The Association of MicroRNA-21 with Carotid Artery Disease and Ischemic Stroke: From Pathophysiology to Clinical Implications and Potential Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Inclusion Criteria
- Experimental (animal or clinical) studies evaluating miR-21 as a biomarker or therapeutic target in ischemic stroke and ACD;
- Observational studies linking miR-21 expression to ischemic stroke or carotid disease prognosis;
- Reviews and meta-analyses (only for backward citation searching);
- Case reports demonstrating clinical relevance (e.g., diagnostic or prognostic value).
2.1.2. Exclusion Criteria
- Mechanistic-only studies without diagnostic, prognostic, or therapeutic context;
- Case reports unrelated to diagnosis, treatment, or prognosis;
- Editorials, commentaries, letters to the editor, and non-peer-reviewed abstracts;
- Reviews and meta-analyses.
2.2. Literature Search Strategy
2.3. Search Syntax
- (a)
- PubMed
- (b)
- Scopus
2.4. Data Extraction and Thematic Synthesis
2.5. Conceptual Framework Application
2.6. Review of the Literature
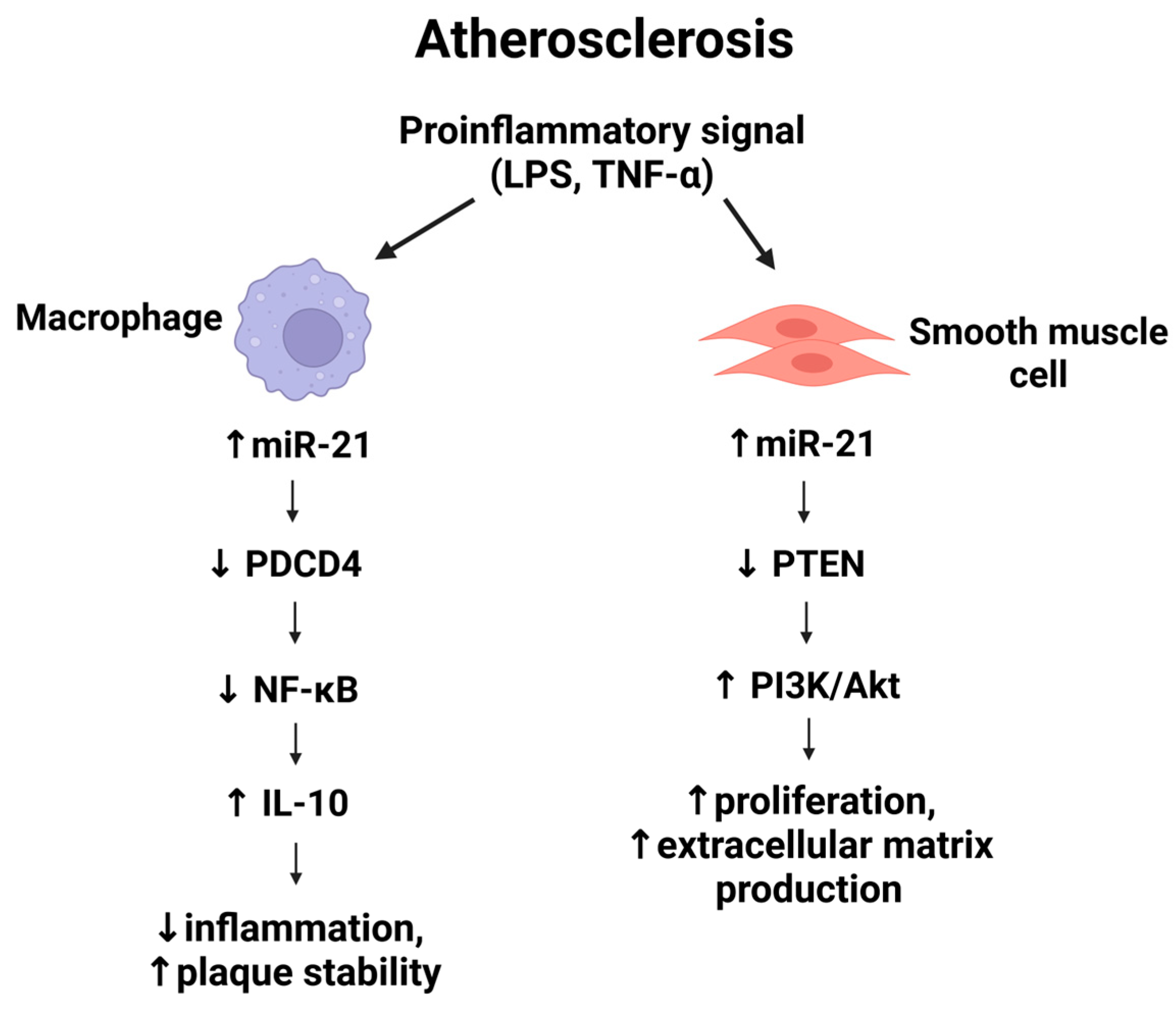
3. Mechanistic Role of miR-21 in Atherosclerosis
4. MiR-21 in Vascular Inflammation
5. MiR-21 and Vascular Smooth Muscle Cell Proliferation
6. MiR-21 and Endothelial Dysfunction
7. MiR-21 and Plaque Instability
8. MiR-21 in Ischemic Stroke Models
9. Therapeutic Modulation of miR-21
10. Biomarker Potential of miR-21
11. Discussion and Limitations
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Lu, S.; Jie, Y.; Chao, W.; Zhu, W.; Huang, D. Comprehensive Analysis of the Ischemic Stroke Burden at Global, Regional, and National Levels (1990–2021): Trends, Influencing Factors, and Future Projections. Front. Neurol. 2025, 16, 1492691. [Google Scholar] [CrossRef]
- Liu, J.; Xu, A.; Zhao, Z.; Ren, B.; Gao, Z.; Fang, D.; Hei, B.; Sun, J.; Bao, X.; Ma, L.; et al. Epidemiology and Future Trend Predictions of Ischemic Stroke Based on the Global Burden of Disease Study 1990–2021. Commun. Med. 2025, 5, 273. [Google Scholar] [CrossRef]
- Dossabhoy, S.; Arya, S. Epidemiology of Atherosclerotic Carotid Artery Disease. Semin. Vasc. Surg. 2021, 34, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Li, R.; Zhang, S.; Li, D.; Dong, B.; Zhou, H.; Jing, L.; Tian, Y.; Liu, S. High Burden of Carotid Atherosclerosis in Rural Northeast China: A Population-Based Study. Front. Neurol. 2021, 12, 597992. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, Y.; Xia, Y.; Liu, Y.; Deng, X.; Wang, W.; Wang, Y.; Wang, C.; Wang, G. Global, Regional, and National Epidemiology of Ischemic Stroke from 1990 to 2021. Eur. J. Neurol. 2024, 31, e16481. [Google Scholar] [CrossRef]
- Fu, J.; Deng, Y.; Ma, Y.; Man, S.; Yang, X.; Yu, C.; Lv, J.; Wang, B.; Li, L. National and Provincial-Level Prevalence and Risk Factors of Carotid Atherosclerosis in Chinese Adults. JAMA Netw. Open 2024, 7, e2351225. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, C.; Liu, X.; Yang, S.; Ma, M.; Tang, J.; Yin, T.; Zhao, S.; Tu, W.; Hu, H. Prevalence and Associated Risk Factors of Carotid Plaque and Artery Stenosis in China: A Population-Based Study. Front. Med. 2025, 19, 64–78. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Lan, X.; Wang, L.; Li, H.; Gu, D.; Wang, M.; Liu, J. Global, Regional, and National Trends in Ischaemic Stroke Burden and Risk Factors among Adults Aged 20+ Years (1990–2021): A Systematic Analysis of Data from the Global Burden of Disease Study 2021 with Projections into 2050. Front. Public Health 2025, 13, 1567275. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, W.; Zhang, Y.; Xiong, Y.; Tao, C.; Ma, L.; Ma, J.; You, C.; Wang, C. Global, Regional, and National Burden of Stroke, 1990–2021: A Systematic Analysis for Global Burden of Disease 2021. Stroke 2024, 55, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M.J.; Jasim, N.Y.; Ahmed, M.H.; Ballal, S.; Kumar, A.; Atteri, S.; Vashishth, R.; Rizaev, J.; Alhili, A.; Jawad, M.J.; et al. Critical Roles of miR-21 in Promotion of Angiogenesis: Friend or Foe? Clin. Exp. Med. 2025, 25, 66. [Google Scholar] [CrossRef]
- Artimovič, P.; Špaková, I.; Macejková, E.; Pribulová, T.; Rabajdová, M.; Mareková, M.; Zavacká, M. The Ability of MicroRNAs to Regulate the Immune Response in Ischemia/Reperfusion Inflammatory Pathways. Genes Immun. 2024, 25, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Bian, Z. MicroRNA-21 Is a Versatile Regulator and Potential Treatment Target in Central Nervous System Disorders. Front. Mol. Neurosci. 2022, 15, 842288. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Mu, Z.; Jiang, H.; Zhang, S.; Pang, Y.; Jin, H.; Chen, J.; Jia, C.; Guo, H. MiR-21-5p Protects against Ischemic Stroke by Targeting IL-6R. Ann. Transl. Med. 2023, 11, 101. [Google Scholar] [CrossRef]
- Mohammed, A.; Shaker, O.G.; Khalil, M.A.F.; Gomaa, M.; Fathy, S.A.; Abu-El-Azayem, A.K.; Samy, A.; Aboelnor, M.I.; Gomaa, M.S.; Zaki, O.M.; et al. Long non-coding RNA NBAT1, TUG1, miRNA-335, and miRNA-21 as potential biomarkers for acute ischemic stroke and their possible correlation to thyroid hormones. Front. Mol. Biosci. 2022, 9, 914506. [Google Scholar] [CrossRef]
- Xiang, Y.; Guo, J.; Peng, Y.F.; Tan, T.; Huang, H.T.; Luo, H.C.; Wei, Y.S. Association of miR-21, miR-126 and miR-605 gene polymorphisms with ischemic stroke risk. Oncotarget 2017, 8, 95755–95763. [Google Scholar] [CrossRef]
