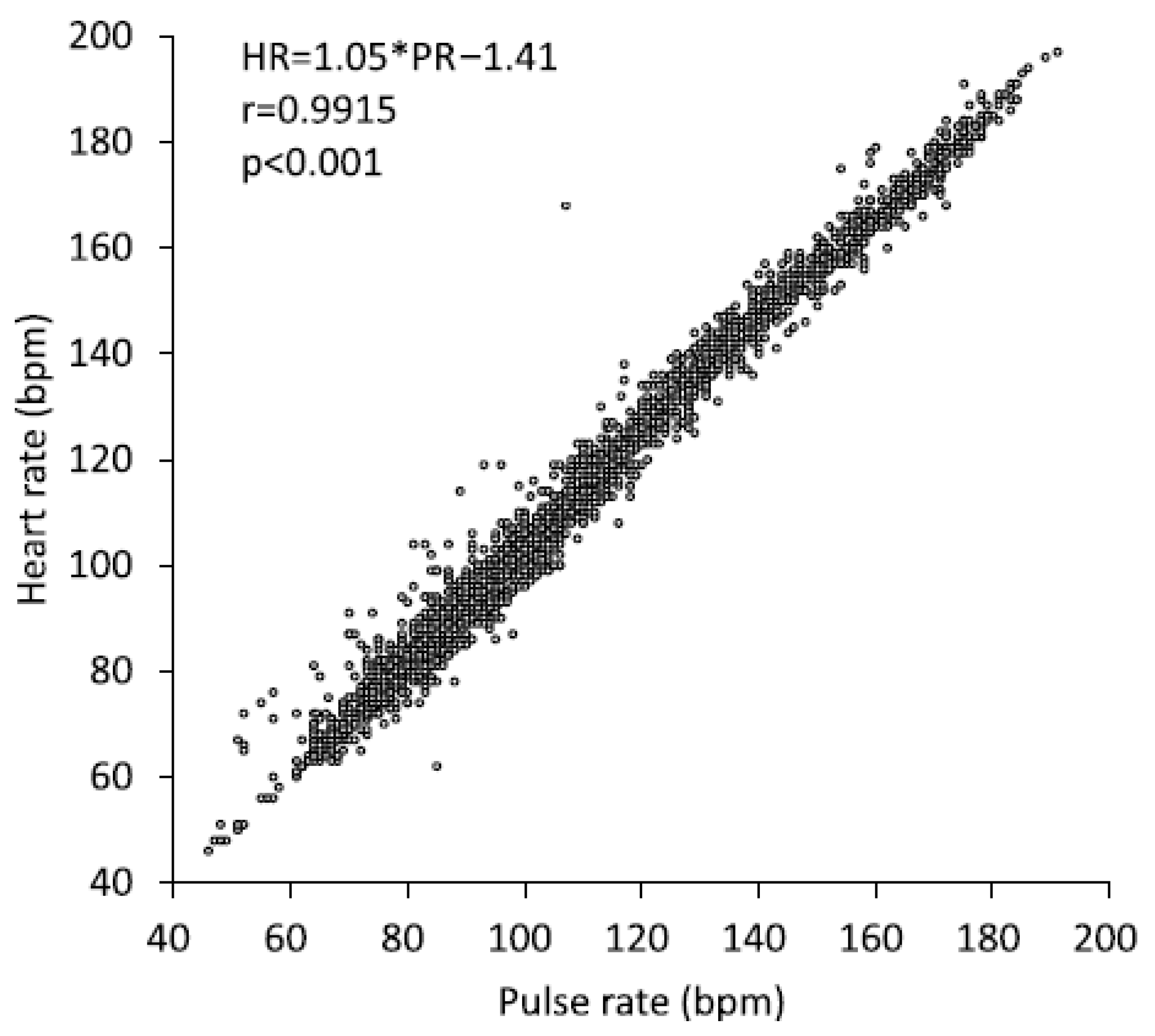
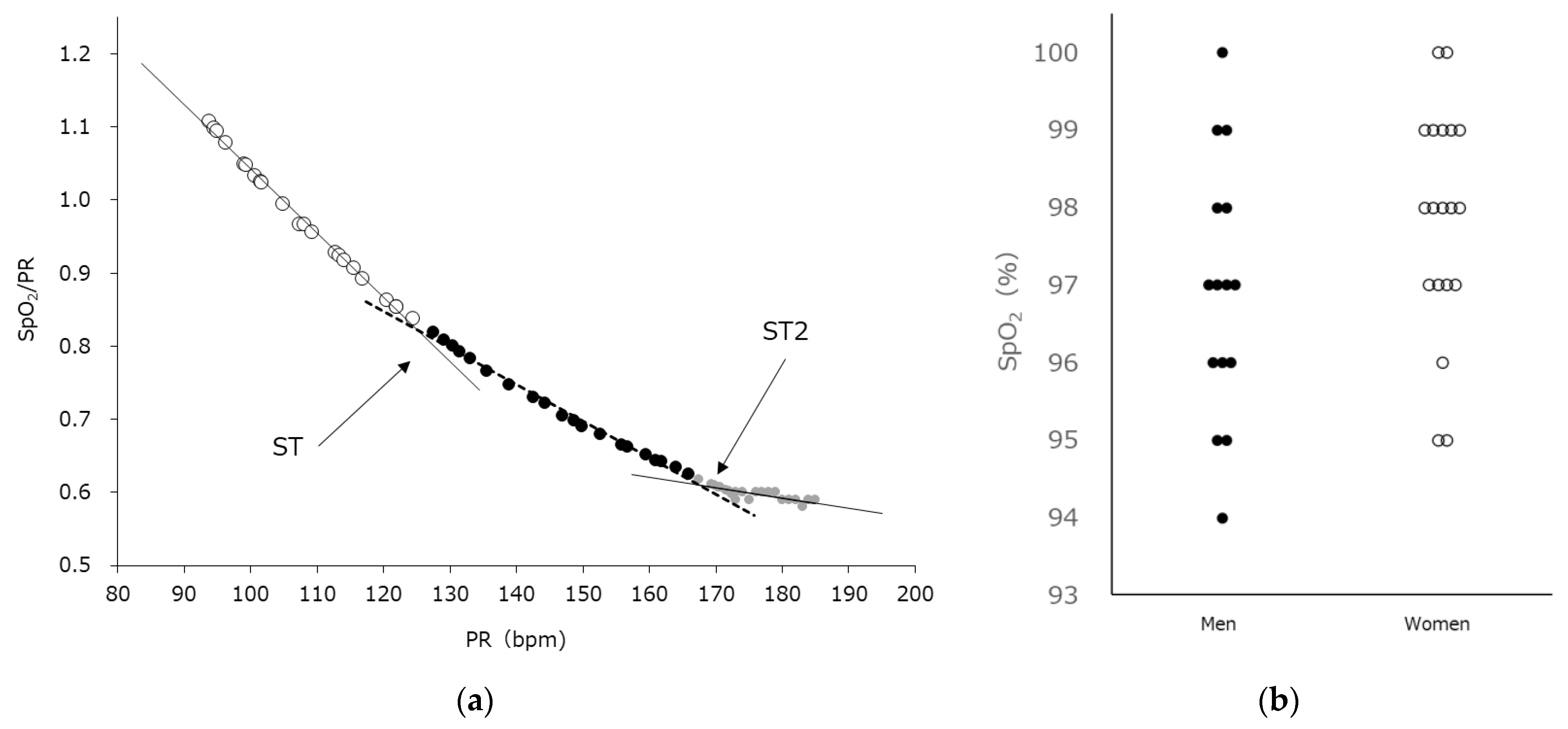
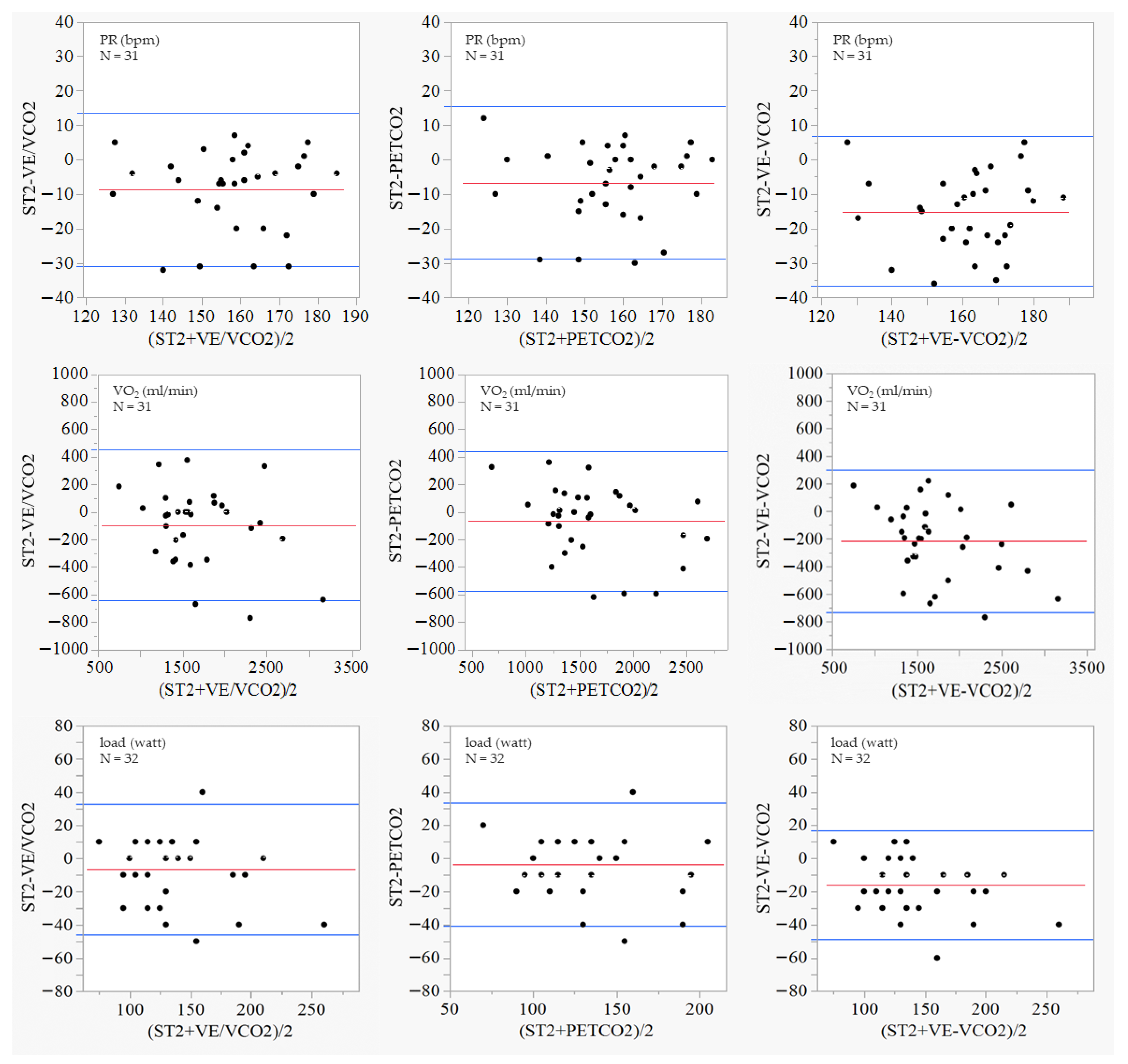
| Characteristics | Unit | All (N = 34) | Men (N = 15) | Women (N = 19) |
|---|---|---|---|---|
| Age | Years | 41.2 ± 9.4 | 36.7 ± 10.2 | 44.8 ± 10.2 |
| Height | Cm | 164.9 ± 8.3 | 171.3 ± 6.4 | 159.7 ± 6.4 |
| Weight | Kg | 60.2 ± 9.4 | 65.6 ± 8.4 | 55.8 ± 8.4 |
| BMI | kg/m2 | 22.1 ± 2.5 | 22.3 ± 2.4 | 21.9 ± 2.4 |
| Gender | Characteristics | N | Pulse Rate (bpm) | VO2 (mL/min) | Load (watts) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| All | VE/VCO2 | 31 | 162.3 ± 15.8 | 1747.1 ± 577.5 | 140.0 ± 42.4 |
| PETCO2 | 31 | 160.3 ± 15.7 | 1660.5 ± 535.8 | 132.9 ± 34.8 | |
| VE-VCO2-Slope | 32 | 168.9 ± 15.3 | 1849.7 ± 594.7 | 148.8 ± 42.3 | |
| SpO2-Slope (ST2) | 34 | 153.4 ± 15.2 | 1632.9 ± 481.8 | 132.6 ± 36.0 | |
| Men | VE/VCO2 | 13 | 166.2 ± 17.8 | 2188.5 ± 571.6 | 170.8 ± 46.6 |
| PETCO2 | 12 | 163.8 ± 18.3 | 2097.5 ± 514.1 | 161.7 ± 34.3 | |
| VE-VCO2-Slope | 13 | 173.4 ± 17.2 | 2333.6 ± 568.7 | 183.1 ± 42.3 | |
| SpO2-Slope (ST2) | 15 | 153.9 ± 15.4 | 1948.5 ± 492.7 | 156.7 ± 37.7 | |
| Women | VE/VCO2 | 18 | 159.5 ± 12.0 | 1428.2 ± 318.2 | 117.8 ± 19.9 |
