Metabolic Reprogramming Through Polyphenol Networks: A Systems Approach to Metabolic Inflammation and Insulin Resistance
Abstract
1. Introduction
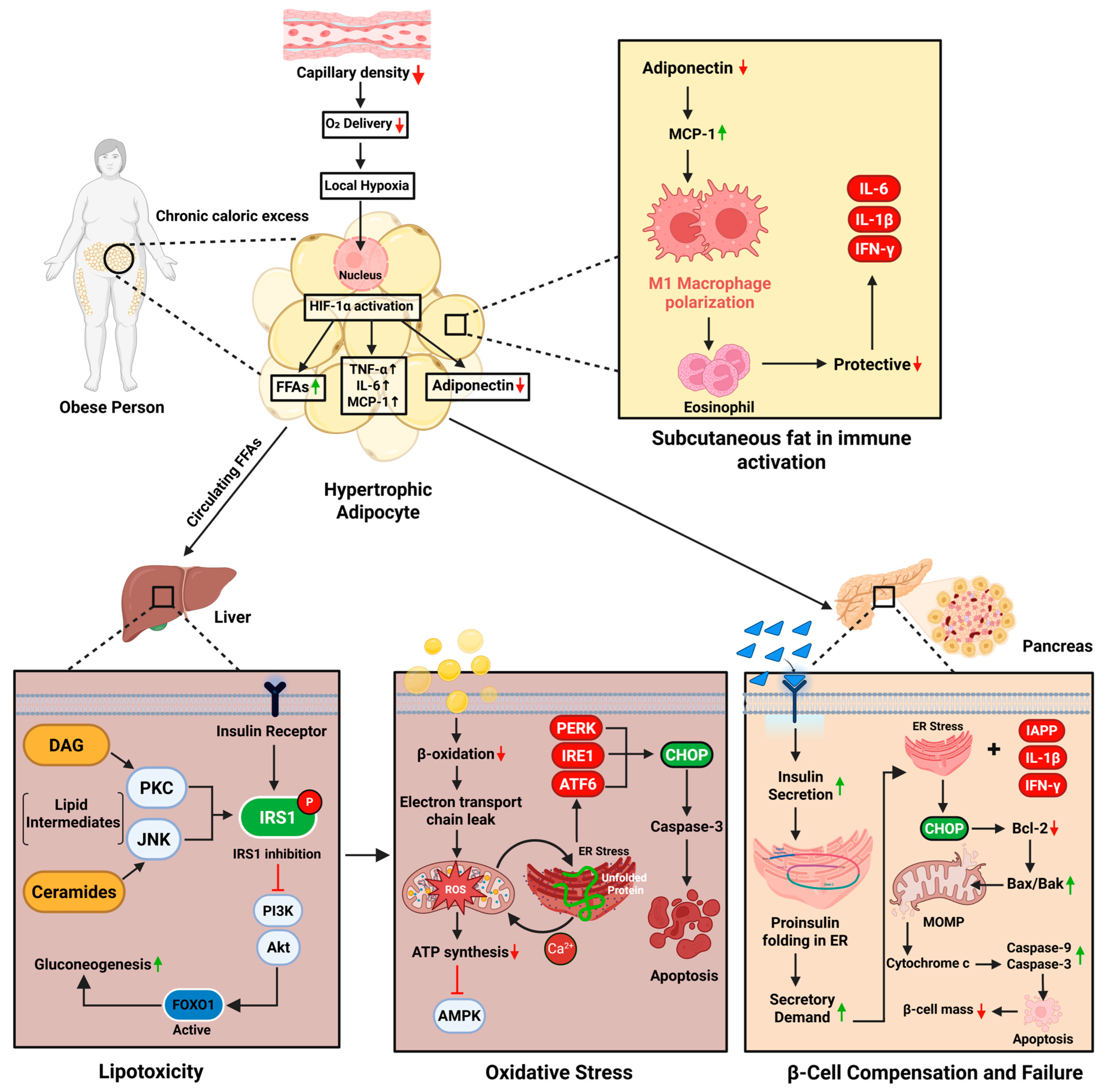
2. Pathophysiology of Obesity-Induced Diabetes
2.1. Lipotoxicity and Free Fatty Acid-Mediated Insulin Resistance
2.2. Oxidative Stress, Mitochondrial Dysfunction, and ER Disruption
2.3. β-Cell Compensation and Failure
2.4. Adipose Tissue Dysfunction and Immune Activation
3. Citrus Polyphenols in Metabolic Reprogramming
3.1. Sour Yet Sweet Salvation: How Citrus Polyphenols Rewire Diabetic Metabolism
3.2. Citrus Polyphenols and Inflammatory Reprogramming in Diabesity
3.3. Role of Citrus in Mitochondrial Health and Endoplasmic Reticulum Stress: Restoring Protein Homeostasis
3.4. Free Radicals, Oxidative Stress, and Citrus Polyphenols: A Natural Line of Defense
3.5. Translating Mechanisms to Humans: Clinical Evidence of Citrus Polyphenol-Driven Metabolic Reprogramming
| Compound(s) | Study Design and Population | Intervention Details (Dose and Duration) | Reported Outcomes | Mechanism of Action | Source |
|---|---|---|---|---|---|
| Neoeriocitrin, Naringin, Neohesperidin | RCT, overweight adults with MASLD (n = 80) | Bergamot extract (500–1000 mg/day, 12 weeks) | ↓ Liver fat content (−18%), ↓ body weight (−5% vs. placebo) | Enhances bile flow; antioxidant activity reduces oxidative stress | [166] |
| Hesperidin, Naringin, Neohesperidin | RCT, metabolic syndrome (n = 95) | Mixed citrus extracts (500 mg/day, 8 weeks) | ↑ Endothelial function (FMD ↑12%), improved vascular tone | Antioxidant effects improve vascular inflammation and nitric oxide availability | [167] |
| Hesperidin → Hesperetin; SCFAs | Clinical trial, healthy volunteers (n = 40) | Citrus fruit extract (500 mg/day, 4 weeks) | Gut microbiota modulation: ↑ Bifidobacterium, ↑ SCFA production; ↓ systemic inflammation | Hesperidin metabolized to hesperetin → SCFA-mediated endothelial protection and anti-inflammatory response | [168] |
| Flavones, Flavanones, Oleuropein | RCT, high-CV-risk adults (n = 120) | Citrus + olive polyphenol mix (500 mg/day, 12 weeks) | ↓ Cardiovascular risk biomarkers; ↓ hs-CRP (−20%); improved metabolic-inflammatory profile | Antioxidant activity; NF-κB inhibition | [169] |
| Hesperidin, Naringin, Oleuropein | RCT, adults with dyslipidemia (n = 72) | Citrus + olive leaf extracts (500 mg/day, 10 weeks) | ↓ LDL oxidation (−12%), ↓ TNF-α (−18%), ↓ IL-6 (−15%) | Free radical scavenging; cytokine modulation | [170] |
| Hesperidin | RCT, obese adults (n = 64) | Orange juice (Citrus sinensis, ~500 mL/day, 12 weeks) | ↓ BMI (−1.2 kg/m2), ↓ waist circumference (−3.4 cm), ↓ IL-1β, IL-6, TNF-α | Inhibits pro-inflammatory cytokine release; antioxidant endothelial protection | [171] |
| Hesperidin | RCT, NAFLD patients (n = 82) | Hesperidin 1 g/day + lifestyle changes (12 weeks) | ↓ Liver fat (−22%), ↓ ALT (−30%), ↓ TG (−18%), ↓ weight (−4 kg) | NF-κB inhibition; ↓ TNF-α, ↓ hs-CRP | [172] |
| Hesperidin (meta-analysis) | Meta-analysis of RCTs (n = 525 metabolic subjects) | Hesperidin (500–1000 mg/day, 4–12 weeks) | ↓ TG, ↓ TC, ↓ LDL (especially in BMI >30); ↓ TNF-α, ↓ IL-6 at higher doses | Anti-inflammatory; lipid-lowering | [173] |
| Orange juice (flavonoids) | 4-week RCT, MASLD patients (n = 62) | Orange juice (500 mL/day) | ↓ Liver steatosis (by FibroScan), ↓ GGT (−10%) | Antioxidant effect; modest anti-inflammatory | [174] |
| Flavonoid-enriched orange juice | RCT, metabolic syndrome patients (n = 48) | Enriched OJ (500 mL/day, 6 weeks) | ↑ Antioxidant status (TAC ↑15%), improved glycemic trend | ↓ CRP, ↓ endothelial inflammation | [175] |
| Hesperidin | RCT, vascular function study (n = 24 metabolic syndrome patients) | Hesperidin 500 mg/day, 3 weeks | ↑ FMD (+12%), ↓ IL-6 (−15%), ↓ TNF-α (−12%) | ↑ NO bioavailability; ↓ inflammatory cytokines | [22] |
| Eriomin® (Eriocitrin) | Crossover RCT, prediabetes patients (n = 103) | Eriomin® 200–500 mg/day, 12 weeks | ↓ FBG (−5.5 mg/dL), ↓ HOMA-IR (−18%), ↑ GLP-1 (+15%), ↑ adiponectin (+20%) | ↓ IL-6, TNF-α, hs-CRP | [176] |
| Polyphenols incl. Naringenin | Meta-analysis in NAFLD patients (12 RCTs, n ≈ 950) | Various flavonoids (6–12 weeks) | ↓ BMI, ↓ ALT (−12%), ↓ AST (−10%), ↓ TG (−18%), ↓ TNF-α (−14%) | Anti-inflammatory; metabolic reprogramming | [177] |
4. Role of Lipoproteins in Diabetes and the Impact of Citrus Polyphenols
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ATF3 | Activating transcription factor 3 |
| BMI | Body mass index |
| CPT1 | Carnitine palmitoyltransferase 1 |
| CRP/hs-CRP | C-reactive protein/high-sensitivity C-reactive protein |
| ER | Endoplasmic reticulum |
| FA | Fatty acid |
| FAS | Fatty acid synthase |
| FMD | Flow-mediated dilation |
| FOXO1 | Forkhead box protein O1 |
| GGT | Gamma-glutamyl transferase |
| GLP-1 | Glucagon-like peptide-1 |
| GLUT4 | Glucose transporter type 4 |
| HbA1c | Glycated hemoglobin A1c |
| HDL | High-density lipoprotein |
| HFD | High-fat diet |
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase |
| HOMA-IR | Homeostatic model assessment of insulin resistance |
| IL-1β, IL-6 | Interleukin-1 beta, Interleukin-6 |
| IRS1 | Insulin receptor substrate 1 |
| JNK | c-Jun N-terminal kinase |
| LDL | Low-density lipoprotein |
| MAPK | Mitogen-activated protein kinase |
| MASLD | Metabolic dysfunction–associated steatotic liver disease |
| MCD | Methionine-choline deficient diet |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 inflammasome |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| OXPHOS | Oxidative phosphorylation |
| PERK | PKR-like ER kinase |
| IRE1 | Inositol-requiring enzyme 1 |
| ATF6 | Activating transcription factor 6 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PI3K/AKT | Phosphoinositide 3-kinase/protein kinase B pathway |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| ROS | Reactive oxygen species |
| SCFA | Short-chain fatty acid |
| SOD | Superoxide dismutase |
| SREBP1c | Sterol regulatory element-binding protein 1c |
| TFEB | Transcription factor EB |
| TG/TC | Triglycerides/Total cholesterol |
| TNF-α | Tumor necrosis factor-alpha |
| T2DM | Type 2 diabetes mellitus |
