Bone Mineral Density and Serum Levels of Bone Remodeling Markers in Ankylosing Spondylitis Treated with Anti TNF-α Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Subjects
2.3. Clinical Setting
2.4. Bone Mineral Density (BMD) Measurements
2.5. Quantification of Serum DKK-1, SOST, BMP-6, and Cytokines Levels
2.6. Statistical Analysis
3. Results
3.1. Characteristics of AS Patients
3.2. Comparison of Characteristics Between AS vs. Healthy Donors
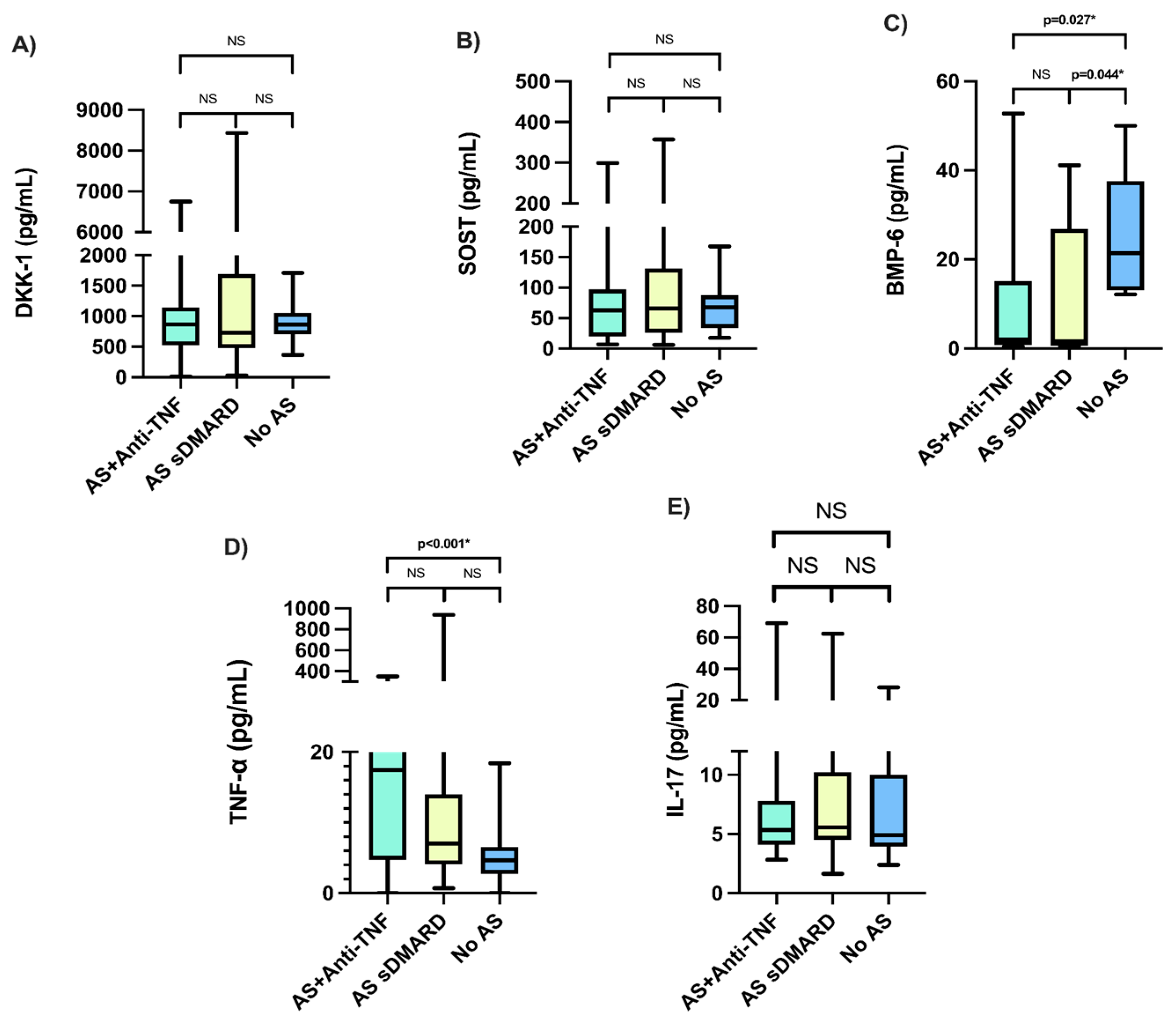
3.3. Comparison of Characteristics, Serum DKK-1, SOST, BMP-6, and Cytokines in AS Study Groups
3.4. Post Hoc Analysis
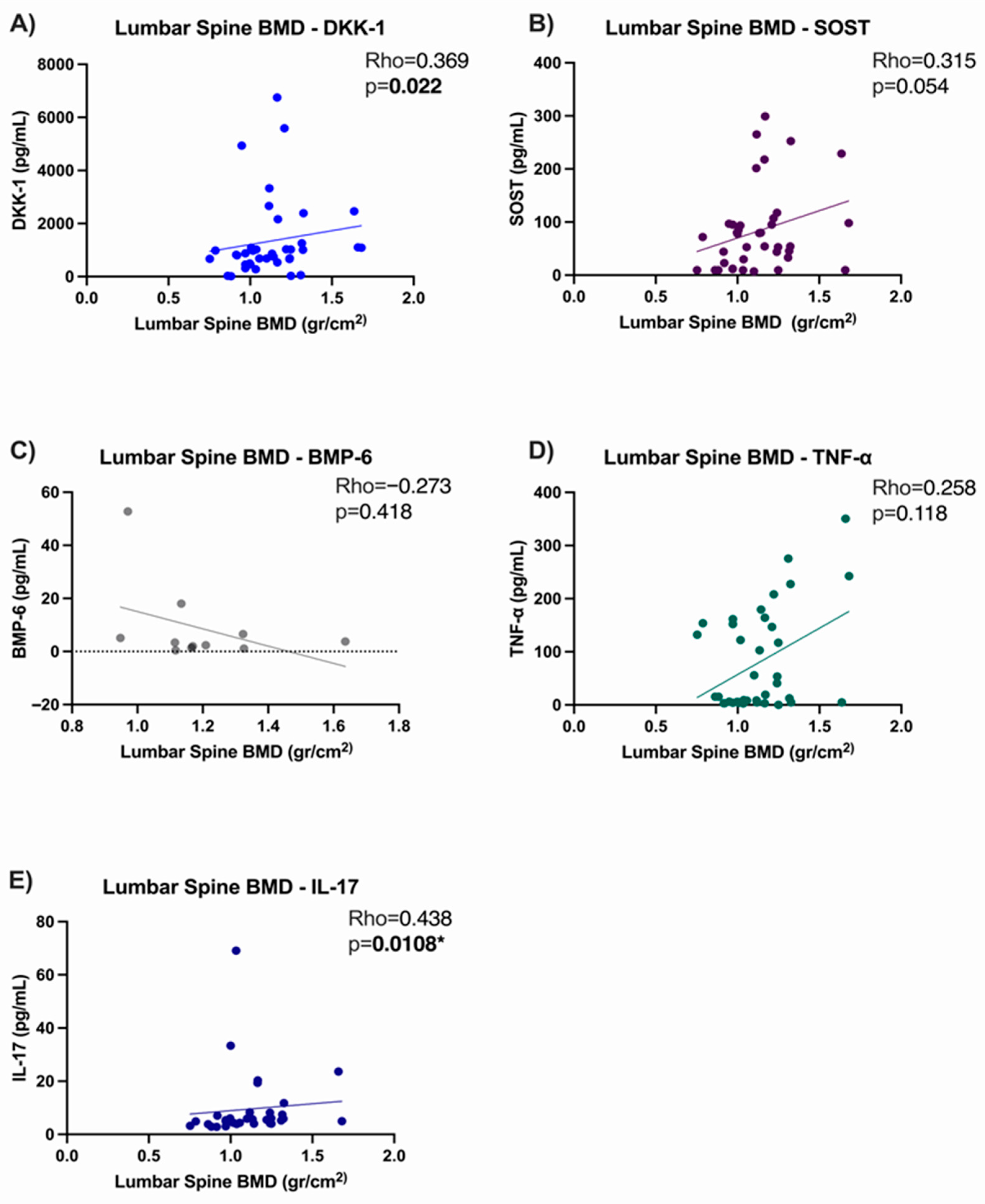
3.5. Correlation Analysis
4. Discussion
Limitations and Strengths of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | ankylosing spondylitis |
| csDMARDs | conventional synthetic disease-modifying antirheumatic drugs |
| bDMARDs | biologic disease-modifying antirheumatic drugs |
| TNF-α | tumor necrosis factor-alpha |
| IL-17 | interleukin-17 |
| BMD | bone mineral density |
| DKK-1 | dickkopf-1 |
| SOST | sclerostin |
| BMP-6 | bone morphogenetic protein-6 |
| INTEC | Institute of Experimental and Clinical Therapeutics |
| BASDAI | bath ankylosing spondylitis disease activity index |
| BASFI | bath ankylosing spondylitis functional index |
| DXA | dual-energy X-ray absorptiometry |
| ELISA | enzyme-linked immunosorbent assay |
| NSAID | non-steroidal anti-inflammatory drugs |
| BMI | body mass index |
References
- Raychaudhuri, S.P.; Deodhar, A. The classification and diagnostic criteria of ankylosing spondylitis. J. Autoimmun. 2014, 48–49, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Taurog, J.D.; Chhabra, A.; Colbert, R.A. Ankylosing spondylitis and axial spondyloarthritis. N. Engl. J. Med. 2016, 374, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Cordero, G.; Enríquez-Sosa, F.; Gomez-Ruiz, C.; Gonzalez-Diaz, V.; Castillo-Ortiz, J.D.; Duran-Barragán, S.; Duran-Ortiz, J.S.; Espinosa-Morales, R.; Gamez-Nava, J.I.; Gonzalez-Lopez, L.; et al. Recommendations of the Mexican College of Rheumatology for the management of spondyloarthritis. Reum. Clin. 2021, 17, 37–45. [Google Scholar] [CrossRef]
- de Morales, J.M.G.R.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef]