| PETCO2 | 19 | 158.1 ± 10.0 | 1384.6 ± 330.6 | 114.7 ± 19.8 | |
| VE-VCO2-Slope | 19 | 165.9 ± 11.1 | 1518.6 ± 329.6 | 125.3 ± 21.4 | |
| SpO2-Slope (ST2) | 19 | 153.1 ± 15.6 | 1383.8 ± 297.0 | 113.7 ± 20.3 |
| Gender | Characteristics | N | Pulse Rate (bpm) | VO2 (mL/min) | Load (watts) | |||
|---|---|---|---|---|---|---|---|---|
| Diff | 95% CI | Diff | 95% CI | Diff | 95% CI | |||
| All | VE/VCO2 | 31 | −8.6 | −12.8 to −4.4 | −98.6 | −200.8 to 3.5 | −6.5 | −13.8 to 0.9 |
| PETCO2 | 31 | −6.7 | −10.8 to −2.6 | −66.2 | −161.1 to 28.8 | −3.5 | −10.5 to 3.4 | |
| VE-VCO2-Slope | 32 | −15.1 | −19.1 to −11.1 | −216.3 | −311.8 to −120.8 | −15.9 | −21.9 to −9.9 | |
| Men | VE/VCO2 | 13 | −11.2 | −19.7 to −2.7 | −190.4 | −411.1 to 30.3 | −10.0 | −26.2 to 6.2 |
| PETCO2 | 12 | −9.3 | −18.1 to −0.6 | −169.8 | −379.9 to 40.4 | −7.5 | −24.7 to 9.7 | |
| VE-VCO2-Slope | 13 | −18.4 | −25.1 to −11.6 | −335.5 | −527.6 to −143.4 | −22.3 | −33.9 to −10.7 | |
| Women | VE/VCO2 | 18 | −6.7 | −11.1 to 2.2 | −32.4 | −120.8 to 56.0 | −3.9 | −10.7 to 3.0 |
| PETCO2 | 19 | −5.0 | −9.4 to −0.6 | −0.7 | −88.6 to 87.1 | −1.1 | −6.6 to 4.5 | |