| UPR | Unfolded protein response |
| UCP2 | Uncoupling protein 2 |
| VLDL | Very-low-density lipoprotein |
References
- Allocca, S.; Monda, A.; Messina, A.; Casillo, M.; Sapuppo, W.; Monda, V.; Polito, R.; Di Maio, G.; Monda, M.; La Marra, M. Endocrine and Metabolic Mechanisms Linking Obesity to Type 2 Diabetes: Implications for Targeted Therapy. Healthcare 2025, 13, 1437. [Google Scholar] [CrossRef]
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity; MD Text.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [PubMed]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Kocatepe, D.; Büyükkol, D.C.; Hinislioğlu, K.N. Obesity Prevalence in World and Türkiye. North. J. Health Sci. 2025, 1, 26–32. [Google Scholar]
- Shimu, S.J. A 10-Year Retrospective Quantitative Analysis of The CDC Database: Examining the Prevalence of Depression in the Us Adult Urban Population. Front. Health Inform. 2024, 13, 2676–7104. [Google Scholar]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. Int. Sch. Res. Not. 2013, 2013, 139239. [Google Scholar]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front. Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef] [PubMed]
- Gkrinia, E.M.M.; Belančić, A. The Mechanisms of Chronic Inflammation in Obesity and Potential Therapeutic Strategies: A Narrative Review. Curr. Issues Mol. Biol. 2025, 47, 357. [Google Scholar] [CrossRef]
- Gudise, V.; Chowdhury, B. Molecular mechanisms and the vital roles of resistin, TLR 4, and NF-κB in treating type 2 diabetic complications. Beni-Suef Univ. J. Basic. Appl. Sci. 2020, 9, 54. [Google Scholar] [CrossRef]
- Kim, J.K. Endothelial nuclear factor κB in obesity and aging: Is endothelial nuclear factor κB a master regulator of inflammation and insulin resistance? Circulation 2012, 125, 1081–1083. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef]
- Sergi, D.; Zauli, E.; Celeghini, C.; Previati, M.; Zauli, G. Ceramides as the molecular link between impaired lipid metabolism, saturated fatty acid intake and insulin resistance: Are all saturated fatty acids to be blamed for ceramide-mediated lipotoxicity? Nutr. Res. Rev. 2024, 38, 256–266. [Google Scholar] [CrossRef]
- Römer, A.; Linn, T.; Petry, S.F. Lipotoxic impairment of mitochondrial function in β-cells: A review. Antioxidants 2021, 10, 293. [Google Scholar] [CrossRef]
- Corkey, B.E. Reactive oxygen species: Role in obesity and mitochondrial energy efficiency. Philos. Trans. R. Soc. B 2023, 378, 20220210. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, N.; Vezza, T.; Muntane, J.; Rocha, M.; Victor, V.M. Mitochondrial dysfunction and mitophagy in type 2 diabetes: Pathophysiology and therapeutic targets. Antioxid. Redox Signal. 2023, 39, 278–320. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Majumdar, A. Endoplasmic Reticulum Stress and Unfolded Protein Response in Metabolic Syndrome. In Biochemical Mechanisms for Metabolic Syndrome; Springer: Berlin/Heidelberg, Germany, 2024; pp. 203–222. [Google Scholar]
- Ghosh, R.; Colon-Negron, K.; Papa, F.R. Endoplasmic reticulum stress, degeneration of pancreatic islet β-cells, and therapeutic modulation of the unfolded protein response in diabetes. Mol. Metab. 2019, 27, S60–S68. [Google Scholar] [CrossRef]
- Aufi, S.S.A.; Hasnin, K. Diabesity An Emerging Epidemic and Advances in Treatment. Barind Med. Coll. J. 2024, 10, 29–34. [Google Scholar] [CrossRef]
- Mohib, M.; Afnan, K.; Paran, T.Z.; Khan, S.; Sarker, J.; Hasan, N.; Hasan, I.; Sagor, A.T. Beneficial role of citrus fruit polyphenols against hepatic dysfunctions: A review. J. Diet. Suppl. 2018, 15, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.-A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernandez Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxidative Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef]
- Xue, Y.; Huang, Z.; Chen, X.; Jia, G.; Zhao, H.; Liu, G. Naringin induces skeletal muscle fiber type transformation via AMPK/PGC-1α signaling pathway in mice and C2C12 myotubes. Nutr. Res. 2021, 92, 99–108. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic properties of naringenin: A citrus fruit polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef]
- Hammerschmidt, P.; Brüning, J.C. Contribution of specific ceramides to obesity-associated metabolic diseases. Cell. Mol. Life Sci. 2022, 79, 395. [Google Scholar] [CrossRef]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal kinase (JNK) in obesity and type 2 diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-T.; Kim, S.-J.; Park, S.-H.; Lee, D.; Park, T.-S. Hepatic expression of the serine palmitoyltransferase subunit Sptlc2 reduces lipid droplets in the liver by activating VLDL secretion. J. Lipid Atheroscler. 2020, 9, 291. [Google Scholar] [CrossRef]
- Elkanawati, R.Y.; Sumiwi, S.A.; Levita, J. Impact of lipids on insulin resistance: Insights from human and animal studies. Drug Des. Dev. Ther. 2024, 18, 3337–3360. [Google Scholar] [CrossRef]
- Sokolowska, E.; Blachnio-Zabielska, A. The role of ceramides in insulin resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef]
- Schrauwen, P.; Hesselink, M.K. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 2004, 53, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol.-Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Mohib, M.M.; Rabe, S.; Nolze, A.; Rooney, M.; Ain, Q.; Zipprich, A.; Gekle, M.; Schreier, B. Eplerenone, a mineralocorticoid receptor inhibitor, reduces cirrhosis associated changes of hepatocyte glucose and lipid metabolism. Cell Commun. Signal. 2024, 22, 614. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725.e6. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Weng, J. Hepatocyte NLRP3 interacts with PKCε to drive hepatic insulin resistance and steatosis. Sci. Bull. 2023, 68, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef]
- Huang, W.; Metlakunta, A.; Dedousis, N.; Zhang, P.; Sipula, I.; Dube, J.J.; Scott, D.K.; O’Doherty, R.M. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 2010, 59, 347–357. [Google Scholar] [CrossRef]
- Ziolkowska, S.; Binienda, A.; Jabłkowski, M.; Szemraj, J.; Czarny, P. The Interplay between Insulin Resistance, Inflammation, Oxidative Stress, Base Excision Repair and Metabolic Syndrome in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2021, 22, 11128. [Google Scholar] [CrossRef]