- Gamez-Nava, J.; de la Cerda-Trujillo, L.; Vazquez-Villegas, M.; Cons-Molina, F.; Alcaraz-Lopez, M.; Zavaleta-Muñiz, S.; Rocha-Muñoz, A.; Martinez-Garcia, E.; Corona-Sanchez, E.; Salazar-Paramo, M.; et al. Association between bone turnover markers, clinical variables, spinal syndesmophytes and bone mineral density in Mexican patients with ankylosing spondylitis. Scand. J. Rheumatol. 2016, 45, 480–490. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; Michopoulos, A.; Yovos, J.G. PTH and PTHR1 in osteocytes: New insights into old partners. Hormones 2017, 16, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Yavropoulou, M.P.; Yovos, J.G. The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones 2007, 6, 279–294. [Google Scholar] [CrossRef]
- Omran, A.; Atanasova, D.; Landgren, F.; Magnusson, P. Sclerostin: From molecule to clinical biomarker. Int. J. Mol. Sci. 2022, 23, 4751. [Google Scholar] [CrossRef]
- Liao, H.T.; Lin, Y.F.; Tsai, C.Y.; Chou, T.C. Bone morphogenetic proteins and Dickkopf-1 in ankylosing spondylitis. Scand. J. Rheumatol. 2018, 47, 56–61. [Google Scholar] [CrossRef]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar]
- Calin, A.; Garrett, S.; Whitelock, H.; Kennedy, L.G.; O’Hea, J.; Mallorie, P.; Jenkinson, T. A new approach to defining functional ability in ankylosing spondylitis: The development of the Bath Ankylosing Spondylitis Functional Index. J. Rheumatol. 1994, 21, 2281–2285. [Google Scholar]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- Tamayo, J.; Díaz, R.; Lazcano-Ponce, E.; Muñoz, M.; Huitrón, G.; Halley, E.; Díaz-Montiel, J.C.; Mudgal, J.; Hernández-Ávila, M.; Salmerón, J. Reference values for areal bone mineral density among a healthy Mexican population. Salud Publica Mex. 2009, 51, S56–S83. [Google Scholar] [CrossRef]
- van der Weijden, M.A.C.; Claushuis, T.A.M.; Nazari, T.; Lems, W.F.; Dijkmans, B.A.C.; van der Horst-Bruinsma, I.E. High prevalence of low bone mineral density in patients within 10 years of onset of ankylosing spondylitis: A systematic review. Clin. Rheumatol. 2012, 31, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Pray, C.; Feroz, N.I.; Haroon, N.N. Bone mineral density and fracture risk in ankylosing spondylitis: A meta-analysis. Calcif. Tissue Int. 2017, 101, 182–192. [Google Scholar] [CrossRef]
