Comparison of HyFoSy, HyCoSy and X-Ray Hysterosalpingography in the Assessment of Tubal Patency in Women with Infertility: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Risk of Bias Assessment
2.8. Statistical Analysis
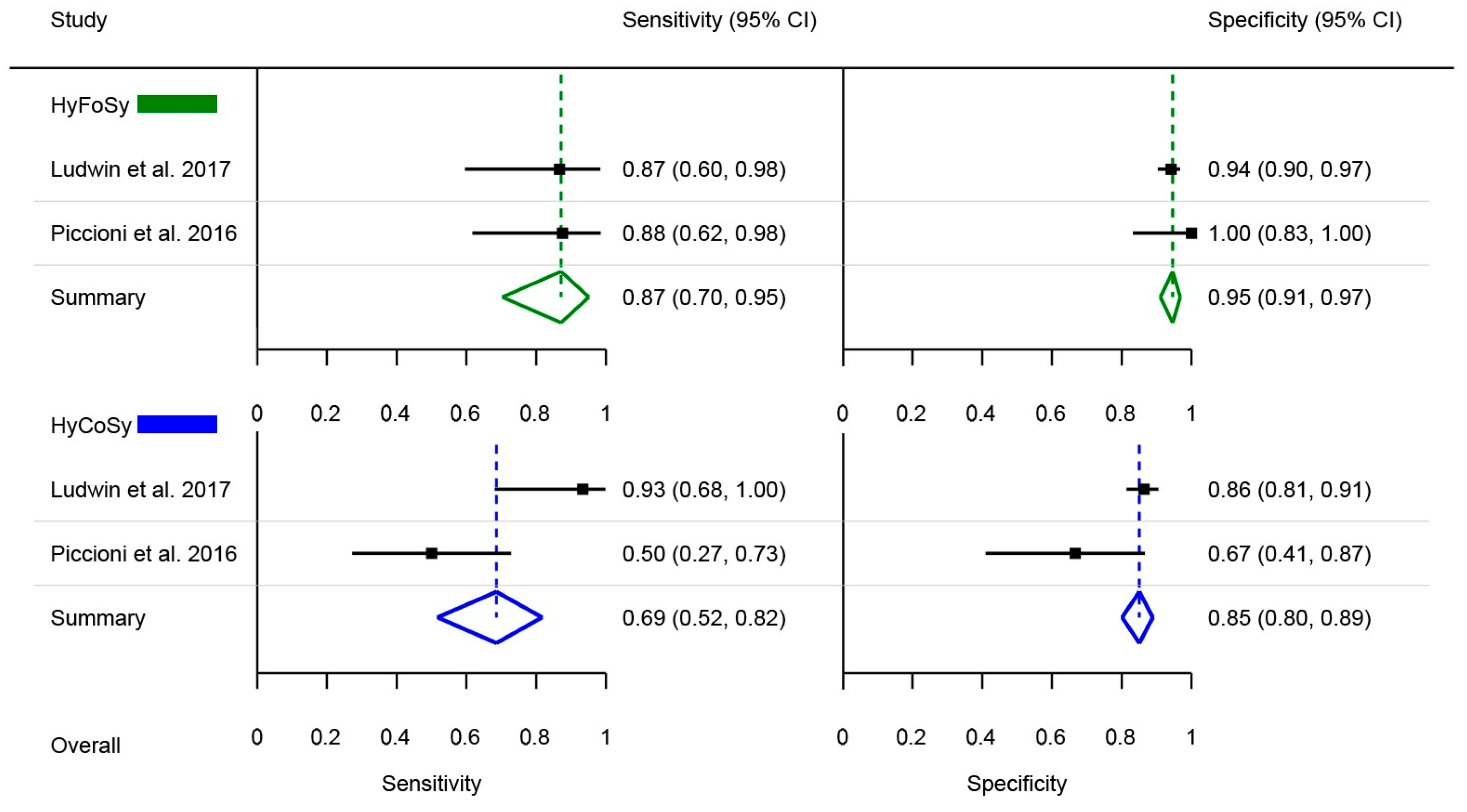
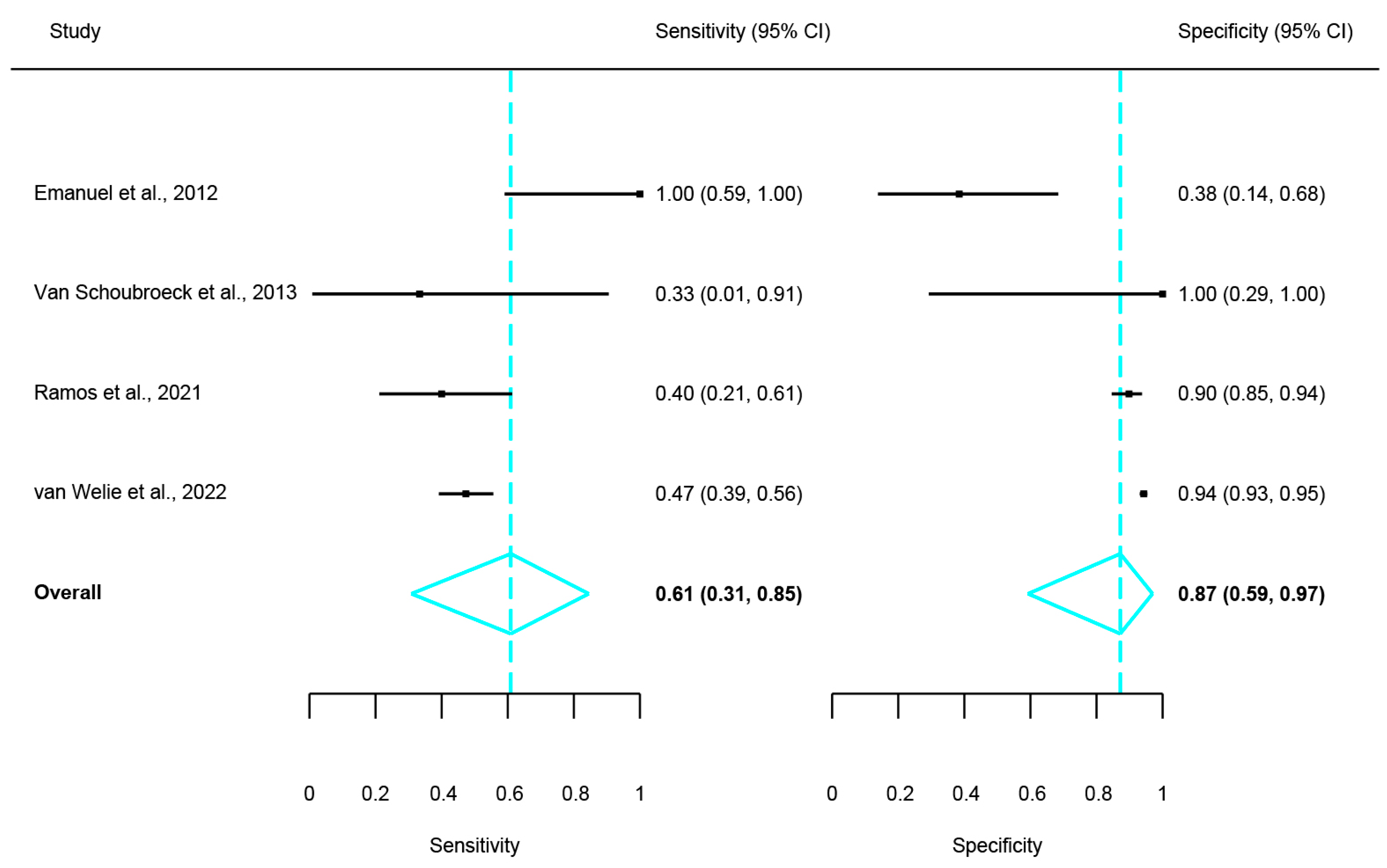
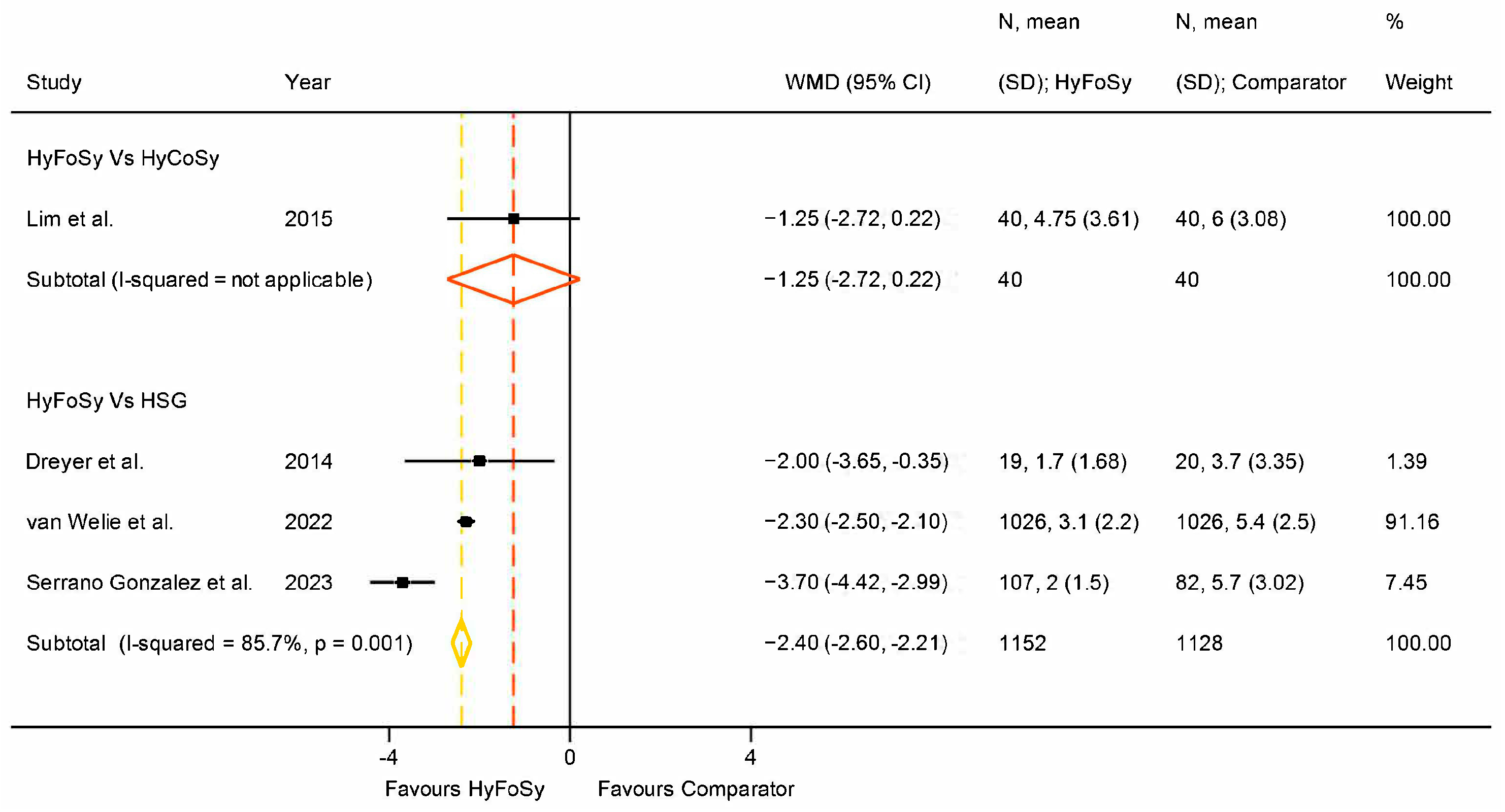
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HyFoSy | Hysterosalpingo-Foam Sonography |
| HyCoSy | Hysterosalpingo-Contrast Sonography |
| HSG | Hysterosalpingography |
References
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef]
- Ramos, J.; Pellicer, N.; Fernández-Sánchez, M. Hysterosalpingography is obsolete: Hysterosalpingo-contrast foam sonography should be the alternatived. Reprod. Biomed. Online 2022, 45, 839–842. [Google Scholar] [CrossRef]
- Dun, E.C.; Nezhat, C.H. Tubal factor infertility: Diagnosis and management in the era of assisted reproductive technology. Obstet. Gynecol. Clin. N. Am. 2012, 39, 551–566. [Google Scholar] [CrossRef]
- Rajesh, H.; Lim, S.L.; Yu, S.L. Hysterosalpingo-foam sonography: Patient selection and perspectives. Int. J. Womens Health 2017, 9, 23–32. [Google Scholar] [CrossRef]
- Saunders, R.D.; Shwayder, J.M.; Nakajima, S.T. Current methods of tubal patency assessment. Fertil. Steril. 2011, 95, 2171–2179. [Google Scholar] [CrossRef]
- Maxim, A.R.; Gligor, O.H.; Badea, R.I. Comparison of Hystero-salpingography and Hysterosalpingo-Contrast Sonography for tubal patency testing: Technical success, pain perception, side effects and complications. Med. Ultrason. 2021, 23, 283–288. [Google Scholar] [CrossRef]
- Lim, C.P.; Hasafa, Z.; Bhattacharya, S.; Maheshwari, A. Should a hysterosalpingogram be a first-line investigation to diagnose female tubal subfertility in the modern subfertility workup? Hum. Reprod. 2011, 26, 967–971. [Google Scholar] [CrossRef]
- Graziano, A.; Lo Monte, G.; Soave, I.; Caserta, D.; Moscarini, M.; Marci, R. Sonohysterosalpingography: A suitable choice in infertility workup. J. Med. Ultrason. 2013, 40, 225–229. [Google Scholar] [CrossRef]