- Gruben, N.; Shiri-Sverdlov, R.; Koonen, D.P.; Hofker, M.H. Nonalcoholic fatty liver disease: A main driver of insulin resistance or a dangerous liaison? Biochim. Biophys. Acta 2014, 1842, 2329–2343. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, R.; Xiong, Y.; Du, F.; Zhu, S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017, 16, 203. [Google Scholar] [CrossRef]
- Ly, L.D.; Xu, S.; Choi, S.K.; Ha, C.M.; Thoudam, T.; Cha, S.K.; Wiederkehr, A.; Wollheim, C.B.; Lee, I.K.; Park, K.S. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017, 49, e291. [Google Scholar] [CrossRef]
- Morawietz, H.; Brendel, H.; Diaba-Nuhoho, P.; Catar, R.; Perakakis, N.; Wolfrum, C.; Bornstein, S.R. Cross-Talk of NADPH Oxidases and Inflammation in Obesity. Antioxidants 2023, 12, 1589. [Google Scholar] [CrossRef]
- Vilas-Boas, E.A.; Almeida, D.C.; Roma, L.P.; Ortis, F.; Carpinelli, A.R. Lipotoxicity and β-Cell Failure in Type 2 Diabetes: Oxidative Stress Linked to NADPH Oxidase and ER Stress. Cells 2021, 10, 3328. [Google Scholar] [CrossRef]
- Wang, S.W.; Sheng, H.; Bai, Y.-F.; Weng, Y.-Y.; Fan, X.-Y.; Lou, L.-J.; Zhang, F. Neohesperidin enhances PGC-1α-mediated mitochondrial biogenesis and alleviates hepatic steatosis in high fat diet fed mice. Nutr. Diabetes 2020, 10, 27. [Google Scholar] [CrossRef]
- Morrow, N.M.; Burke, A.C.; Samsoondar, J.P.; Seigel, K.E.; Wang, A.; Telford, D.E.; Sutherland, B.G.; O’Dwyer, C.; Steinberg, G.R.; Fullerton, M.D.; et al. The citrus flavonoid nobiletin confers protection from metabolic dysregulation in high-fat-fed mice independent of AMPK. J. Lipid Res. 2020, 61, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, X.; Wang, M.; Zhang, X.; Zhuang, R.; Wu, F.; Li, W.; Zhu, W.; Zhang, B. Liver-Specific Bmal1 Depletion Reverses the Beneficial Effects of Nobiletin on Liver Cholesterol Homeostasis in Mice Fed with High-Fat Diet. Nutrients 2023, 15, 2547. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Cha, B.-Y.; Saito, K.; Yamakawa, H.; Choi, S.-S.; Yamaguchi, K.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.-T. Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem. Pharmacol. 2010, 79, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.; Zou, J.; Wang, Y.-H.; Xu, M.-X.; Huang, W.; Yu, D.-J.; Zhang, L.; Zhang, Y.-Y.; Sun, X.-D. Naringenin Attenuates Non-Alcoholic Fatty Liver Disease by Enhancing Energy Expenditure and Regulating Autophagy via AMPK. Front. Pharmacol. 2021, 12, 687095. [Google Scholar] [CrossRef]
- Liu, X.; Wang, N.; Fan, S.; Zheng, X.; Yang, Y.; Zhu, Y.; Lu, Y.; Chen, Q.; Zhou, H.; Zheng, J. The citrus flavonoid naringenin confers protection in a murine endotoxaemia model through AMPK-ATF3-dependent negative regulation of the TLR4 signalling pathway. Sci. Rep. 2016, 6, 39735. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, S.; Biswas, M. Naringin ameliorates high-fat diet-induced hepatotoxicity and dyslipidemia in experimental rat model via modulation of anti-oxidant enzymes, AMPK and SERBP-1c signaling pathways. Toxicol. Rep. 2025, 14, 102062. [Google Scholar] [CrossRef]
- Pengnet, S.; Sumarithum, P.; Phongnu, N.; Prommaouan, S.; Kantip, N.; Phoungpetchara, I.; Malakul, W. Naringin attenuates fructose-induced NAFLD progression in rats through reducing endogenous triglyceride synthesis and activating the Nrf2/HO-1 pathway. Front. Pharmacol. 2022, 13, 1049818. [Google Scholar] [CrossRef]
- Guan, L.; Guo, L.; Zhang, H.; Liu, H.; Zhou, W.; Zhai, Y.; Yan, X.; Men, X.; Peng, L. Naringin Protects against Non-Alcoholic Fatty Liver Disease by Promoting Autophagic Flux and Lipophagy. Mol. Nutr. Food Res. 2024, 68, e2200812. [Google Scholar] [CrossRef]
- Tian, M.; Han, Y.-B.; Zhao, C.-C.; Liu, L.; Zhang, F.-L. Hesperidin alleviates insulin resistance by improving HG-induced oxidative stress and mitochondrial dysfunction by restoring miR-149. Diabetol. Metab. Syndr. 2021, 13, 50. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Massot-Cladera, M.; Garcia-Cerdà, P.; Pérez-Cano, F.J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats. Nutrients 2019, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Morshedzadeh, N.; Ahmadi, A.R.; Behrouz, V.; Mir, E. A narrative review on the role of hesperidin on metabolic parameters, liver enzymes, and inflammatory markers in nonalcoholic fatty liver disease. Food Sci. Nutr. 2023, 11, 7523–7533. [Google Scholar] [CrossRef]
- Yeh, C.H.; Shen, Z.Q.; Wang, T.W.; Kao, C.H.; Teng, Y.C.; Yeh, T.K.; Lu, C.K.; Tsai, T.F. Hesperetin promotes longevity and delays aging via activation of Cisd2 in naturally aged mice. J. Biomed. Sci. 2022, 29, 53. [Google Scholar] [CrossRef]
- Durço, A.O.; de Souza, D.S.; Heimfarth, L.; Miguel-Dos-Santos, R.; Rabelo, T.K.; Barreto, T.d.O.; Rhana, P.; Santana, M.N.S.; Braga, W.F.; Cruz, J.d.S.; et al. d-Limonene Ameliorates Myocardial Infarction Injury by Reducing Reactive Oxygen Species and Cell Apoptosis in a Murine Model. J. Nat. Prod. 2019, 82, 3010–3019. [Google Scholar] [CrossRef] [PubMed]
- Valerii, M.C.; Turroni, S.; Ferreri, C.; Zaro, M.; Sansone, A.; Dalpiaz, A.; Botti, G.; Ferraro, L.; Spigarelli, R.; Bellocchio, I.; et al. Effect of a Fiber D-Limonene-Enriched Food Supplement on Intestinal Microbiota and Metabolic Parameters of Mice on a High-Fat Diet. Pharmaceutics 2021, 13, 1753. [Google Scholar] [CrossRef]
- Hiramitsu, M.; Shimada, Y.; Kuroyanagi, J.; Inoue, T.; Katagiri, T.; Zang, L.; Nishimura, Y.; Nishimura, N.; Tanaka, T. Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci. Rep. 2014, 4, 3708. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Yoshida, T.; Nii, Y.; Okahisa, N.; Iwata, S.; Tsukayama, M.; Hashimoto, R.; Taniguchi, Y.; Sakaue, H.; Hosaka, T.; et al. Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr. Metab. 2014, 11, 32. [Google Scholar] [CrossRef]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine 2014, 21, 793–799. [Google Scholar] [CrossRef]
- Wang, Q.; Ou, Y.; Hu, G.; Wen, C.; Yue, S.; Chen, C.; Xu, L.; Xie, J.; Dai, H.; Xiao, H.; et al. Naringenin attenuates non-alcoholic fatty liver disease by down-regulating the NLRP3/NF-κB pathway in mice. Br. J. Pharmacol. 2020, 177, 1806–1821. [Google Scholar]