- Capaci, K.; Hepguler, S.; Argin, M.; Tas, I. Bone mineral density in mild and advanced ankylosing spondylitis. Yonsei Med. J. 2003, 44, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Fakhreldin, S.; Abdallah, M.M.; Al-Tohamy, M.Y.; Zayed, H.S. Bone mineral density in ankylosing spondylitis: Relation to disease activity, functional capacity, spinal mobility and radiological damage. Egypt. Rheumatol. 2020, 42, 297–301. [Google Scholar] [CrossRef]
- Gulyás, K.; Horváth, Á.; Végh, E.; Pusztai, A.; Szentpétery, Á.; Pethö, Z.; Váncsa, A.; Bodnár, N.; Csomor, P.; Hamar, A.; et al. Effects of 1-year anti-TNF-α therapies on bone mineral density and bone biomarkers in rheumatoid arthritis and ankylosing spondylitis. Clin. Rheumatol. 2020, 39, 167–175. [Google Scholar] [CrossRef]
- Haroon, N.N.; Sriganthan, J.; Al Ghanim, N.; Inman, R.D.; Cheung, A.M. Effect of TNF-alpha inhibitor treatment on bone mineral density in patients with ankylosing spondylitis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2014, 44, 155–161. [Google Scholar] [CrossRef]
- Lowery, J.W.; Rosen, V. The BMP pathway and its inhibitors in the skeleton. Physiol. Rev. 2018, 98, 2431–2452. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.H.; Hatzopoulos, A.K. Bone morphogenetic protein signaling in inflammation. Exp. Biol. Med. 2019, 244, 147–156. [Google Scholar] [CrossRef]
- Yamazaki, M.; Fukushima, H.; Shin, M.; Katagiri, T.; Doi, T.; Takahashi, T.; Jimi, E. Tumor necrosis factor alpha represses bone morphogenetic protein (BMP) signaling by interfering with the DNA binding of Smads through the activation of NF-kappaB. J. Biol. Chem. 2009, 284, 35987–35995. [Google Scholar] [CrossRef] [PubMed]
- Siderius, M.; Spoorenberg, A.; Kroese, F.G.M.; van der Veer, E.; Arends, S. After an initial balance favoring collagen formation and mineralization, bone turnover markers return to pre-treatment levels during long-term TNF-α inhibition in patients with ankylosing spondylitis. PLoS ONE 2023, 18, e0283579. [Google Scholar] [CrossRef] [PubMed]
- Arends, S.; Spoorenberg, A.; Brouwer, E.; van der Veer, E. Clinical studies on bone-related outcome and the effect of TNF-α blocking therapy in ankylosing spondylitis. Curr. Opin. Rheumatol. 2014, 26, 259–268. [Google Scholar] [CrossRef]
- Arends, S.; van der Veer, E.; Kallenberg, C.G.; Brouwer, E.; Spoorenberg, A. Baseline predictors of response to TNF-α blocking therapy in ankylosing spondylitis. Curr. Opin. Rheumatol. 2012, 24, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Lee, S.H.; Lee, H.T.; Lee, J.U.; Son, J.Y.; Shin, W.; Heo, Y.-S. Structural biology of the TNFα antagonists used in the treatment of rheumatoid arthritis. Int. J. Mol. Sci. 2018, 19, 768. [Google Scholar] [CrossRef]