| VE-VCO2-Slope | 19 | −12.8 | −18.0 to −7.6 | −134.7 | −226.5 to −43.0 | −11.6 | −18.1 to −5.1 | |
| Gender | Characteristics | N | Pulse Rate (bpm) | VO2 (mL/min) | Load (watts) | |||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |||
| All | VE/VCO2 | 31 | 0.74 | <0.01 | 0.88 | <0.01 | 0.88 | <0.01 |
| PETCO2 | 31 | 0.75 | <0.01 | 0.88 | <0.01 | 0.84 | <0.01 | |
| VE-VCO2-Slope | 32 | 0.74 | <0.01 | 0.90 | <0.01 | 0.92 | <0.01 | |
| Men | VE/VCO2 | 13 | 0.54 | 0.057 | 0.78 | <0.01 | 0.82 | <0.01 |
| PETCO2 | 12 | 0.58 | 0.048 | 0.77 | <0.01 | 0.67 | 0.017 | |
| VE-VCO2-Slope | 13 | 0.73 | <0.01 | 0.83 | <0.01 | 0.89 | <0.01 | |
| Women | VE/VCO2 | 18 | 0.86 | <0.01 | 0.84 | <0.01 | 0.77 | <0.01 |
| PETCO2 | 19 | 0.87 | <0.01 | 0.84 | <0.01 | 0.84 | <0.01 | |
| VE-VCO2-Slope | 19 | 0.79 | <0.01 | 0.82 | <0.01 | 0.79 | <0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, M.; Ushio, K.; Tsubokawa, M.; Fukuhara, K.; Iwamoto, Y.; Iwaki, D.; Nakashima, Y.; Nakamura, T.; Mikami, Y. A Novel Method to Determine the Respiratory Compensation Point from Percutaneous Oxygen Saturation of Healthy Adults During a Ramp-Incremental Test: A Cross-Sectional Study. Med. Sci. 2025, 13, 192. https://doi.org/10.3390/medsci13030192