- Liu, C.; Huang, H.; Li, Y.; Zhao, H. The relationship of long non-coding RNA maternally expressed gene 3 with microRNA-21 and their correlation with acute ischemic stroke risk, disease severity and recurrence risk. Clin. Neurol. Neurosurg. 2021, 210, 106940. [Google Scholar] [CrossRef]
- Li, C.; Fei, K.; Tian, F.; Gao, C.; Yang, S. Adipose-derived mesenchymal stem cells attenuate ischemic brain injuries in rats by modulating miR-21-3p/MAT2B signaling transduction. Croat. Med. J. 2019, 60, 439–448. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Liao, Y.-C.; Wang, Y.-S.; Lin, H.-F.; Lin, R.-T.; Juo, S.-H. Serum microRNA-21 and microRNA-221 as Potential Biomarkers for Cerebrovascular Disease. J. Vasc. Res. 2013, 50, 346–354. [Google Scholar] [CrossRef]
- Lei, G.; Wu, X.; Zhang, S.; Tong, X.; Zhou, G. Acupuncture Therapy Modulating “Du” Channel Attenuates Ischemic Stroke-Induced Disorders by Modulating REST-Mediated miR-21/PDCD4 Signaling Transduction. J. Mol. Neurosci. 2024, 74, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Yu, X. An Investigation of the Correlation between miRNA-21-5p and the Classification and Short-Term Prognosis of Acute Ischemic Cerebrovascular Disease. Altern. Ther. Health Med. 2023, 29, 6–11. [Google Scholar] [PubMed]
- Wang, W.; Li, D.B.; Li, R.Y.; Zhou, X.; Yu, D.J.; Lan, X.Y.; Li, J.P.; Liu, J.L. Diagnosis of Hyperacute and Acute Ischaemic Stroke: The Potential Utility of Exosomal MicroRNA-21-5p and MicroRNA-30a-5p. Cerebrovasc. Dis. 2018, 45, 204–212. [Google Scholar] [CrossRef]
- Vibo, R.; Jõgi, K.; Remm, A.; Rebane, A.; Kõrv, J. Different Expression Patterns of Inflammation-Related Genes and Serum MicroRNAs in Young-Onset Ischemic Stroke. Sci. Rep. 2024, 14, 23845. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fan, C.L.; Ma, L.J.; Liu, T.; Wang, C.; Song, J.X.; Lv, Q.S.; Pan, H.; Zhang, C.N.; Wang, J.J. Distinctive Expression Signatures of Serum MicroRNAs in Ischaemic Stroke and Transient Ischaemic Attack Patients. Thromb. Haemost. 2017, 117, 992–1001. [Google Scholar] [CrossRef]
- Korvenlaita, N.; Gómez-Budia, M.; Scoyni, F.; Pistono, C.; Giudice, L.; Eamen, S.; Loppi, S.; de Sande, A.H.; Huremagic, B.; Bouvy-Liivrand, M.; et al. Dynamic Release of Neuronal Extracellular Vesicles Containing miR-21a-5p Is Induced by Hypoxia. J. Extracell. Vesicles 2023, 12, e12297. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hu, X.; Li, L.; Fang, Y.; Yang, Y.; Gu, J.; Xu, J.; Chu, L. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in Ischemic Stroke Mice via Upregulation of MiR-21-5p. Biomolecules 2022, 12, 883. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J. Identification of miRNA-21 and miRNA-24 in Plasma as Potential Early Stage Markers of Acute Cerebral Infarction. Mol. Med. Rep. 2014, 10, 971–976. [Google Scholar] [CrossRef]
- Jin, H.; Li, D.Y.; Chernogubova, E.; Sun, C.; Busch, A.; Eken, S.M.; Saliba-Gustafsson, P.; Winter, H.; Winski, G.; Raaz, U.; et al. Local Delivery of miR-21 Stabilizes Fibrous Caps in Vulnerable Atherosclerotic Lesions. Mol. Ther. 2018, 26, 1040–1055. [Google Scholar] [CrossRef]
- Raskurazhev, A.A.; Tanashyan, M.M.; Shabalina, A.A.; Kuznetsova, P.I.; Kornilova, A.A.; Burmak, A.G. Micro-RNA in Patients with Carotid Atherosclerosis. Hum. Physiol. 2020, 46, 880–885. [Google Scholar] [CrossRef]
- Lopez, M.S.; Morris-Blanco, K.C.; Ly, N.; Maves, C.; Dempsey, R.J.; Vemuganti, R. MicroRNA miR-21 Decreases Post-Stroke Brain Damage in Rodents. Transl. Stroke Res. 2022, 13, 483–493. [Google Scholar] [CrossRef]
- Buller, B.; Liu, X.; Wang, X.; Zhang, R.L.; Zhang, L.; Hozeska-Solgot, A.; Chopp, M.; Zhang, Z.G. MicroRNA-21 protects neurons from ischemic death. FEBS J. 2010, 277, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Moradi, Z.; Rabiei, Z.; Anjomshoa, M.; Amini-Farsani, Z.; Massahzadeh, V.; Asgharzade, S. Neuroprotective Effect of Wild Lowbush Blueberry (Vaccinium angustifolium) on Global Cerebral Ischemia/Reperfusion Injury in Rats: Downregulation of iNOS/TNF-α and Upregulation of miR-146a/miR-21 Expression. Phytother. Res. 2021, 35, 6428–6440. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Le, D.; Wang, C.; Mao, G. Pterostilbene Attenuates Ischemic Stroke by Modulating miR-21-5p/PDCD4 Axis In Vivo and In Vitro. J. Funct. Foods 2020, 75, 104275. [Google Scholar] [CrossRef]
- Unal, S.; Arapi, B.; Omeroglu, S.N.; Rouhi, V.; Guven, M. The Upregulation of miR-21-5p in Atherosclerotic Plaque. Mol. Biol. Rep. 2025, 52, 686. [Google Scholar] [CrossRef]
- Huang, R.; Huang, Y.; Zeng, G.; Li, M.; Jin, Y. Ursodeoxycholic Acid Inhibits Intimal Hyperplasia, Vascular Smooth Muscle Cell Excessive Proliferation, Migration via Blocking miR-21/PTEN/AKT/mTOR Signaling Pathway. Cell Cycle 2020, 19, 918–932. [Google Scholar] [CrossRef]
- Bahar, A.; Akbar, M.; Bintang, A.K.; Massi, M.N.; Ladju, R.B.; Bukhari, A.; Satriotomo, I. Expression of MicroRNA-21 in Acute Ischemic Stroke: Relationship with Inflammatory Cytokines, Clinical Severity, and Clinical Outcome. F1000Research 2024, 13, 1142. [Google Scholar] [CrossRef]
- Barwari, T.; Rienks, M.; Mayr, M. MicroRNA-21 and the Vulnerability of Atherosclerotic Plaques. Mol. Ther. 2018, 26, 938–940. [Google Scholar] [CrossRef]