- Wu, L.; Niu, Y.; Ren, B.; Wang, S.; Song, Y.; Wang, X.; Zhao, K.; Yue, Z.; Li, Y.; Gao, J. Naringenin Promotes Gastrointestinal Motility in Mice by Impacting the SCF/c-Kit Pathway and Gut Microbiota. Foods 2024, 13, 2520. [Google Scholar] [PubMed]
- Zygmunt, K.; Faubert, B.; MacNeil, J.; Tsiani, E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem. Biophys. Res. Commun. 2010, 398, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Gao, D.M.; Mohamed, S.; Chen, J.; Zhang, J.; Zhou, X.Y.; Zhou, N.J.; Xie, J.; Jiang, H. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch. Biochem. Biophys. 2012, 518, 61–70. [Google Scholar] [CrossRef]
- Dong, L.; Lou, W.; Xu, C.; Wang, J. Naringenin cationic lipid-modified nanoparticles mitigate MASLD progression by modulating lipid homeostasis and gut microbiota. J. Nanobiotechnology 2025, 23, 168. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Meng, J.; Yao, S.; Xiao, F.; Li, S.; Shi, H.; Cui, C.; Chen, K.; Luo, X.; Ye, Y.; et al. Naringenin improves muscle endurance via activation of the Sp1-ERRγ transcriptional axis. Cell Rep. 2023, 42, 113288. [Google Scholar] [CrossRef]
- Jia, S.; Hu, Y.; Zhang, W.; Zhao, X.; Chen, Y.; Sun, C.; Li, X.; Chen, K. Hypoglycemic and hypolipidemic effects of neohesperidin derived from Citrus aurantium L. in diabetic KK-A(y) mice. Food Funct. 2015, 6, 878–886. [Google Scholar]
- Wu, H.; Liu, Y.; Chen, X.; Zhu, D.; Ma, J.; Yan, Y.; Si, M.; Li, X.; Sun, C.; Yang, B.; et al. Neohesperidin Exerts Lipid-Regulating Effects in vitro and in vivo via Fibroblast Growth Factor 21 and AMP-Activated Protein Kinase/Sirtuin Type 1/Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1α Signaling Axis. Pharmacology 2017, 100, 115–126. [Google Scholar]
- Jamal, A.; Brettle, H.; Jamil, D.A.; Tran, V.; Diep, H.; Bobik, A.; van der Poel, C.; Vinh, A.; Drummond, G.R.; Thomas, C.J.; et al. Reduced Insulin Resistance and Oxidative Stress in a Mouse Model of Metabolic Syndrome following Twelve Weeks of Citrus Bioflavonoid Hesperidin Supplementation: A Dose-Response Study. Biomolecules 2024, 14, 637. [Google Scholar]
- Yuk, T.; Kim, Y.; Yang, J.; Sung, J.; Jeong, H.S.; Lee, J.; Dini, L. Nobiletin Inhibits Hepatic Lipogenesis via Activation of AMP-Activated Protein Kinase. Evid. Based Complement. Altern. Med. 2018, 2018, 7420265. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, K.A.; Luchian, I.; Goriuc, A.; Antoci, L.M.; Ciobanu, C.G.; Popescu, R.; Vlad, C.E.; Blaj, M.; Foia, L.G. Mitochondrial Dysfunction, Oxidative Stress, and Therapeutic Strategies in Diabetes, Obesity, and Cardiovascular Disease. Antioxidants 2023, 12, 658. [Google Scholar] [CrossRef]
- Argaev-Frenkel, L.; Rosenzweig, T. Redox Balance in Type 2 Diabetes: Therapeutic Potential and the Challenge of Antioxidant-Based Therapy. Antioxidants 2023, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; De Franco, E.; Arvan, P.; Cnop, M. Pathological β-Cell Endoplasmic Reticulum Stress in Type 2 Diabetes: Current Evidence. Front. Endocrinol. 2021, 12, 650158. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Lee, J.; Ha, J.; Kang, I.; Choe, W. Endoplasmic Reticulum Stress and Its Impact on Adipogenesis: Molecular Mechanisms Implicated. Nutrients 2023, 15, 5082. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.R.; Chae, H.J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Veluthakal, R.; Esparza, D.; Hoolachan, J.M.; Balakrishnan, R.; Ahn, M.; Oh, E.; Jayasena, C.S.; Thurmond, D.C. Mitochondrial Dysfunction, Oxidative Stress, and Inter-Organ Miscommunications in T2D Progression. Int. J. Mol. Sci. 2024, 25, 1504. [Google Scholar]
- Benito-Vicente, A.; Jebari-Benslaiman, S.; Galicia-Garcia, U.; Larrea-Sebal, A.; Uribe, K.B.; Martin, C. Molecular mechanisms of lipotoxicity-induced pancreatic β-cell dysfunction. Int. Rev. Cell Mol. Biol. 2021, 359, 357–402. [Google Scholar]
- Biondi, G.; Marrano, N.; Borrelli, A.; Rella, M.; Palma, G.; Calderoni, I.; Siciliano, E.; Lops, P.; Giorgino, F.; Natalicchio, A. Adipose Tissue Secretion Pattern Influences β-Cell Wellness in the Transition from Obesity to Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 5522. [Google Scholar] [CrossRef]
- Ježek, P.; Jabůrek, M.; Holendová, B.; Plecitá-Hlavatá, L. Fatty Acid-Stimulated Insulin Secretion vs. Lipotoxicity. Molecules 2018, 23, 1483. [Google Scholar]
- Kim, J.W.; Yoon, K.H. Glucolipotoxicity in Pancreatic β-Cells. Diabetes Metab. J. 2011, 35, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Abderrahmani, A.; Renard, E. Pharmacological inhibitors of β-cell dysfunction and death as therapeutics for diabetes. Front. Endocrinol. 2023, 14, 1076343. [Google Scholar] [CrossRef]
- Brozzi, F.; Eizirik, D.L. ER stress and the decline and fall of pancreatic beta cells in type 1 diabetes. Ups. J. Med. Sci. 2016, 121, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Diane, A.; Allouch, A.; Mu-U-Min, R.B.A.; Al-Siddiqi, H.H. Endoplasmic reticulum stress in pancreatic β-cell dysfunctionality and diabetes mellitus: A promising target for generation of functional hPSC-derived β-cells in vitro. Front. Endocrinol. 2024, 15, 1386471. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yao, D.; Yang, H.; Wei, Y.; Peng, Y.; Ding, Y.; Shu, L. Puerarin Protects Pancreatic β-Cells in Obese Diabetic Mice via Activation of GLP-1R Signaling. Mol. Endocrinol. 2016, 30, 361–371. [Google Scholar]
- Yusta, B.; Baggio, L.L.; Estall, J.L.; Koehler, J.A.; Holland, D.P.; Li, H.; Pipeleers, D.; Ling, Z.; Drucker, D.J. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006, 4, 391–406. [Google Scholar]
- Li, Y.; Zhu, J.; Yue, C.; Song, S.; Tian, L.; Wang, Y. Recent advances in pancreatic α-cell transdifferentiation for diabetes therapy. Front. Immunol. 2025, 16, 1551372. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Huynh, P.M.; Wang, F.; An, Y.A. Hypoxia signaling in the adipose tissue. J. Mol. Cell Biol. 2025, 16, mjae039. [Google Scholar] [CrossRef]
- Mirabelli, M.; Misiti, R.; Sicilia, L.; Brunetti, F.S.; Chiefari, E.; Brunetti, A.; Foti, D.P. Hypoxia in Human Obesity: New Insights from Inflammation towards Insulin Resistance-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 9802. [Google Scholar]