- Stacey, C.; Bown, C.; Manhire, A.; Rose, D. HyCoSy–as good as claimed? Br. J. Radiol. 2000, 73, 133–136. [Google Scholar] [CrossRef]
- Bohîlțea, R.-E.; Mihai, B.-M.; Stănică, C.-D.; Gheorghe, C.-M.; Berceanu, C.; Dima, V.; Bohîlțea, A.-T.; Neagu, S.; Vlădăreanu, R. Technical Tips and Tricks after 10 Years of HyFoSy for Tubal Patency Testing. J. Clin. Med. 2022, 11, 5946. [Google Scholar] [CrossRef]
- Emanuel, M.H.; Exalto, N. Hysterosalpingo-foam sonography (HyFoSy): A new technique to visualize tubal patency. Ultrasound Obstet. Gynecol. 2011, 37, 498–499. [Google Scholar] [CrossRef]
- Tsakos, E.; Xydias, E.M.; Emmanouil, V.; Koutini, M.; Ntanika, A.; Prior, M.; Lykeridou, K.; Sarris, I.; Ziogas, A.C. O-271 Application of HyFoSy in the assessment of fallopian tube patency compared to HyCoSy and HS; results of a systematic review and meta-analysis. Hum. Reprod. 2023, 38, dead093.325. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, J.B.R.A.; Whiting, P.; Vlassov, V.V.; Leeflang, M.M.G.; Deeks, J.J. Chapter 9: Assessing methodological quality. In Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, Version 1.0.0; Deeks, J.J.B.P., Gatsonis, C., Eds.; The Cochrane Collaboration: London, UK, 2010; Available online: https://srdta.cochrane.org/ (accessed on 30 March 2025).
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Emanuel, M.H.; van Vliet, M.; Weber, M.; Exalto, N. First experiences with hysterosalpingo-foam sonography (HyFoSy) for office tubal patency testing. Hum. Reprod. 2012, 27, 114–117. [Google Scholar] [CrossRef]
- Van Schoubroeck, D.; Van Den Bosch, T.; Meuleman, C.; Tomassetti, C.; D’Hooghe, T.; Timmerman, D. The use of a new gel foam for the evaluation of tubal patency. Gynecol. Obstet. Investig. 2013, 75, 152–156. [Google Scholar] [CrossRef]