- Dogan, S.; Kimyon, G.; Ozkan, H.; Kacmaz, F.; Camdeviren, B.; Karaaslan, I. TNF-alpha, IL-6, IL-10 and fatty acids in rheumatoid arthritis patients receiving cDMARD and bDMARD therapy. Clin. Rheumatol. 2022, 41, 2341–2349. [Google Scholar] [CrossRef]
- Schulz, M.; Dotzlaw, H.; Neeck, G. Ankylosing spondylitis and rheumatoid arthritis: Serum levels of TNF-α and its soluble receptors during the course of therapy with etanercept and infliximab. Biomed Res. Int. 2014, 2014, 675108. [Google Scholar] [CrossRef]
- Zamora-Atenza, C.; Diaz-Torne, C.; Geli, C.; Diaz-Lopez, C.; Ortiz, M.A.; Moya, P.; Castellví, I.; Nieto, J.C.; Cantó, E.; Casademont, J.; et al. Adalimumab regulates intracellular TNFα production in patients with rheumatoid arthritis. Arthritis Res. Ther. 2014, 16, R153. [Google Scholar] [CrossRef]
- Ustun, N.; Tok, F.; Kalyoncu, U.; Motor, S.; Yuksel, R.; E Yagiz, A.; Guler, H.; Turhanoglu, A.D. Sclerostin and Dkk-1 in patients with ankylosing spondylitis. Acta Reum. Port. 2014, 39, 146–151. [Google Scholar] [PubMed]
- Descamps, E.; Molto, A.; Borderie, D.; Lories, R.; Richard, C.M.; Pons, M.; Roux, C.; Briot, K. Changes in bone formation regulator biomarkers in early axial spondyloarthritis. Rheumatology 2021, 60, 1185–1194. [Google Scholar] [CrossRef]
- Korkosz, M.; Gąsowski, J.; Leszczyński, P.; Pawlak-Buś, K.; Jeka, S.; Siedlar, M.; Grodzicki, T. Effect of tumour necrosis factor-α inhibitor on serum level of dickkopf-1 protein and bone morphogenetic protein-7 in ankylosing spondylitis patients with high disease activity. Scand. J. Rheumatol. 2014, 43, 43–48. [Google Scholar] [CrossRef]
- Sikorska, D.; Rutkowski, R.; Łuczak, J.; Samborski, W.; Witowski, J. No effect of anti-TNF-α treatment on serum IL-17 in patients with rheumatoid arthritis. Cent. Eur. J. Immunol. 2018, 43, 270–275. [Google Scholar] [CrossRef]
- Katz, L.H.; Kopylov, U.; Fudim, E.; Yavzori, M.; Picard, O.; Ungar, B.; Eliakim, R.; Ben-Horin, S.; Chowers, Y. Expression of IL-2, IL-17 and TNF-alpha in patients with Crohn’s disease treated with anti-TNF antibodies. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 491–498. [Google Scholar] [CrossRef]