- Canfrán-Duque, A.; Rotllan, N.; Zhang, X.; Fernández-Fuertes, M.; Ramírez-Hidalgo, C.; Araldi, E.; Daimiel, L.; Busto, R.; Fernández-Hernando, C.; Suárez, Y. Macrophage Deficiency of miR-21 Promotes Apoptosis, Plaque Necrosis, and Vascular Inflammation during Atherogenesis. EMBO Mol. Med. 2017, 9, 1244–1262. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, M.; Schober, A. Macrophage MicroRNAs as Therapeutic Targets for Atherosclerosis, Metabolic Syndrome, and Cancer. Int. J. Mol. Sci. 2018, 19, 1756. [Google Scholar] [CrossRef] [PubMed]
- Wassaifi, S.; Kaeffer, B.; Zarrouk, S. Cellular Phenotypic Transformation During Atherosclerosis: The Potential Role of miRNAs as Biomarkers. Int. J. Mol. Sci. 2025, 26, 2083. [Google Scholar] [CrossRef]
- Euler, G.; Parahuleva, M.S. Monocytic microRNAs—Novel Targets in Atherosclerosis Therapy. Br. J. Pharmacol. 2025, 182, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Lightbody, R.J.; Taylor, J.M.W.; Dempsie, Y.; Graham, A. MicroRNA Sequences Modulating Inflammation and Lipid Accumulation in Macrophage “Foam” Cells: Implications for Atherosclerosis. World J. Cardiol. 2020, 12, 303–333. [Google Scholar] [CrossRef]
- Gao, L.; Zeng, H.; Zhang, T.; Mao, C.; Wang, Y.; Han, Z.; Chen, K.; Zhang, J.; Fan, Y.; Gu, J.; et al. MicroRNA-21 Deficiency Attenuated Atherogenesis and Decreased Macrophage Infiltration by Targeting Dusp-8. Atherosclerosis 2019, 291, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.; Enrick, M.; Diaz, A.; Yin, L. Is miR-21 a Therapeutic Target in Cardiovascular Disease? Int. J. Drug Discov. Pharmacol. 2023, 2, 26–36. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Mrakic-Sposta, S.; Micheloni, S.; Prattichizzo, F.; Ceriello, A. Glucose-Sensing MicroRNA-21 Disrupts ROS Homeostasis and Impairs Antioxidant Responses in Cellular Glucose Variability. Cardiovasc. Diabetol. 2018, 17, 105. [Google Scholar] [CrossRef]
- Huang, S.; Xu, T.; Huang, X.; Li, S.; Qin, W.; Chen, W.; Zhang, Z. miR-21 Regulates Vascular Smooth Muscle Cell Function in Arteriosclerosis Obliterans of Lower Extremities through AKT and ERK1/2 Pathways. Arch. Med. Sci. 2019, 15, 1490–1497. [Google Scholar] [CrossRef]
- Wang, G.; Luo, Y.; Gao, X.; Liang, Y.; Yang, F.; Wu, J.; Fang, D.; Luo, M. MicroRNA Regulation of Phenotypic Transformations in Vascular Smooth Muscle: Relevance to Vascular Remodeling. Cell. Mol. Life Sci. 2023, 80, 144. [Google Scholar] [CrossRef]
- Sun, P.; Tang, L.N.; Li, G.Z.; Xu, Z.L.; Xu, Q.H.; Wang, M.; Li, L. Effects of miR-21 on the Proliferation and Migration of Vascular Smooth Muscle Cells in Rats with Atherosclerosis via the Akt/ERK Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cai, Y.; Yang, F.; Yang, Y.; Cui, Z.; Shi, D.; Bai, R. Vascular Smooth Muscle Cell Phenotypic Switching in Atherosclerosis. Heliyon 2024, 10, e37727. [Google Scholar] [CrossRef]
- Araldi, E.; Suárez, Y. MicroRNAs as Regulators of Endothelial Cell Functions in Cardiometabolic Diseases. Biochim. Biophys. Acta 2016, 1861, 2094–2103. [Google Scholar] [CrossRef]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial Dysfunction: The Early Predictor of Atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef]
- Marin, T.; Gongol, B.; Chen, Z.; Woo, B.; Subramaniam, S.; Chien, S.; Shyy, J.Y. Mechanosensitive MicroRNAs—Role in Endothelial Responses to Shear Stress and Redox State. Free Radic. Biol. Med. 2013, 64, 61–68. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, K.C.; Wu, W.; Subramaniam, S.; Shyy, J.Y.; Chiu, J.J.; Li, J.Y.; Chien, S. MicroRNA-21 Targets Peroxisome Proliferator-Activated Receptor-Alpha in an Autoregulatory Loop to Modulate Flow-Induced Endothelial Inflammation. Proc. Natl. Acad. Sci. USA 2011, 108, 10355–10360. [Google Scholar] [CrossRef]
- Portius, D.; Sobolewski, C.; Foti, M. MicroRNAs-Dependent Regulation of PPARs in Metabolic Diseases and Cancers. PPAR Res. 2017, 2017, 7058424. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Saeedy, S.A.G.; Roodi, P.B.; Saedisomeolia, A. The Association between Zinc and Endothelial Adhesion Molecules ICAMs and VCAM-1 and Nuclear Receptors PPAR-α and PPAR-γ: A Systematic Review on Cell Culture, Animal and Human Studies. Microvasc. Res. 2021, 138, 104217. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Iaconetti, C.; Eyileten, C.; Yasuda, M.; Albanese, M.; Polimeni, A.; Sabatino, J.; Sorrentino, S.; Postula, M.; Indolfi, C. Flow-Responsive Noncoding RNAs in the Vascular System: Basic Mechanisms for the Clinician. J. Clin. Med. 2022, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Baker, M.B.; Moore, J.P.; Searles, C.D. miR-21 Is Induced in Endothelial Cells by Shear Stress and Modulates Apoptosis and eNOS Activity. Biochem. Biophys. Res. Commun. 2010, 393, 643–648. [Google Scholar] [CrossRef]
- Fernández-Hernando, C.; Suárez, Y. MicroRNAs in Endothelial Cell Homeostasis and Vascular Disease. Curr. Opin. Hematol. 2018, 25, 227–236. [Google Scholar] [CrossRef]
- Sabatel, C.; Malvaux, L.; Bovy, N.; Deroanne, C.; Lambert, V.; Gonzalez, M.L.; Colige, A.; Rakic, J.M.; Noël, A.; Martial, J.A.; et al. MicroRNA-21 Exhibits Antiangiogenic Function by Targeting RhoB Expression in Endothelial Cells. PLoS ONE 2011, 6, e16979. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Juo, S.H. MicroRNAs in Atherosclerosis. Kaohsiung J. Med. Sci. 2012, 28, 631–640. [Google Scholar] [CrossRef]
- Loftus, I. Mechanisms of Plaque Rupture. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; Chapter 4. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534259/ (accessed on 11 July 2025).