- Dreyer, K.; Out, R.; Hompes, P.G.; Mijatovic, V. Hysterosalpingo-foam sonography, a less painful procedure for tubal patency testing during fertility workup compared with (serial) hysterosalpingography: A randomized controlled trial. Fertil. Steril. 2014, 102, 821–825. [Google Scholar] [CrossRef]
- Lim, S.L.; Jung, J.J.; Yu, S.L.; Rajesh, H. A comparison of hysterosalpingo-foam sonography (HyFoSy) and hysterosalpingo-contrast sonography with saline medium (HyCoSy) in the assessment of tubal patency. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 168–172. [Google Scholar] [CrossRef]
- Piccioni, M.G.; Riganelli, L.; Filippi, V.; Fuggetta, E.; Colagiovanni, V.; Imperiale, L.; Caccetta, J.; Panici, P.B. Sonohysterosalpingography: Comparison of foam and saline solution. J. Clin. Ultrasound. 2017, 45, 67–71. [Google Scholar] [CrossRef]
- Ludwin, I.; Ludwin, A.; Wiechec, M.; Nocun, A.; Banas, T.; Basta, P.; Pitynski, K. Accuracy of hysterosalpingo-foam sonography in comparison to hysterosalpingo-contrast sonography with air/saline and to laparoscopy with dye. Hum. Reprod. 2017, 32, 758–769. [Google Scholar] [CrossRef]
- Ramos, J.; Caligara, C.; Santamaría-López, E.; González-Ravina, C.; Prados, N.; Carranza, F.; Blasco, V.; Fernández-Sánchez, M. Diagnostic Accuracy Study Comparing Hysterosalpingo-Foam Sonography and Hysterosalpingography for Fallopian Tube Patency Assessment. J. Clin. Med. 2021, 10, 4169. [Google Scholar] [CrossRef] [PubMed]
- van Welie, N.; van Rijswijk, J.; Dreyer, K.; van Hooff, M.H.A.; de Bruin, J.P.; Verhoeve, H.R.; Mol, F.; van Baal, W.M.; Traas, M.A.F.; van Peperstraten, A.M.; et al. Can hysterosalpingo-foam sonography replace hysterosalpingography as first-choice tubal patency test? A randomized non-inferiority trial. Hum. Reprod. 2022, 37, 969–979. [Google Scholar] [CrossRef]