- Vasiliadis, E.S.; Evangelopoulos, D.S.; Kaspiris, A.; Benetos, I.S.; Vlachos, C.; Pneumaticos, S.G. The role of sclerostin in bone diseases. J. Clin. Med. 2022, 11, 806. [Google Scholar] [CrossRef]
- Rossini, M.; Viapiana, O.; Idolazzi, L.; Ghellere, F.; Fracassi, E.; Troplini, S.; Povino, M.R.; Kunnathully, V.; Adami, S.; Gatti, D. Higher level of dickkopf-1 is associated with low bone mineral density and higher prevalence of vertebral fractures in patients with ankylosing spondylitis. Calcif. Tissue Int. 2016, 98, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Dong, Y.; Zheng, M.; Kang, H.; Wang, P.; Zhu, M.; Song, K.; Wu, W.; Li, F. IL-17 promotes osteoclast-induced bone loss by regulating glutamine-dependent energy metabolism. Cell Death Dis. 2024, 15, 111. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, J.M.; Grahnemo, L.; Engdahl, C.; Drevinge, C.; Gustafsson, K.L.; Corciulo, C.; Lawenius, L.; Iwakura, Y.; Sjögren, K.; Lagerquist, M.K.; et al. Interleukin 17A: A Janus-faced regulator of osteoporosis. Sci. Rep. 2020, 10, 5692. [Google Scholar] [CrossRef] [PubMed]
- Croes, M.; Öner, F.C.; van Neerven, D.; Sabir, E.; Kruyt, M.C.; Blokhuis, T.J.; Dhert, W.J.; Alblas, J. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone 2016, 84, 262–270. [Google Scholar] [CrossRef]
- Ehnert, S.; Baur, J.; Schmitt, A.; Neumaier, M.; Lucke, M.; Dooley, S.; Vester, H.; Wildemann, B.; Stöckle, U.; Nussler, A.K.; et al. TGF-β1 as possible link between loss of bone mineral density and chronic inflammation. PLoS ONE 2010, 5, e14073. [Google Scholar] [CrossRef]
- van Bezooijen, R.L.; Svensson, J.P.; Eefting, D.; Visser, A.; van der Horst, G.; Karperien, M.; Quax, P.H.; Vrieling, H.; E Papapoulos, S.; Dijke, P.T.; et al. Wnt but not BMP signaling is involved in the inhibitory action of sclerostin on BMP-stimulated bone formation. J. Bone Min. Res. 2007, 22, 19–28. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | AS Patients (n = 76) |
|---|---|
| Sociodemographic Characteristics | |
| Male Gender, n (%) | 46 (60.5) |
| Age (Years), Median (Min–Max) | 49 (18–69) |
| BMI (kg/m2), Median (Min–Max) | 26.5 (16.40–40.86) |
| Disease Characteristics | |
| Disease Evolution (Years), Median (Min–Max) | 10 (1–33) |
| BASDAI (Score), Median (Min–Max) | 5.35 (0.4–9.5) |
| BASFI (Score), Median (Min–Max) | 3.9 (0–10) |
| Bone Mineral Density | |
| BMD in Lumbar Spine, L1–L4 (gr/cm2), Median (Min–Max) | 1.11 (0.75–1.68) |