- Begum, M.; Choubey, M.; Tirumalasetty, M.B.; Arbee, S.; Mohib, M.M.; Wahiduzzaman, M.; Mamun, M.A.; Uddin, M.B.; Mohiuddin, M.S. Adiponectin: A promising target for the treatment of diabetes and its complications. Life 2023, 13, 2213. [Google Scholar] [CrossRef]
- Corvera, S. Cellular Heterogeneity in Adipose Tissues. Annu. Rev. Physiol. 2021, 83, 257–278. [Google Scholar] [CrossRef]
- Massier, L.; Jalkanen, J.; Elmastas, M.; Zhong, J.; Wang, T.; Nono Nankam, P.A.; Frendo-Cumbo, S.; Bäckdahl, J.; Subramanian, N.; Sekine, T.; et al. An integrated single cell and spatial transcriptomic map of human white adipose tissue. Nat. Commun. 2023, 14, 1438. [Google Scholar] [CrossRef]
- Sultana, A.; Jahan Shimu, S.; Jakanta Faika, M.; Islam, T.; Ferdous, N.; Nessa, A. Bangladeshi parents’ knowledge and awareness about cervical cancer and willingness to vaccinate female family members against human papilloma virus: A cross sectional study. Int. J. Community Med. Public. Health 2023, 10, 3446–3453. [Google Scholar] [CrossRef]
- Khin, P.P.; Lee, J.H.; Jun, H.-S. Pancreatic beta-cell dysfunction in type 2 diabetes. Eur. J. Inflamm. 2023, 21. [Google Scholar] [CrossRef]
- Chen, B.; Li, T.; Wu, Y.; Song, L.; Wang, Y.; Bian, Y.; Qiu, Y.; Yang, Z. Lipotoxicity: A New Perspective in Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2025, 18, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, V.; Sanz-Lamora, H.; Arias, G.; Marrero, P.F.; Haro, D.; Relat, J. Metabolic impact of flavonoids consumption in obesity: From central to peripheral. Nutrients 2020, 12, 2393. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Mirzaei, A.; Najjar Khalilabad, S.; Askari, V.R.; Baradaran Rahimi, V. Promising influences of hesperidin and hesperetin against diabetes and its complications: A systematic review of molecular, cellular, and metabolic effects. Excli J. 2023, 22, 1235–1263. [Google Scholar]
- Russo, B.; Picconi, F.; Malandrucco, I.; Frontoni, S. Flavonoids and insulin-resistance: From molecular evidences to clinical trials. Int. J. Mol. Sci. 2019, 20, 2061. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The therapeutic effects and mechanisms of quercetin on metabolic diseases: Pharmacological data and clinical evidence. Oxidative Med. Cell. Longev. 2021, 2021, 6678662. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, X.; Zhang, L.; Bian, H.X.; Xu, N.; Bao, B.; Liu, J. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: A mechanism including AMPKα1/SIRT1. J. Lipid Res. 2014, 55, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Chen, G.W.; Chen, Y.C.; Shen, C.K.; Lu, D.Y.; Yang, L.Y.; Chen, J.H.; Yeh, W.L. Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. Nutrients 2021, 14, 67. [Google Scholar] [CrossRef]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; Enjoji, M. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef]
- Lee, J.; Hong, S.-W.; Rhee, E.-J.; Lee, W.-Y. GLP-1 receptor agonist and non-alcoholic fatty liver disease. Diabetes Metab. J. 2012, 36, 262. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Sun, Y.; Zhang, G.; Bai, J.; Guo, J.; Su, X.; Du, H.; Cao, X.; Yang, J. Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutr. Diabetes 2019, 9, 28. [Google Scholar] [CrossRef]
- Nery, M.; Ferreira, P.S.; Gonçalves, D.R.; Spolidorio, L.C.; Manthey, J.A.; Cesar, T.B. Physiological effects of tangeretin and heptamethoxyflavone on obese C57BL/6J mice fed a high-fat diet and analyses of the metabolites originating from these two polymethoxylated flavones. Food Sci. Nutr. 2021, 9, 1997–2009. [Google Scholar] [CrossRef]
- La Spina, M.; Galletta, E.; Azzolini, M.; Gomez Zorita, S.; Parrasia, S.; Salvalaio, M.; Salmaso, A.; Biasutto, L. Browning effects of a chronic pterostilbene supplementation in mice fed a high-fat diet. Int. J. Mol. Sci. 2019, 20, 5377. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Gu, Y.; Jiang, Z.; Zhou, Z. Tangeretin prevents obesity by modulating systemic inflammation, fat browning, and gut microbiota in high-fat diet-induced obese C57BL/6 mice. J. Nutr. Biochem. 2022, 101, 108943. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, M.; Niu, R.; Gu, X.; Hao, E.; Hou, X.; Deng, J.; Bai, G. The combination of cinnamaldehyde and kaempferol ameliorates glucose and lipid metabolism disorders by enhancing lipid metabolism via AMPK activation. J. Funct. Foods 2021, 83, 104556. [Google Scholar] [CrossRef]
- Park, J.E.; Yoo, J.; Han, J.S. HM-Chromanone Alleviates Hyperglycemia by Activating AMPK and PI3K/AKT Pathways in Mice Fed a High-Fat Diet. Nutrients 2024, 16, 3972. [Google Scholar]
- Chang, W.-T.; Huang, S.-C.; Cheng, H.-L.; Chen, S.-C.; Hsu, C.-L. Rutin and gallic acid regulates mitochondrial functions via the SIRT1 pathway in C2C12 myotubes. Antioxidants 2021, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R.P.; Zhen, W.; Zhou, K.; Liu, D. The flavonoid kaempferol ameliorates streptozotocin-induced diabetes by suppressing hepatic glucose production. Molecules 2018, 23, 2338. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Shimu, S.J.; Islam, S. Gender Differences in Drug Addiction: Neurobiological, Social, and Psychological Perspectives in Women–A Systematic Review. J. Primeasia 2025, 6, 1–13. [Google Scholar]
- Gao, Z.; Hwang, D.; Bataille, F.; Lefevre, M.; York, D.; Quon, M.J.; Ye, J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J. Biol. Chem. 2002, 277, 48115–48121. [Google Scholar] [CrossRef] [PubMed]
- Kanety, H.; Feinstein, R.; Papa, M.Z.; Hemi, R.; Karasik, A. Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J. Biol. Chem. 1995, 270, 23780–23784. [Google Scholar] [CrossRef]