- González, L.S.; Pérez-Medina, T.; Olalla, B.B.; Royuela, A.; Cuesta, M.d.L.R.D.L.; de la Mata, D.S.; Domínguez-Franjo, E.; Calles-Sastre, L.; Engels, V. Is hysterosalpingo-foam sonography (HyFoSy) more tolerable in terms of pain and anxiety than hysterosalpingography (HSG)? A prospective real-world setting multicentre study. BMC Womens Health 2022, 22, 41. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Steinkeler, J.A.; Woodfield, C.A.; Lazarus, E.; Hillstrom, M.M. Female infertility: A systematic approach to radiologic imaging and diagnosis. Radiographics 2009, 29, 1353–1370. [Google Scholar] [CrossRef] [PubMed]
- Tsevat, D.G.; Wiesenfeld, H.C.; Parks, C.; Peipert, J.F. Sexually transmitted diseases infertility. Am. J. Obstet. Gynecol. 2017, 216, 1–9. [Google Scholar] [CrossRef]
- Tuddenham, S.; Hamill, M.M.; Ghanem, K.G. Diagnosis and Treatment of Sexually Transmitted Infections: A Review. JAMA 2022, 327, 161–172. [Google Scholar] [CrossRef]
- Engels, V.; Medina, M.; Antolín, E.; Ros, C.; Amaro, A.; De-Guirior, C.; Manzour, N.; Sotillo, L.; De la Cuesta, R.; Rodríguez, R.; et al. Feasibility tolerability safety of hysterosalpingo-foam sonography (hyfosy) multicenter prospective Spanish study. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102004. [Google Scholar] [CrossRef]
- Tanaka, K.; Chua, J.; Cincotta, R.; Ballard, E.L.; Duncombe, G. Hysterosalpingo-foam sonography (HyFoSy): Tolerability, safety and the occurrence of pregnancy post-procedure. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Exalto, N.; Stassen, M.; Emanuel, M.H. Safety aspects and side-effects of ExEm-gel and foam for uterine cavity distension and tubal patency testing. Reprod. Biomed. Online 2014, 29, 534–540. [Google Scholar] [CrossRef][Green Version]
- Melcer, Y.; Shamir-Kaholi, N.; Vainer-Rotbart, S.; Pekar-Zlotin, M.; Youngster, M.; Gat, I.; Maymon, R. Spontaneous pregnancy rates in infertile women after sequential hydrosonography and hysterosalpingo-foam sonography. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 271, 219–222. [Google Scholar] [CrossRef]