- Fan, X.; Wang, E.; Wang, X.; Cong, X.; Chen, X. MicroRNA-21 Is a Unique Signature Associated with Coronary Plaque Instability in Humans by Regulating MMP-9 via RECK. Exp. Mol. Pathol. 2014, 96, 145–154. [Google Scholar] [CrossRef]
- Salaudeen, M.A.; Bello, N.; Danraka, R.N.; Ammani, M.L. Understanding the Pathophysiology of Ischemic Stroke: The Basis of Current Therapies and Opportunity for New Ones. Biomolecules 2024, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Huang, W.; Rao, J.; Yuan, J. miR-21 Regulates Ischemic Neuronal Injury via the p53/Bcl-2/Bax Signaling Pathway. Aging 2021, 13, 22242–22255. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Luo, Z.; Peng, M.; Yan, H.; Yi, D.; Du, Z.; Liu, J. Expression Profile of Circulating miRNAs in Patients with Atrial Fibrillation-Dominated Cardioembolic Stroke: A Systematic Review and Bioinformatics Analysis. Heliyon 2024, 10, e35201. [Google Scholar] [CrossRef]
- Burlacu, C.-C.; Ciobanu, D.; Badulescu, A.-V.; Chelaru, V.-F.; Mitre, A.-O.; Capitanescu, B.; Hermann, D.M.; Popa-Wagner, A. Circulating MicroRNAs and Extracellular Vesicle-Derived MicroRNAs as Predictors of Functional Recovery in Ischemic Stroke Patients: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 251. [Google Scholar] [CrossRef]
- Edwardson, M.A.; Shivapurkar, N.; Li, J.; Khan, M.; Smith, J.; Giannetti, M.L.; Fan, R.; Dromerick, A.W. Expansion of Plasma MicroRNAs over the First Month Following Human Stroke. J. Cereb. Blood Flow Metab. 2023, 43, 2130–2143. [Google Scholar] [CrossRef]
- Eyileten, C.; Wicik, Z.; De Rosa, S.; Mirowska-Guzel, D.; Soplinska, A.; Indolfi, C.; Jastrzebska-Kurkowska, I.; Czlonkowska, A.; Postula, M. MicroRNAs as Diagnostic and Prognostic Biomarkers in Ischemic Stroke—A Comprehensive Review and Bioinformatic Analysis. Cells 2018, 7, 249. [Google Scholar] [CrossRef]
- Sheedy, F.J. Turning 21: Induction of miR-21 as a Key Switch in the Inflammatory Response. Front. Immunol. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, W.; Wang, S.; Xie, W.; Li, H.; Ning, B. microRNA-21 regulates astrocytic reaction post-acute phase of spinal cord injury through modulating TGF-β signaling. Aging 2018, 10, 1474–1488. [Google Scholar] [CrossRef]
- Sawant, H.; Sun, B.; Mcgrady, E.; Bihl, J.C. Role of miRNAs in neurovascular injury and repair. J. Cereb. Blood Flow Metab. 2024, 44, 1693–1708. [Google Scholar] [CrossRef]
- Berchtold, D.; Priller, J.; Meisel, C.; Meisel, A. Interaction of microglia with infiltrating immune cells in the different phases of stroke. Brain Pathol. 2020, 30, 1208–1218. [Google Scholar] [CrossRef]
- Slota, J.A.; Booth, S.A. MicroRNAs in Neuroinflammation: Implications in Disease Pathogenesis, Biomarker Discovery and Therapeutic Applications. Non-Coding RNA 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.B.; Hochfelder, C.G.; Lamberty, B.G.; Meays, B.M.; Morsey, B.M.; Kelso, M.L.; Fox, H.S.; Yelamanchili, S.V. Traumatic brain injury increases levels of miR-21 in extracellular vesicles: Implications for neuroinflammation. FEBS Open Bio 2016, 6, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Price, L.; Wilson, C.; Grant, G. Blood–Brain Barrier Pathophysiology following Traumatic Brain Injury. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016; Chapter 4. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326726/ (accessed on 11 July 2025).
- Yao, X.; Wang, Y.; Zhang, D. microRNA-21 Confers Neuroprotection Against Cerebral Ischemia-Reperfusion Injury and Alleviates Blood-Brain Barrier Disruption in Rats via the MAPK Signaling Pathway. J. Mol. Neurosci. 2018, 65, 43–53. [Google Scholar] [CrossRef]
- Deng, X.; Zhong, Y.; Gu, L.; Shen, W.; Guo, J. MiR-21 Involve in ERK-Mediated Upregulation of MMP9 in the Rat Hippocampus Following Cerebral Ischemia. Brain Res. Bull. 2013, 94, 56–62. [Google Scholar] [CrossRef]
- Li, H.J.; Pan, Y.B.; Sun, Z.L.; Sun, Y.Y.; Yang, X.T.; Feng, D.F. Inhibition of miR-21 ameliorates excessive astrocyte activation and promotes axon regeneration following optic nerve crush. Neuropharmacology 2018, 137, 33–49. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Buckwalter, M.S. Astrocytes: Integrative Regulators of Neuroinflammation in Stroke and Other Neurological Diseases. Neurotherapeutics 2016, 13, 685–701. [Google Scholar] [CrossRef]
- He, F.; Guan, W. The Role of miR-21 as a Biomarker and Therapeutic Target in Cardiovascular Disease. Clin. Chim. Acta 2025, 574, 120304. [Google Scholar] [CrossRef]
- Schober, A.; Maleki, S.S.; Nazari-Jahantigh, M. Regulatory Non-Coding RNAs in Atherosclerosis. In Prevention and Treatment of Atherosclerosis; Von Eckardstein, A., Binder, C.J., Eds.; Handbook of Experimental Pharmacology Series; Springer: Cham, Switzerland, 2020; Volume 270. [Google Scholar] [CrossRef]