- Martínez Báez, A.; Ayala, G.; Pedroza-Saavedra, A.; González-Sánchez, H.M.; Chihu Amparan, L. Phosphorylation codes in IRS-1 and IRS-2 are associated with the activation/inhibition of insulin canonical signaling pathways. Curr. Issues Mol. Biol. 2024, 46, 634–649. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.-I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Apaijit, K.; Pakdeechote, P.; Maneesai, P.; Meephat, S.; Prasatthong, P.; Bunbupha, S. Hesperidin Alleviates Vascular Dysfunction and Remodelling in High-Fat/High-Fructose Diet-Fed Rats by Modulating Oxidative Stress, Inflammation, AdipoR1, and eNOS Expression. Tissue Cell 2022, 78, 101901. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-Y.; Lu, C.-H.; Wu, C.-H.; Li, K.-J.; Kuo, Y.-M.; Hsieh, S.-C.; Yu, C.-L. The development of maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules 2020, 25, 5591. [Google Scholar] [CrossRef]
- Syed, A.A.; Reza, M.I.; Shafiq, M.; Kumariya, S.; Singh, P.; Husain, A.; Hanif, K.; Gayen, J.R. Naringin ameliorates type 2 diabetes mellitus-induced steatohepatitis by inhibiting RAGE/NF-κB mediated mitochondrial apoptosis. Life Sci. 2020, 257, 118118. [Google Scholar] [CrossRef]
- Cepas, V.; Collino, M.; Mayo, J.C.; Sainz, R.M. Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants 2020, 9, 142. [Google Scholar] [CrossRef]
- Al-Aubaidy, H.A.; Dayan, A.; Deseo, M.A.; Itsiopoulos, C.; Jamil, D.; Hadi, N.R.; Thomas, C.J. Twelve-week mediterranean diet intervention increases citrus bioflavonoid levels and reduces inflammation in people with type 2 diabetes mellitus. Nutrients 2021, 13, 1133. [Google Scholar] [CrossRef]
- Passaro, A.; Sanz, J.M.; Naumovski, N.; Sergi, D. The complex interplay between oxinflammation, mitochondrial dysfunction and lipotoxicity: Focus on their role in the pathogenesis of skeletal muscle insulin resistance and modulation by dietary fatty acids. Adv. Redox Res. 2024, 11, 100100. [Google Scholar] [CrossRef]
- Cavaliere, G.; Cimmino, F.; Trinchese, G.; Catapano, A.; Petrella, L.; D’Angelo, M.; Lucchin, L.; Mollica, M.P. From obesity-induced low-grade inflammation to lipotoxicity and mitochondrial dysfunction: Altered multi-crosstalk between adipose tissue and metabolically active organs. Antioxidants 2023, 12, 1172. [Google Scholar] [PubMed]
- Kicinska, A.; Jarmuszkiewicz, W. Flavonoids and mitochondria: Activation of cytoprotective pathways? Molecules 2020, 25, 3060. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.; Feraco, A.; Camajani, E.; Gorini, S.; Lombardo, M.; Caprio, M. Nutraceuticals in Brown Adipose Tissue Activation. Cells 2022, 11, 3996. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Muoio, D.M.; Neufer, P.D. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab. 2012, 15, 595–605. [Google Scholar] [CrossRef]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.-F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Lemos, G.d.O.; Torrinhas, R.S.; Waitzberg, D.L. Nutrients, physical activity, and mitochondrial dysfunction in the setting of metabolic syndrome. Nutrients 2023, 15, 1217. [Google Scholar] [CrossRef]
- Ouyang, Y.B.; Xu, L.J.; Emery, J.F.; Lee, A.S.; Giffard, R.G. Overexpressing GRP78 influences Ca2+ handling and function of mitochondria in astrocytes after ischemia-like stress. Mitochondrion 2011, 11, 279–286. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef]
- Alotaibi, G.; Alkhammash, A. Pharmacological landscape of endoplasmic reticulum stress: Uncovering therapeutic avenues for metabolic diseases. Eur. J. Pharmacol. 2025, 998, 177509. [Google Scholar] [CrossRef] [PubMed]
- Lenghel, A.; Gheorghita, A.M.; Vacaru, A.M.; Vacaru, A.-M. What is the sweetest UPR flavor for the β-cell? That is the question. Front. Endocrinol. 2021, 11, 614123. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J. Endoplasmic reticulum (ER) stress and its role in pancreatic β-cell dysfunction and senescence in type 2 diabetes. Int. J. Mol. Sci. 2022, 23, 4843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Du, J.; Zeng, H. Interplay between endoplasmic reticulum stress and non-coding RNAs in cancer. J. Hematol. Oncol. 2020, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Amodio, G.; Pagliara, V.; Moltedo, O.; Remondelli, P. Structural and functional significance of the endoplasmic reticulum unfolded protein response transducers and chaperones at the mitochondria–ER contacts: A cancer perspective. Front. Cell Dev. Biol. 2021, 9, 641194. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating MAPK and AKT signal transduction pathways in endometriosis cells. MHR: Basic. Sci. Reprod. Med. 2017, 23, 842–854. [Google Scholar]
- Li, X.; Lin, Q.; Gou, F.; Zhu, J.; Yu, M.; Hong, Q.; Hu, C. Effects of hesperidin on mitochondrial function, mitochondria-associated endoplasmic reticulum membranes and IP3R–MCU calcium axis in the intestine of piglets exposed to deoxynivalenol. Food Funct. 2024, 15, 6459–6474. [Google Scholar] [CrossRef] [PubMed]
- Gou, F.; Lin, Q.; Tu, X.; Zhu, J.; Li, X.; Chen, S.; Hu, C. Hesperidin alleviated intestinal barrier injury, mitochondrial dysfunction, and disorder of endoplasmic reticulum mitochondria contact sites under oxidative stress. J. Agric. Food Chem. 2024, 72, 16276–16286. [Google Scholar] [CrossRef]
- Khoi, C.-S.; Lin, T.-Y.; Chiang, C.-K. Targeting Insulin Resistance, Reactive Oxygen Species, Inflammation, Programmed Cell Death, ER Stress, and Mitochondrial Dysfunction for the Therapeutic Prevention of Free Fatty Acid-Induced Vascular Endothelial Lipotoxicity. Antioxidants 2024, 13, 1486. [Google Scholar] [CrossRef]
- Karunakaran, U.; Park, K.G. A systematic review of oxidative stress and safety of antioxidants in diabetes: Focus on islets and their defense. Diabetes Metab. J. 2013, 37, 106–112. [Google Scholar] [CrossRef]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (dys) function and insulin resistance: From pathophysiological molecular mechanisms to the impact of diet. Front. Physiol. 2019, 10, 449821. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and oxidative stress: An integral, updated and critical overview of their metabolic interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Gabbar, M.A.; Abdel-Twab, S.M.; Fahmy, E.M.; Ebaid, H.; Alhazza, I.M.; Ahmed, O.M. Antidiabetic Potency, Antioxidant Effects, and Mode of Actions of Citrus reticulata Fruit Peel Hydroethanolic Extract, Hesperidin, and Quercetin in Nicotinamide/Streptozotocin-Induced Wistar Diabetic Rats. Oxid. Med. Cell Longev. 2020, 2020, 1730492. [Google Scholar] [PubMed]