- Piccioni, M.G.; Tabacco, S.; Merlino, L.; Del Negro, V.; Mazzeo, A.; Logoteta, A.; Del Prete, F.; Riganelli, L.; Giannini, A.; Monti, M. Does hysterosalpingo-foam sonography have any therapeutic effect? A systematic review. Minerva Ginecol. 2020, 72, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Lamari, I.; Xydias, E.M.; Tsakos, E.; Thanasas, I.; Ziogas, A.C. Spontaneous Pregnancy in a Hysterosalpingo-Foam-Sonography (HyFoSy) Cycle: A Case Report and Review of the Available Literature. Cureus 2023, 15, e37640. [Google Scholar] [CrossRef]
- Van Schoubroeck, D.; Van den Bosch, T.; Ameye, L.; Boes, A.S.; D’Hooghe, T.; Timmerman, D. Pain during Fallopian-tube patency testing by hysterosalpingo-foam sonography. Ultrasound Obstet. Gynecol. 2015, 45, 346–350. [Google Scholar] [CrossRef]
- Exalto, N.; Emanuel, M.H. Clinical Aspects of HyFoSy as Tubal Patency Test in Subfertility Workup. Biomed. Res. Int. 2019, 2019, 4827376. [Google Scholar] [CrossRef]
- Xydias, E.M.; Liasidi, P.N.; Papageorgouli, D.; Tsakos, E.; Gouliopoulos, N.; Thanasas, I.; Daponte, A.; Ziogas, A.C. Three-dimensional transvaginal ultrasound versus MRI in the diagnosis and classification of congenital uterine anomalies: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 312, 114560. [Google Scholar] [CrossRef]
- Ziogas, A.; Xydias, E.; Tsakos, E. Novel Methods in the Diagnosis of PCOS: The Role of 3D Ultrasonographic Modalities. IntechOpen 2022, 1, 103–122. [Google Scholar] [CrossRef]
- Hardel, A.-S.; Marie, H.F.S.; Lorrain, S.; Iacobelli, S.; Lazaro, G.; Boukerrou, M.; Tran, P.L. Factors associated with non-visualisation of tubal patency during Hysterosalpingo-Foam-Sonography. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102379. [Google Scholar] [CrossRef]

| Study | Year | Country | Recruitment Period | Location | Sample | Mean Age ± SD (y) | Mean BMI ± SD | Mean Infertility Duration ± SD (y) | Index Test | Comparator | Reference Test | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emanuel et al. [20] | 2012 | The Netherlands | In 2010 | Single-centre | 10 | 34 ± 3.8 | 24.5 ± 4.8 | 2.2 ± 1.4 | HyFoSy | HSG | N/A | Inter-method agreement |