- Chipont, A.; Esposito, B.; Challier, I.; Montabord, M.; Tedgui, A.; Mallat, Z.; Loyer, X.; Potteaux, S. MicroRNA-21 Deficiency Alters the Survival of Ly-6Clo Monocytes in ApoE-/- Mice and Reduces Early-Stage Atherosclerosis-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Khoshnam, S.E.; Winlow, W.; Farbood, Y.; Moghaddam, H.F.; Farzaneh, M. Emerging Roles of microRNAs in Ischemic Stroke: As Possible Therapeutic Agents. J. Stroke 2017, 19, 166–187. [Google Scholar] [CrossRef]
- Wasserman, A.H.; Abolibdeh, B.; Hamdan, R.; Hong, C.C. Stem-Cell Derived Exosomal microRNAs as Biomarkers and Therapeutics for Pediatric Cardiovascular Disease. Curr. Treat. Options Cardiovasc. Med. 2025, 27, 32. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.G.; Van Balkom, B.W.; Schiffelers, R.M.; Bouten, C.V.; Verhaar, M.C. Extracellular Vesicles: Potential Roles in Regenerative Medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef]
- Mehdizadeh, S.; Mamaghani, M.; Hassanikia, S.; Pilehvar, Y.; Ertas, Y.N. Exosome-Powered Neuropharmaceutics: Unlocking the Blood–Brain Barrier for Next-Gen Therapies. J. Nanobiotechnol. 2025, 23, 329. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, Z.; Webster, K.A.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; et al. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem Cells Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef]
- Mainali, S.; Nepal, G.; Shumilov, K.; Webb, A.; Fadda, P.; Mirebrahimi, D.; Hamed, M.; Nana-Sinkam, P.; Worrall, B.B.; Woo, D.; et al. MicroRNA Expression Profile in Acute Ischemic Stroke. Int. J. Mol. Sci. 2025, 26, 747. [Google Scholar] [CrossRef]
- Edwardson, M.A.; Zhong, X.; Fiandaca, M.S.; Evans, P.A.; Nie, Z.; Laskowitz, D.T.; Federoff, H.J.; Quinn, T.J.; Hu, S.C. Plasma MicroRNA Markers of Upper Limb Recovery Following Human Stroke. Sci. Rep. 2018, 8, 12558. [Google Scholar] [CrossRef]
- Mompeón, A.; Ortega-Paz, L.; Vidal-Gómez, X.; Sabaté-Tenas, M.; Bayés-Genís, A.; Suades, R.; Crespo, J.; Mateo, J.; Chiva-Blanch, G.; Sionis, A.; et al. Disparate miRNA Expression in Serum and Plasma of Patients with Acute Myocardial Infarction: A Systematic and Paired Comparative Analysis. Sci. Rep. 2020, 10, 5373. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Marumo, M.; Ekawa, K.; Daimon, T. Differences in Serum and Plasma Levels of MicroRNAs and Their Time-Course Changes after Blood Collection. Pract. Lab. Med. 2024, 39, e00376. [Google Scholar] [CrossRef]
- Accardi, G.; Bono, F.; Cammarata, G.; Aiello, A.; Herrero, M.T.; Alessandro, R.; Augello, G.; Carru, C.; Colomba, P.; Costa, M.A.; et al. miR-126-3p and miR-21-5p as Hallmarks of Bio-Positive Ageing; Correlation Analysis and Machine Learning Prediction in Young to Ultra-Centenarian Sicilian Population. Cells 2022, 11, 1505. [Google Scholar] [CrossRef]
- Sharma, S.; Eghbali, M. Influence of Sex Differences on MicroRNA Gene Regulation in Disease. Biol. Sex Differ. 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Krichevsky, A.M.; Gabriely, G. miR-21: A Small Multi-Faceted RNA. J. Cell. Mol. Med. 2009, 13, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Telkoparan-Akillilar, P.; Chichiarelli, S.; Tucci, P.; Saso, L. Integration of MicroRNAs with Nanomedicine: Tumor Targeting and Therapeutic Approaches. Front. Cell Dev. Biol. 2025, 13, 1569101. [Google Scholar] [CrossRef]
- de Sousa, M.C.; Calo, N.; Sobolewski, C.; Gjorgjieva, M.; Clément, S.; Maeder, C.; Dolicka, D.; Fournier, M.; Vinet, L.; Montet, X.; et al. Mir-21 Suppression Promotes Mouse Hepatocarcinogenesis. Cancers 2021, 13, 4983. [Google Scholar] [CrossRef]
- Rhim, J.; Baek, W.; Seo, Y.; Kim, J.H. From Molecular Mechanisms to Therapeutics: Understanding MicroRNA-21 in Cancer. Cells 2022, 11, 2791. [Google Scholar] [CrossRef]
- Martino, M.T.D.; Tagliaferri, P.; Tassone, P. MicroRNA in Cancer Therapy: Breakthroughs and Challenges in Early Clinical Applications. J. Exp. Clin. Cancer Res. 2025, 44, 126. [Google Scholar] [CrossRef]
- Androsavich, J.R.; Chau, B.N.; Bhat, B.; Linsley, P.S.; Walter, N.G. Disease-Linked microRNA-21 Exhibits Drastically Reduced mRNA Binding and Silencing Activity in Healthy Mouse Liver. RNA 2012, 18, 1510–1526. [Google Scholar] [CrossRef]
- Mollajan, E.; Yazdani, S.; Ghasemzadeh, M.; Mozhgani, S.-H. miR-21 in Cardiovascular Disease: New Insights and Emerging Therapeutic Potential. Discov. Appl. Sci. 2025, 7, 447. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Zhou, Q.; Wang, Y.; Liu, X.; Liu, Z.; Zhan, Z. MicroRNA-21 Prevents Excessive Inflammation and Cardiac Dysfunction after Myocardial Infarction through Targeting KBTBD7. Cell Death Dis. 2018, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Huang, Z. Recent Progress in MicroRNA-Based Delivery Systems for the Treatment of Human Disease. ExRNA 2019, 1, 24. [Google Scholar] [CrossRef]
- Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25, 1469. [Google Scholar] [CrossRef]
- Song, R.; Zhang, L. MicroRNAs and Therapeutic Potentials in Acute and Chronic Cardiac Disease. Drug Discov. Today 2024, 29, 104179. [Google Scholar] [CrossRef]