| Low BMD in Lumbar Spine, L1–L4, n (%) | 24 (31.6) |
| BMD in Total Hip (gr/cm2), Median (Min–Max) | 0.94 (0.63–1.30) |
| Low BMD in Total Hip, n (%) | 16 (21.1) |
| Results of BMD, n (%) | |
| Osteoporosis, n (%) | 8 (10.5) |
| Osteopenia, n (%) | 16 (21.1) |
| Characteristics | AS Patients (n = 76) | Healthy Donors (n = 30) | p |
|---|---|---|---|
| Sociodemographic Characteristics | |||
| Male Gender, n (%) | 46 (60.5) | 16 (53.3) | 0.498 |
| Age (Years), Median (Min–Max) | 49 (18–69) | 46 (23–64) | 0.096 |
| BMI (kg/m2), Median (Min–Max) | 26.5 (16.40–40.86) | 28.75 (20.19–40.96) | 0.014 |
| Bone Mineral Density | |||
| BMD in Lumbar Spine, L1–L4 (gr/cm2), Median (Min–Max) | 1.11 (0.75–1.68) | 1.19 (0.92–1.44) | 0.101 |
| Low BMD in Lumbar Spine, L1–L4, n (%) | 24 (31.6) | 2 (6.7) | 0.024 |
| BMD in Total Hips (gr/cm2), Median (Min–Max) | 0.94 (0.634–1.30) | 1.06 (0.81–1.34) | <0.001 |
| Low BMD in Total Hips, n (%) | 16 (21.1) | 1 (3.3) | 0.077 |
| Serum Bone turnover markers and cytokines levels | |||
| DKK-1, (pg/mL), Median (Min–Max) | 815.90 (13.77–8434.50) | 862.60 (368.0–1710.0) | 0.742 |
| SOST, (pg/mL), Median (Min–Max) | 65.82 (6.48–357.32) | 67.85 (17.54–167.80) | 0.757 |
| BMP-6, (pg/mL), Median (Min–Max) | 2.05 (0.41–52.77) | 21.44 (12.17–50.0) | 0.001 |
| TNF-α, (pg/mL), Median (Min–Max) | 8.87 (0.01–938.19) | 4.66 (0.01–18.4) | <0.001 |
| IL-17, (pg/mL), Median (Min–Max) | 5.44 (1.65–69.07) | 4.92 (2.40–28.19) | 0.371 |
| Characteristics | Anti-TNF + csDMARD (n = 38) | csDMARD (n = 38) | p |
|---|---|---|---|
| Sociodemographic Characteristics | |||
| Male Gender, n (%) | 27 (71.1) | 19 (50) | 0.050 |
| Age (years), Median (Min–Max) | 43 (18–69) | 53 (24–66) | 0.007 |
| BMI (kg/m2), Median (Min–Max) | 25.88 (16.59–40.86) | 27.38 (16.40–39.94) | 0.309 |
| Disease Evolution (Years), Median (Min–Max) | 10 (1–33) | 10 (1–30) | 0.625 |
| Clinical Disease Index | |||
| BASDAI (Score), Median (Min–Max) | 5.1 (0.4–9.5) | 5.9 (1.4–9.2) | 0.285 |
| BASFI (Score), Median (Min–Max) | 3.7 (0–9.5) | 3.9 (0.8–10.0) | 0.394 |
| Bone Mineral Density | |||
| BMD in Lumbar Spine, L1–L4 (gr/cm2), Median (Min–Max) | 1.11 (0.75–1.68) | 1.10 (0.79–1.55) | 0.930 |
| Low BMD in Lumbar Spine, L1–L4 | 12 (31.6) | 12 (31.6) | 1.000 |
| BMD in Total Hips (gr/cm2), Median (Min–Max) | 0.94 (0.66–1.30) | 0.93 (0.63–1.26) | 0.876 |
| Low BMD in Total Hips, n (%) | 8 (21.1) | 8 (21.1) | 1.000 |