- Korac, B.; Kalezic, A.; Pekovic-Vaughan, V.; Korac, A.; Jankovic, A. Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol. 2021, 42, 101887. [Google Scholar] [CrossRef]
- Park, H.J.; Jung, U.J.; Cho, S.J.; Jung, H.K.; Shim, S.; Choi, M.S. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose- and lipid-regulating enzymes in db/db mice. J. Nutr. Biochem. 2013, 24, 419–427. [Google Scholar]
- Kim, B.M.; Cho, B.O.; Jang, S.I. Anti-obesity effects of Diospyros lotus leaf extract in mice with high-fat diet-induced obesity. Int. J. Mol. Med. 2019, 43, 603–613. [Google Scholar]
- L Suraweera, T.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- Tsai, H.L.; Chang, S.K.; Chang, S.J. Antioxidant content and free radical scavenging ability of fresh red pummelo [Citrus grandis (L.) Osbeck] juice and freeze-dried products. J. Agric. Food Chem. 2007, 55, 2867–2872. [Google Scholar] [CrossRef]
- Namkhah, Z.; Naeini, F.; Mahdi Rezayat, S.; Mehdi, Y.; Mansouri, S.; Javad Hosseinzadeh-Attar, M. Does naringenin supplementation improve lipid profile, severity of hepatic steatosis and probability of liver fibrosis in overweight/obese patients with NAFLD? A randomised, double-blind, placebo-controlled, clinical trial. Int. J. Clin. Pract. 2021, 75, e14852. [Google Scholar]
- Naeini, F.; Namkhah, Z.; Tutunchi, H.; Rezayat, S.M.; Mansouri, S.; Jazayeri-Tehrani, S.A.; Yaseri, M.; Hosseinzadeh-Attar, M.J. Effects of naringenin supplementation in overweight/obese patients with non-alcoholic fatty liver disease: Study protocol for a randomized double-blind clinical trial. Trials 2021, 22, 801. [Google Scholar] [CrossRef]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: Role of PPARalpha, PPARgamma and LXRalpha. PLoS ONE 2010, 5, e12399. [Google Scholar]
- Sleiman, D.; Al-Badri, M.R.; Azar, S.T. Effect of mediterranean diet in diabetes control and cardiovascular risk modification: A systematic review. Front. Public. Health 2015, 3, 69. [Google Scholar]
- Carpenito, M.; Coletti, F.; Muscoli, S.; Guarino, L.; Di Cristo, A.; Cammalleri, V.; Mega, S.; Emerenziani, S.; Cicala, M.; Fanali, C.; et al. Unveiling the Power of Bergamot: Beyond Lipid-Lowering Effects. Nutrients 2025, 17, 1871. [Google Scholar] [CrossRef]
- Fogacci, F.; Giovannini, M.; Imbalzano, E.; Grandi, E.; Rizzoli, E.; D’Addato, S.; Cicero, A.F.G. Metabolic and vascular effect of a new standardized bergamot phytocomplex: A three-arm, placebocontrolled, double-blind clinical trial. Arch. Med. Sci. 2023, 19, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Ferro, Y.; Maurotti, S.; Mazza, E.; Pujia, R.; Sciacqua, A.; Musolino, V.; Mollace, V.; Pujia, A.; Montalcini, T. Citrus Bergamia and Cynara Cardunculus Reduce Serum Uric Acid in Individuals with Non-Alcoholic Fatty Liver Disease. Medicina 2022, 58, 1728. [Google Scholar] [CrossRef]
- Jalili, F.; Moradi, S.; Talebi, S.; Mehrabani, S.; Ghoreishy, S.M.; Wong, A.; Jalalvand, A.R.; Kermani, M.A.H.; Jalili, C. The effects of citrus flavonoids supplementation on endothelial function: A systematic review and dose-response meta-analysis of randomized clinical trials. Phytother. Res. 2024, 38, 2847–2859. [Google Scholar] [CrossRef]
- Sost, M.M.; Ahles, S.; Verhoeven, J.; Verbruggen, S.; Stevens, Y.; Venema, K. A Citrus Fruit Extract High in Polyphenols Beneficially Modulates the Gut Microbiota of Healthy Human Volunteers in a Validated In Vitro Model of the Colon. Nutrients 2021, 13, 3915. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Macarro, M.; Rodríguez, J.P.M.; Morell, E.B.; Pérez-Piñero, S.; Victoria-Montesinos, D.; García-Muñoz, A.M.; García, F.C.; Sánchez, J.C.; López-Román, F.J. Effect of a Combination of Citrus Flavones and Flavanones and Olive Polyphenols for the Reduction of Cardiovascular Disease Risk: An Exploratory Randomized, Double-Blind, Placebo-Controlled Study in Healthy Subjects. Nutrients 2020, 12, 1475. [Google Scholar] [CrossRef]
- Victoria-Montesinos, D.; Sánchez-Macarro, M.; Gabaldón-Hernández, J.A.; Abellán-Ruiz, M.S.; Querol-Calderón, M.; Luque-Rubia, A.J.; Bernal-Morell, E.; Ávila-Gandía, V.; López-Román, F.J. Effect of Dietary Supplementation with a Natural Extract of Sclerocarya birrea on Glycemic Metabolism in Subjects with Prediabetes: A Randomized Double-Blind Placebo-Controlled Study. Nutrients 2021, 13, 1948. [Google Scholar] [CrossRef]
- Morand, C.; DuBray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef]
- Yari, Z.; Movahedian, M.; Imani, H.; Alavian, S.M.; Hedayati, M.; Hekmatdoost, A. The effect of hesperidin supplementation on metabolic profiles in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 2569–2577. [Google Scholar] [CrossRef]
- Khorasanian, A.S.; Fateh, S.T.; Gholami, F.; Rasaei, N.; Gerami, H.; Khayyatzadeh, S.S.; Shiraseb, F.; Asbaghi, O. The effects of hesperidin supplementation on cardiovascular risk factors in adults: A systematic review and dose-response meta-analysis. Front. Nutr. 2023, 10, 1177708. [Google Scholar] [CrossRef]
- Notarnicola, M.; Tutino, V.; De Nunzio, V.; Cisternino, A.M.; Cofano, M.; Donghia, R.; Giannuzzi, V.; Zappimbulso, M.; Milella, R.A.; Giannelli, G.; et al. Daily Orange Consumption Reduces Hepatic Steatosis Prevalence in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: Exploratory Outcomes of a Randomized Clinical Trial. Nutrients 2024, 16, 3191. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Bosch-Sierra, N.; Valle, C.G.-D.; Salazar, J.D.; Marqués-Cardete, R.; Sáez, G.; Morillas, C.; Bañuls, C. Effects of a flavonoid-enriched orange juice on antioxidant capacity, lipid profile, and inflammation in obese patients: A randomized placebo-controlled trial. Food Res. Int. 2025, 217, 116759. [Google Scholar]
- Cesar, T.B.; Ramos, F.M.M.; Ribeiro, C.B. Nutraceutical Eriocitrin (Eriomin) Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation: A Crossover-Randomized Clinical Trial. J. Med. Food 2022, 25, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Song, L.; Wang, X.H. Efficacy of dietary polyphenol supplement in patients with non-alcoholic fatty liver disease: A network meta-analysis. Front. Nutr. 2025, 12, 1582861. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fang, Z.; Luo, Q.; Wang, X.; Warda, M.; Das, A.; Oldoni, F.; Luo, F. Unlocking the Mysteries of VLDL: Exploring Its Production, Intracellular Trafficking, and Metabolism as Therapeutic Targets. Lipids Health Dis. 2024, 23, 14. [Google Scholar] [CrossRef]