- Bazan, H.A.; Hatfield, S.A.; Brug, A.; Brooks, A.J.; Lightell, D.J., Jr.; Woods, T.C. Carotid Plaque Rupture Is Accompanied by an Increase in the Ratio of Serum circR-284 to miR-221 Levels. Circ. Cardiovasc. Genet. 2017, 10, e001720. [Google Scholar] [CrossRef] [PubMed]
- Jasineviciute, I.; Hasan, M.N.; Grigas, J.; Pautienius, A.; Stankevicius, A.; Zymantiene, J.; Miura, N. microRNAs Are Abundant and Stable in Platelet-Rich Fibrin and Other Autologous Blood Products of Canines. Int. J. Mol. Sci. 2023, 24, 770. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Perich, L.; Puigoriol-Illamola, D.; Bashir, S.; Terceño, M.; Silva, Y.; Gubern-Mérida, C.; Serena, J. Clinical Parameters and Epigenetic Biomarkers of Plaque Vulnerability in Patients with Carotid Stenosis. Int. J. Mol. Sci. 2022, 23, 5149. [Google Scholar] [CrossRef]
- Dolz, S.; Górriz, D.; Tembl, J.I.; Sánchez, D.; Fortea, G.; Parkhutik, V.; Lago, A. Circulating MicroRNAs as Novel Biomarkers of Stenosis Progression in Asymptomatic Carotid Stenosis. Stroke 2017, 48, 10–16. [Google Scholar] [CrossRef]
- Pointner, A.; Krammer, U.D.B.; Tomeva, E.; Magnet, U.; Hippe, B.; Jacob, U.; Haslberger, A.G. Lifestyle-Driven Variations in Nutrimiromic MicroRNA Expression Patterns across and beyond Genders. Life 2024, 14, 390. [Google Scholar] [CrossRef]
- Jenike, A.E.; Halushka, M.K. miR-21: A Non-Specific Biomarker of All Maladies. Biomark. Res. 2021, 9, 18. [Google Scholar] [CrossRef]
- Borja-Gonzalez, M.; Casas-Martinez, J.C.; McDonagh, B.; Goljanek-Whysall, K. Inflamma-miR-21 Negatively Regulates Myogenesis during Ageing. Antioxidants 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hu, B.; Jadhav, R.R.; Jin, J.; Zhang, H.; Cavanagh, M.M.; Akondy, R.S.; Ahmed, R.; Weyand, C.M.; Goronzy, J.J. Activation of miR-21-Regulated Pathways in Immune Aging Selects against Signatures Characteristic of Memory T Cells. Cell Rep. 2018, 25, 2148–2162.e5. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Giuliani, A.; Matacchione, G.; Rippo, M.R.; Sabbatinelli, J.; Bonafè, M. miR-21 and miR-146a: The microRNAs of Inflammaging and Age-Related Diseases. Ageing Res. Rev. 2021, 70, 101374. [Google Scholar] [CrossRef]
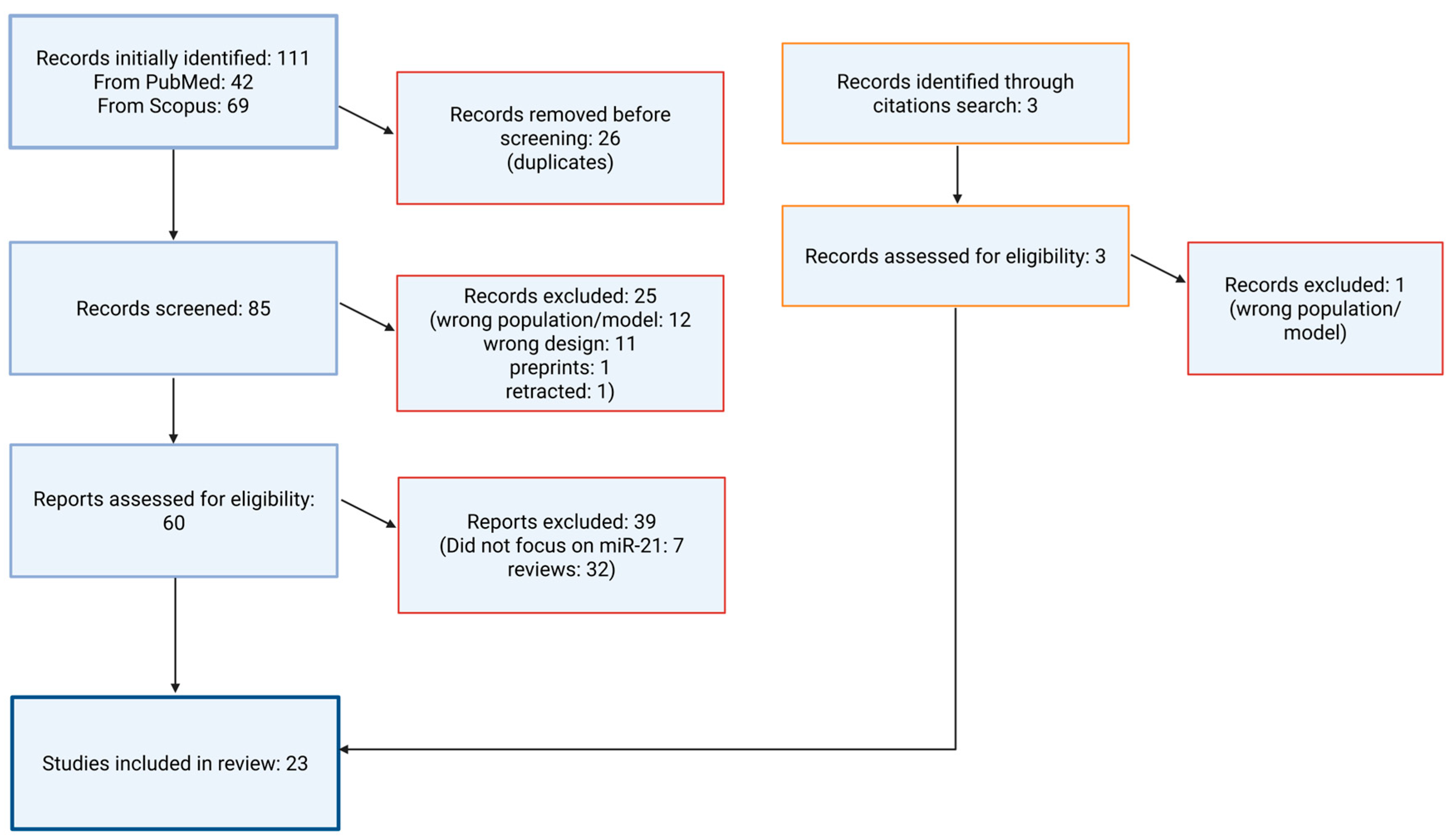
| Database | Search Syntax |
|---|---|
| PubMed | (microRNA-21 OR miR-21 or miRNA-21) and (carotid artery disease or ischemic stroke) and (diagnosis or prognosis or treatment or biomarker or early detection or predictive value or treatment outcome or drug response or targeted therapy) |
| Scopus | Title-Abs-Key (“microRNA-21” or “miR-21” or “miRNA-21”) and Title-Abs-Key (“carotid artery disease” or “ischemic stroke”) and Title-Abs-Key (“diagnosis” or “prognosis” or “treatment” or “biomarker” or “early detection” or “predictive value” or “treatment outcome”or “drug response” or “targeted therapy”) and Limit-To Language, “English”) |
| Author/Year | Study Type | Population/ Model | Translational Stage (T0–T4) | Disease Focus | Pathophysiological Mechanism | Main Findings | Implications |
|---|---|---|---|---|---|---|---|
| Zhan et al., 2023 [13] | Observational clinical + in vitro functional study | Ischemic stroke patients (n = 60), healthy controls (n = 23); OGD-treated HMEC-1 cells | T2 | Ischemic stroke | miR-21-5p suppresses IL-6R; downregulated in patients; IL-6R linked to ischemic injury. | miR-21-5p ↓ in patients (p < 0.001); overexpression improves cell viability and reduces apoptosis. | Potential biomarker and therapeutic target for reperfusion injury. |
| Mohammed et al., 2022 [14] | Clinical observational human and in vitro study | A total of 60 AIS patients vs. 60 healthy controls; HMEC-1 cells under OGD ± miR-21-5p mimic/inhibitor | T1 | Acute ischemic stroke | Four ncRNAs linked to AIS: TUG1 ↑, NBAT1 ↑, miR-21 ↑, miR-335 ↓; correlations with lipid and thyroid profile). | NBAT1: 100% sens/spec; TUG1: 80% sens; miR-335: 73.3% sens, 100% spec. | Novel non-invasive biomarkers; link to atherosclerosis and thyroid function. |
| Xiang et al., 2017 [15] | Genetic association case–control | A total of 592 ischemic stroke patients vs. 456 healthy controls | T2 | Ischemic stroke | miR-21 rs1292037T > C polymorphism; circulating miR-21; miR-126G > A linked to ↓ stroke risk. | miR-21 ↑ in stroke (p < 0.001); miR-126G > A polymorphism associated with reduced stroke risk. | miR-126/miR-21 expression may serve as biomarkers and therapeutic targets. |
| Liu et al., 2021 [16] | Clinical observational correlation study | A total of 170 AIS patients vs. 100 high-risk controls; PBMCs, cytokine ELISA; 36-month follow-up for recurrence | T1 | Acute ischemic stroke | lnc-MEG3 ↑ and miR-21 ↓ regulate inflammation and vascular microenvironment. | lnc-MEG3 ↑ (AUC 0.874), miR-21 ↓ (AUC 0.889) in AIS; correlation with recurrence risk. | Diagnostic/prognostic value; potential targets for reducing post-stroke inflammation and recurrence. |