| Low BMD in Any Region, n (%) | 14 (36.8) | 14 (36.8) | 1.000 |
| Serum Bone Turnover Markers and Cytokine Levels | |||
| DKK-1, (pg/mL), Median (Min–Max) | 868.45 (13.77–6748.21) | 730.85 (31.30–8434.54) | 0.815 |
| SOST, (pg/mL), Median (Min–Max) | 63.09 (7.19–299.12) | 65.82 (6.48–357.32) | 0.771 |
| BMP-6, (pg/mL), Median (Min–Max) | 3.34 (0.41–52.77) | 1.60 (0.58–41.16) | 0.451 |
| TNF-α, (pg/mL), Median (Min–Max) | 17.42 (0.013–350.60) | 7.02 (0.72–938.19) | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Ayala, E.G.; Gamez-Nava, J.I.; Saldaña-Cruz, A.M.; Gonzalez-Ponce, F.; Contreras-Haro, B.; Ramirez-Villafaña, M.; Perez-Guerrero, E.E.; Alcaraz-Lopez, M.F.; Gomez-Ramirez, E.E.; Ponce-Guarneros, J.M.; et al. Bone Mineral Density and Serum Levels of Bone Remodeling Markers in Ankylosing Spondylitis Treated with Anti TNF-α Agents. Med. Sci. 2025, 13, 189. https://doi.org/10.3390/medsci13030189
Alvarez-Ayala EG, Gamez-Nava JI, Saldaña-Cruz AM, Gonzalez-Ponce F, Contreras-Haro B, Ramirez-Villafaña M, Perez-Guerrero EE, Alcaraz-Lopez MF, Gomez-Ramirez EE, Ponce-Guarneros JM, et al. Bone Mineral Density and Serum Levels of Bone Remodeling Markers in Ankylosing Spondylitis Treated with Anti TNF-α Agents. Medical Sciences. 2025; 13(3):189. https://doi.org/10.3390/medsci13030189
Chicago/Turabian StyleAlvarez-Ayala, Efren Gerardo, Jorge Ivan Gamez-Nava, Ana Miriam Saldaña-Cruz, Fabiola Gonzalez-Ponce, Betsabe Contreras-Haro, Melissa Ramirez-Villafaña, Edsaul Emilio Perez-Guerrero, Miriam Fabiola Alcaraz-Lopez, Eli Efrain Gomez-Ramirez, Juan Manuel Ponce-Guarneros, and et al. 2025. "Bone Mineral Density and Serum Levels of Bone Remodeling Markers in Ankylosing Spondylitis Treated with Anti TNF-α Agents" Medical Sciences 13, no. 3: 189. https://doi.org/10.3390/medsci13030189
APA StyleAlvarez-Ayala, E. G., Gamez-Nava, J. I., Saldaña-Cruz, A. M., Gonzalez-Ponce, F., Contreras-Haro, B., Ramirez-Villafaña, M., Perez-Guerrero, E. E., Alcaraz-Lopez, M. F., Gomez-Ramirez, E. E., Ponce-Guarneros, J. M., Rodriguez-Jimenez, N. A., Totsuka-Sutto, S. E., Rocha-Muñoz, A. D., Muñoz-Miranda, L. A., Gonzalez-Lopez, L., & Nava-Valdivia, C. A. (2025). Bone Mineral Density and Serum Levels of Bone Remodeling Markers in Ankylosing Spondylitis Treated with Anti TNF-α Agents. Medical Sciences, 13(3), 189. https://doi.org/10.3390/medsci13030189