- Gluba-Sagr, A.; Olszewski, R.; Franczyk, B.; Młynarska, E.; Rysz-Górzyńska, M.; Rysz, J.; Surma, S.; Sohum, S.; Banach, M.; Toth, P.P. High-Density Lipoproteins. Part 2. Impact of Disease States on Functionality. Am. J. Prev. Cardiol. 2025, 23, 101073. [Google Scholar] [CrossRef]
- Nauman, M.C.; Johnson, J.J. Clinical Application of Bergamot (Citrus Bergamia) for Reducing High Cholesterol and Cardiovascular Disease Markers. Integr. Food Nutr. Metab. 2019, 6, 10.15761/IFNM.1000249. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Assini, J.M.; Lee, J.K.; Allister, E.M.; Sutherland, B.G.; Koppes, J.B.; Sawyez, C.G.; Edwards, J.Y.; Telford, D.E.; Charbonneau, A.; et al. Nobiletin Attenuates VLDL Overproduction, Dyslipidemia, and Atherosclerosis in Mice With Diet-Induced Insulin Resistance. Diabetes 2011, 60, 1446–1457. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Zhang, W.; Liu, C.; Chen, S. Natural Polyphenols in Metabolic Syndrome: Protective Mechanisms and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 6110. [Google Scholar] [CrossRef] [PubMed]
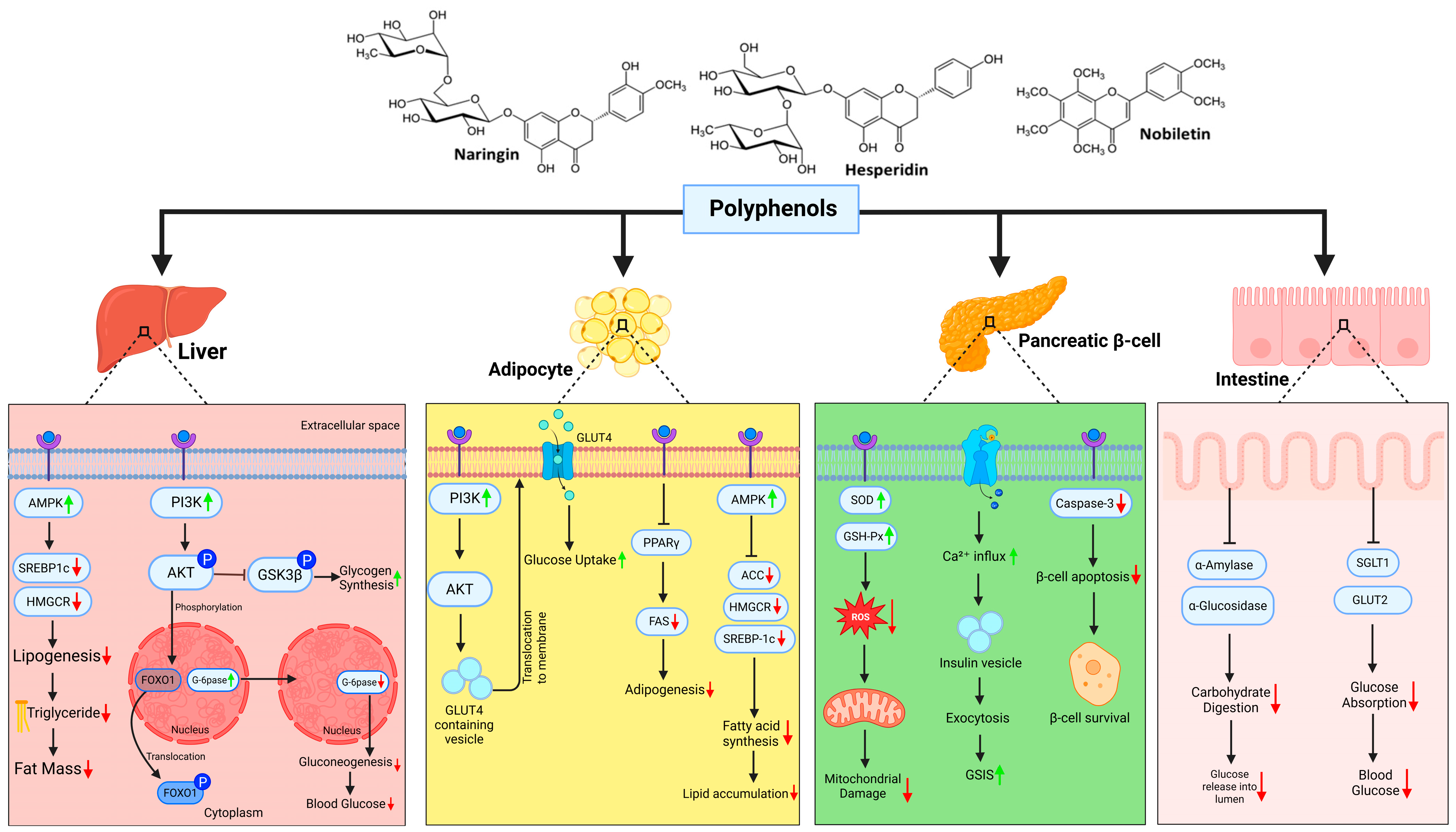


| Compound | Model/System | Intervention Details (Dose and Duration) | Primary Outcomes/Mechanisms | Source |
|---|---|---|---|---|
| Neohesperidin | HFD-fed mice | 50–100 mg/kg/day, 8–12 weeks | ↑ AMPK–PGC-1α → ↑ mitochondrial biogenesis, ↓ steatosis | [46] |
| Nobiletin | HFD-fed mice | 100 mg/kg/day, 8 weeks | ↑ FA oxidation, ↑ energy expenditure; AMPK-independent | [47] |
| Nobiletin | Hepatocytes | 10–50 μM, 24–48 h | Restores Bmal1 → ↑ lipid/OXPHOS metabolism | [48] |
| Nobiletin | Insulin-resistant mice | 50 mg/kg/day, 6 weeks | ↓ VLDL secretion; improved lipid/glucose metabolism | [47] |
| Nobiletin | HepG2 cells | 25 μM, 24 h | ↑ PGC1α, CPT1, UCP2 → ↑ β-oxidation | [47] |
| Nobiletin | ob/ob mice | 100 mg/kg/day, 6 weeks | ↑ GLUT4, ↑ Akt phosphorylation → improved insulin sensitivity | [49] |
| Naringenin | MCD or HFD mice | 50–100 mg/kg/day, 8–12 weeks | ↑ AMPK → ↑ autophagy, ↑ mitochondrial biogenesis | [50] |
| Naringenin | Hepatocytes/mice | 10–50 μM in vitro; 100 mg/kg/day in vivo | ↑ AMPK, ↑ ATF3 → ↓ metabolic inflammation | [51] |
| Naringin | HFD-fed mice | 100 mg/kg/day, 10 weeks | ↑ AMPK → ↓ SREBP-1c/FAS, ↑ redox balance | [52] |
| Naringin | Fructose-fed rats | 40 mg/kg/day, 8 weeks | ↑ Nrf2/HO-1 → antioxidant response; ↓ ChREBP/SREBP-1c | [53] |
| Naringin | HFD mice | 100 mg/kg/day, 12 weeks | ↑ TFEB → lipophagy → ↓ hepatic lipid droplets | [54] |
| Hesperidin | LO2 hepatocytes (HG) | 25–100 μM, 24–48 h | ↑ ATP, restores ΔΨm via AKT/GSK3β | [55] |
| Hesperidin | Hyperlipidemic rats | 100 mg/kg/day, 6 weeks | ↑ SOD, ↑ catalase; preserved mitochondrial enzymes | [56] |
| Hesperidin | Neurons (hyperglycemia) | 25 μM, 24–48 h | Improves ATP/redox; ↓ mitochondrial dysfunction | [57] |
| Hesperetin | Aging mice | 50 mg/kg/day, 8 weeks | ↑ Cisd2 expression → maintenance of metabolic health | [58] |
| Limonene | Mice model | 100 mg/kg/day, 6 weeks | ↑ mitochondrial respiration, ↓ ROS | [59,60] |
| Eriocitrin | HFD rats | 25–50 mg/kg/day, 8 weeks | ↑ mitochondrial biogenesis, ↓ steatosis | [61] |
| Sudachitin | C57BL/6J, db/db mice | 50 mg/kg/day, 8 weeks | ↑ β-oxidation, ↑ mitochondrial biogenesis | [62] |
| Tangeretin | Diabetic rats | 100 mg/kg/day, 6 weeks | ↑ GLUT4, ↑ antioxidant enzymes | [63] |
| Naringenin | NAFLD mice | 100 mg/kg/day, 10 weeks | ↓ NLRP3/NF-κB, ↓ IL-1β → metabolic reprogramming | [64] |
| Naringenin | NAFLD mice (metabolomics) | 100 mg/kg/day, 12 weeks | Modulates gut microbiota → improved host metabolism | [65] |
| Naringenin | Muscle cells | 25–50 μM, 24 h | ↑ p-AMPK → ↑ glucose uptake, ↑ mitochondrial content | [66] |
| Naringin | Hepatocytes, HFD mice | 25 μM in vitro; 100 mg/kg/day, 8 weeks | AMPK–IRS1–MAPK pathway → improved insulin signaling | [67] |
| Naringenin | MASLD mice | 100 mg/kg/day, 12 weeks | ↑ PPAR, ↑ lipid oxidation, gut microbiota shift | [68] |
| Naringenin | Mice (aerobic fitness) | 100 mg/kg/day, 4 weeks | ↑ oxidative fibers, ↑ aerobic metabolism | [69] |
| Naringin | KK-A(y) mice | 100 mg/kg/day, 8 weeks | ↑ AMPK → ↓ glucose/lipids, ↑ insulin sensitivity | [70] |
| Neohesperidin | DIO mice, HepG2 cells | 50–100 mg/kg/day, 12 weeks; 25 μM in vitro | ↑ FGF21, ↑ AMPK → improved lipid regulation | [71] |
| Hesperidin | MASLD mice | 100 mg/kg/day, 8 weeks | ↓ insulin resistance, ↓ oxidative stress | [72] |
| Nobiletin | HepG2 cells | 25 μM, 24 h | ↑ AMPK, ↓ lipogenesis | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimu, S.J.; Mahir, J.U.K.; Shakib, F.A.F.; Ridoy, A.A.; Samir, R.A.; Jahan, N.; Hasan, M.F.; Sazzad, S.; Akter, S.; Mohiuddin, M.S.; et al. Metabolic Reprogramming Through Polyphenol Networks: A Systems Approach to Metabolic Inflammation and Insulin Resistance. Med. Sci. 2025, 13, 180. https://doi.org/10.3390/medsci13030180
Shimu SJ, Mahir JUK, Shakib FAF, Ridoy AA, Samir RA, Jahan N, Hasan MF, Sazzad S, Akter S, Mohiuddin MS, et al. Metabolic Reprogramming Through Polyphenol Networks: A Systems Approach to Metabolic Inflammation and Insulin Resistance. Medical Sciences. 2025; 13(3):180. https://doi.org/10.3390/medsci13030180
Chicago/Turabian StyleShimu, Shakila Jahan, Jawad Ul Karim Mahir, Fardin Al Fahad Shakib, Arafath Amin Ridoy, Ratin Al Samir, Nadia Jahan, Md Fahim Hasan, Sadman Sazzad, Shamima Akter, Mohammad Sarif Mohiuddin, and et al. 2025. "Metabolic Reprogramming Through Polyphenol Networks: A Systems Approach to Metabolic Inflammation and Insulin Resistance" Medical Sciences 13, no. 3: 180. https://doi.org/10.3390/medsci13030180
APA StyleShimu, S. J., Mahir, J. U. K., Shakib, F. A. F., Ridoy, A. A., Samir, R. A., Jahan, N., Hasan, M. F., Sazzad, S., Akter, S., Mohiuddin, M. S., Shawon, M. J. A., Shariare, M. H., Mohib, M. M., & Uddin, M. B. (2025). Metabolic Reprogramming Through Polyphenol Networks: A Systems Approach to Metabolic Inflammation and Insulin Resistance. Medical Sciences, 13(3), 180. https://doi.org/10.3390/medsci13030180