Abe M, Ushio K, Tsubokawa M, Fukuhara K, Iwamoto Y, Iwaki D, Nakashima Y, Nakamura T, Mikami Y. A Novel Method to Determine the Respiratory Compensation Point from Percutaneous Oxygen Saturation of Healthy Adults During a Ramp-Incremental Test: A Cross-Sectional Study. Medical Sciences. 2025; 13(3):192. https://doi.org/10.3390/medsci13030192
Chicago/Turabian StyleAbe, Masatsugu, Kai Ushio, Masaya Tsubokawa, Koki Fukuhara, Yoshitaka Iwamoto, Daisuke Iwaki, Yuki Nakashima, Takeshi Nakamura, and Yukio Mikami. 2025. "A Novel Method to Determine the Respiratory Compensation Point from Percutaneous Oxygen Saturation of Healthy Adults During a Ramp-Incremental Test: A Cross-Sectional Study" Medical Sciences 13, no. 3: 192. https://doi.org/10.3390/medsci13030192
APA StyleAbe, M., Ushio, K., Tsubokawa, M., Fukuhara, K., Iwamoto, Y., Iwaki, D., Nakashima, Y., Nakamura, T., & Mikami, Y. (2025). A Novel Method to Determine the Respiratory Compensation Point from Percutaneous Oxygen Saturation of Healthy Adults During a Ramp-Incremental Test: A Cross-Sectional Study. Medical Sciences, 13(3), 192. https://doi.org/10.3390/medsci13030192