| Li et al., 2019 [17] | Preclinical animal + in vitro study | Male SD rats (n = 126), MCAO model; SH-SY5Y and HMEC-1 cells under OGD/reoxygenation ± miR-21-3p | T0 | Ischemic stroke | ADMSCs suppress miR-21-3p → MAT2B ↑ → apoptosis and inflammation ↓; BBB improved. | ADMSCs reduced infarct size, apoptosis, IL-1β/IL-6/TNF-α; miR-21-3p inhibition increased viability. | Targeting miR-21-3p/MAT2B via ADMSCs or inhibitors may improve stroke recovery. |
| Tsai et al., 2013 [18] | Observational human study | A total of 167 ischemic stroke patients; 66 carotid atherosclerosis subjects; 157 healthy controls | T1 | Ischemic stroke, atherosclerosis | Circulating miR-21 increases with stroke severity. | miR-21 significantly elevated in stroke and atherosclerosis vs. controls; independent predictor. | miR-21 may serve as a biomarker for stroke and carotid atherosclerosis. |
| Lei et al., 2024 [19] | In vivo animal preclinical intervention | Male SD rats, MCAO model; 4 groups (Sham, Surgery, Acupuncture, Nimodipine); 15-day acupuncture; cognitive testing | T0 | Ischemic stroke | Acupuncture ↓ REST → ↑ miR-21-3p → ↓ PDCD4 → ↓ apoptosis, ↑ cognitive function. | Acupuncture reduced REST, increased miR-21-3p, decreased cytokines/apoptosis, and improved cognition. | Acupuncture may aid post-stroke recovery via REST/miR-21-3p axis; potential complementary therapy. |
| Zhang et al., 2023 [20] | Clinical observational biomarker study | A total of 84 AIS patients, 39 TIA patients, 30 healthy controls | T3 | Acute ischemic stroke, TIA | Serum miR-21-5p upregulated with severity and poor outcome. | miR-21-5p ↑ in AIS vs. TIA and controls; correlated with NIHSS and mRS; AUC = 0.710 for AIS vs. TIA. | miR-21-5p may help differentiate AIS from TIA and predict short-term outcome. |
| Wang, 2018 [21] | Clinical biomarker observational study | A total of 143 IS patients (by phase) + 24 controls; plasma exosomes analyzed | T1 | Ischemic stroke | Exosomal miR-21-5p/miR-30a-5p dynamically change with stroke stage. | miR-21-5p ↑ in subacute and recovery phases; best AUC ~0.7 for later phases. | Exosomal miRNAs may aid diagnosis, staging, and timing of therapy in stroke. |
| Vibo et al., 2024 [22] | Clinical observational study | A total of 73 young patients with cryptogenic and LAA stroke; blood samples at onset and 1-year follow-up | T2 | Ischemic stroke (Cryp vs. LAA) | Inflammatory gene/miRNA upregulation (e.g., miR-21) in cryptogenic stroke. | Cryp stroke: ↑ miR-21, ICAM1, TNF, IL1B during acute phase vs. follow-up; correlated with hs-CRP and severity. | Suggests role of miR-21 in inflammation and severity of cryptogenic stroke. |
| Wu et al., 2017 [23] | Clinical cohort + biomarker study | Serum: 50 IS patients, 50 controls (screening); 177 IS, 81 TIA, 42 controls (validation) | T1–T2 | Ischemic stroke, TIA | Circulating miRNAs reflect neurovascular injury, inflammation, stress response. | miR-21-5p ↑ in IS vs. TIA; logistic regression and ROC: predictive and discriminative potential. | miR-21-5p may help assess stroke severity and risk after TIA. |
| Korvenlaita et al., 2023 [24] | Preclinical animal study (biomarker focus) | Male Balb/c mice, permanent MCAO model | T0–T1 | Ischemic stroke, hypoxia | Hypoxia rapidly ↑ miR-21a-5p in EVs; majority remains in non-EV form. | miR-21a-5p = most deregulated neuronal miRNA; EV and non-EV miR-21 correlate with worse outcome. | Circulating miR-21a-5p may predict disability and guide early post-stroke care. |
| Hu et al., 2022 [25] | Preclinical in vivo + in vitro study | Stroke mouse model; HUVECs for angiogenesis assays | T0–T1 | Ischemic stroke recovery | BMSC-derived exosomes deliver miR-21-5p → ↑ angiogenesis via VEGF, Ang-1, Tie-2. | BMSC-Exos ↓ infarct size, ↑ neurological function and micro vessel density; miR-21-5p enhanced EC migration. | BMSC-Exos may enable cell-free stroke therapy by promoting angiogenesis through miR-21-5p. |
| Zhou et al., 2014. [26] | Mechanistic + biomarker study | In vitro OGD (N2A cells); plasma from 68 ACI patients and 21 controls | T1–T2 | Acute cerebral infarction | miR-21 (anti-apoptotic) and miR-24 (pro-apoptotic) regulate Bcl-2, XIAP. | Plasma miR-21 and miR-24 were lower in ACI; both negatively correlated with NIHSS. | miR-21 and miR-24 may serve as early diagnostic/prognostic biomarkers and therapeutic targets. |
| Jin et al., 2018 [27] | Experimental (mechanistic + therapeutic) | Human plaques (n = 20); Apoe−/−, miR-21−/−, and Apoe−/−miR-21−/− mice; local miR-21 delivery | T0–T1 | Atherosclerosis (carotid) | miR-21 modulates SMC proliferation, macrophage activity; REST–miR-21–REST feedback loop. | miR-21 ↓ in unstable plaques; miR-21 deficiency → plaque rupture; local miR-21 stabilized plaques. | miR-21 is a potential therapeutic target for plaque stabilization in atherosclerosis. |
| Raskurazhev et al., 2020 [28] | Observational human case–control study | A total of 25 carotid atherosclerosis patients vs. 11 controls; leukocyte miRNA from blood plasma | T1 | Carotid atherosclerosis | miR-21 inhibits Pdcd4 in VSMCs and macrophages, reducing apoptosis and inflammation. | miR-21-5p/3p downregulated in CA; expression correlated with anti-atherogenic mechanisms. | miR-21 may have diagnostic and protective roles in CA; microRNAs could be future therapeutic targets. |
| Lopez et al., 2022 [29] | Experimental animal intervention study | Adult and aged male/female C57BL/6 mice; transient MCAO model | T1 | Ischemic stroke | miR-21 mimic suppresses pro-apoptotic, inflammatory, and autophagy mRNAs. | miR-21 mimic ↓ infarct volume and improved motor recovery; effective via intracerebral or IV delivery. | Supports miR-21-based neuroprotection as a promising post-stroke therapeutic strategy. |
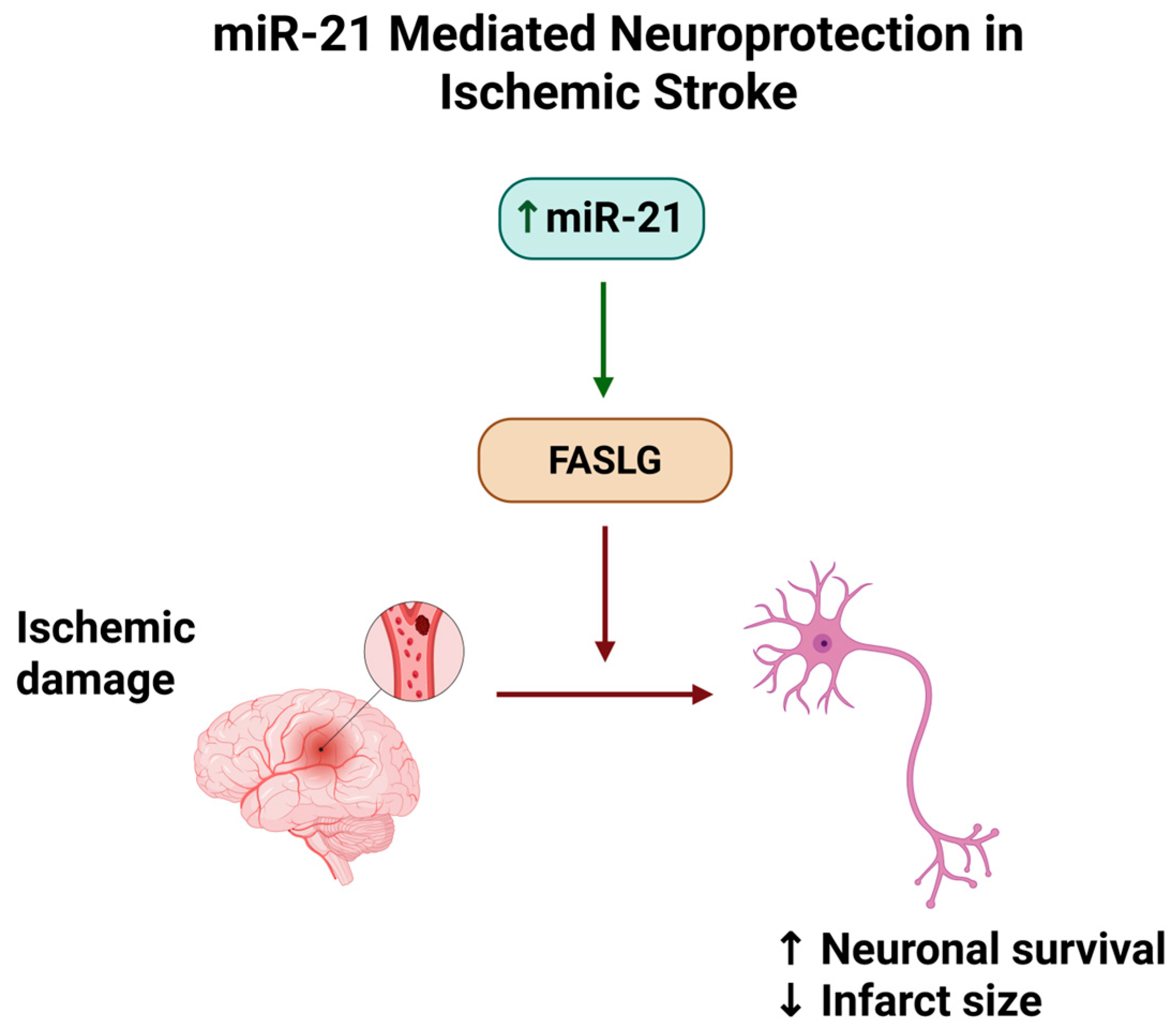
| Buller et al., 2010 [30] | Experimental in vivo + in vitro | Male Wistar rats with embolic MCAO; cultured cortical neurons | T1 | Ischemic stroke | miR-21 upregulation inhibits FASLG, reducing neuronal apoptosis. | miR-21 protected neurons post-stroke by directly targeting pro-apoptotic FASLG. | miR-21/FASLG axis represents a potential therapeutic target for stroke treatment. |
| Moradi et al., 2021 [31] | Preclinical therapeutic intervention | Rat model of cerebral ischemia–reperfusion injury (BCCAO) | T1 | Ischemic stroke | Wild blueberry extract modulates miR-21 and miR-146a; reduces iNOS, TNF-α, and oxidative stress. | Extract ↑ miR-21/miR-146a, ↓ inflammation and oxidative damage; preserved hippocampal neurons. | Diet-derived compounds like blueberry extract may activate protective miRNA pathways post-stroke. |
| Tu et al., 2020 [32] | Preclinical therapeutic study | SH-SY5Y neuronal cells + mouse model of ischemic stroke | T1 | Ischemic stroke | Pterostilbene ↑ miR-21-5p → ↓ PDCD4 → ↓ apoptosis and infarct size. | Pterostilbene reduced infarct size and neuronal apoptosis; effect mediated by miR-21-5p upregulation. | Suggests therapeutic potential of Pterostilbene via miR-21-5p modulation in stroke treatment. |
| Unal et al., 2025 [33] | Observational gene expression | A total of 50 patients with CAD and carotid atherosclerosis; samples from plaques and internal mammary arteries | T1 | Carotid and coronary atherosclerosis | Dysregulated miRNAs drive plaque inflammation and remodeling. | miR-21-5p ↑ 22-fold in plaques vs. healthy tissue (p = 0.0001.) | miR-21-5p may serve as biomarker and therapeutic target in atherosclerosis. |
| Huang et al., 2020 [34] | Preclinical in vivo + in vitro study | SD rats (carotid ligation model); cultured rat vascular smooth muscle cells (VSMCs) | T1 | Atherosclerosis, restenosis | UDCA ↓ miR-21 → ↑ PTEN → ↓ AKT/mTOR → ↓ VSMC proliferation and migration. | UDCA suppressed intimal hyperplasia and VSMC growth; miR-21 overexpression reversed UDCA effects. | UDCA may prevent vascular remodeling via the miR-21/PTEN/AKT/mTOR pathway. |
| Bahar et al., 2024 [35] | Case–control study | A total of 64 acute ischemic stroke patients and 22 age-matched controls (Indonesia) | T2 | Acute ischemic stroke | miR-21 and cytokines (TNF-α, IL-10, ICAM-1, CCL5) involved in inflammation. | miR-21 significantly ↑ in stroke; cytokines elevated; no correlation with NIHSS or mRS scores. | miR-21 may be diagnostic marker, but not predictive of short-term stroke severity/outcome. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sič, A.; Atanasković, M.; Ahmed, A.; Petrović, I.; Simović, F.; Burnjaković, B.; Tonković, U.; Manzar, A.; Shadab, S.; Gajić, S.; et al. The Association of MicroRNA-21 with Carotid Artery Disease and Ischemic Stroke: From Pathophysiology to Clinical Implications and Potential Therapy. Med. Sci. 2025, 13, 172. https://doi.org/10.3390/medsci13030172
Sič A, Atanasković M, Ahmed A, Petrović I, Simović F, Burnjaković B, Tonković U, Manzar A, Shadab S, Gajić S, et al. The Association of MicroRNA-21 with Carotid Artery Disease and Ischemic Stroke: From Pathophysiology to Clinical Implications and Potential Therapy. Medical Sciences. 2025; 13(3):172. https://doi.org/10.3390/medsci13030172
Chicago/Turabian StyleSič, Aleksandar, Marko Atanasković, Alyan Ahmed, Ivan Petrović, Filip Simović, Boris Burnjaković, Una Tonković, Aarish Manzar, Simra Shadab, Selena Gajić, and et al. 2025. "The Association of MicroRNA-21 with Carotid Artery Disease and Ischemic Stroke: From Pathophysiology to Clinical Implications and Potential Therapy" Medical Sciences 13, no. 3: 172. https://doi.org/10.3390/medsci13030172
APA StyleSič, A., Atanasković, M., Ahmed, A., Petrović, I., Simović, F., Burnjaković, B., Tonković, U., Manzar, A., Shadab, S., Gajić, S., Bjelić, D., Ristanović, V. K., & Baralić, M. (2025). The Association of MicroRNA-21 with Carotid Artery Disease and Ischemic Stroke: From Pathophysiology to Clinical Implications and Potential Therapy. Medical Sciences, 13(3), 172. https://doi.org/10.3390/medsci13030172