| Van Schoubroeck et al. [21] | 2013 | Belgium | N/S | Single-centre | 3 | 30.5 ± 4.5 | N/S | N/S | HyFoSy | HSG | Laparoscopic chromo-pertubation | Inter-method agreement |
| Dreyer et al. [22] | 2014 | The Netherlands | Jan 2013–Sept 2013 | Two-centre | 39 | 33 ± 4.8 | 20.8 ± 3.7 | 1.26 ± 0.67 | HyFoSy | HSG | N/A | Procedure pain |
| Lim et al. [23] | 2015 | Singapore | April 2014–Dec 2014 | Single-centre | 40 | 28.8 ± 9.1 | N/S | 1.86 ± 1.5 | HyFoSy | HyCoSy | N/A | Inter-method agreement, procedure pain |
| Piccioni et al. [24] | 2016 | Italy | Sep 2014–Oct 2015 | Single-centre | 37 | 34 ± 0.5 | 26.1 ± 1.2 | 2.4 ± 0.6 | HyFoSy | HyCoSy | Laparoscopic chromo-pertubation | Diagnostic accuracy |
| Ludwin et al. [25] | 2017 | Poland | Nov 2013–July 2015 | Three-centre | 132 | 32.3 ± 4.3 | 22.2 ± 3.66 | 2.6 ± 1.8 | HyFoSy | HyCoSy | Laparoscopic chromo-pertubation | Diagnostic accuracy |
| Ramos et al. [26] | 2021 | Spain | June 2013–Feb 2017 | Single-centre | 106 | 34.71 ± 3.68 | 24.11 ± 4.54 | 2.30 ± 1.70 | HyFoSy | HSG | N/A | Inter-method agreement |
| Van Welie et al. [27] | 2022 | The Netherlands | N/S | 26-centre | 1026 | 33.0 ± 4.45 | 23.7 ± 4.2 | 1.7 ± 0.7 | HyFoSy | HSG | N/A | Inter-method agreement, procedure pain |
| Serrano Gonzalez et al. [28] | 2023 | Spain | Sep 2017–Oct 2018 | Two-centre | 189 | 34.6 ± 3.2 | N/S | N/S | HyFoSy | HSG | N/A | Procedure pain |
| Index Test (HyFoSy) | Comparator Test | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Model | Frequency | Timing | Contrast Type | Contrast/Water Ratio | Contrast Volume, Infusion Rate | Catheter | Comparator | Model | Contrast Agent | Contrast/Air ratio | Contrast Volume, Infusion Rate | Catheter | No of Radiographs |
| Emanuel et al. [20] | N/S | N/S | N/S | ExEm-gel® | 10 mL/10 mL | 20 mL, NS | GIS cervical balloon-less catheter | HSG | N/S | N/S | N/A | N/S | N/S | N/S |
| Van Schoubroeck et al. [21] | Voluson E8 | 6–12 MHz | N/S | ExEm-gel® | 10 mL/10 mL | 2–5 mL, NS | 2 mm pediatric Foley balloon | HSG | N/S | N/S | N/S | N/S | N/S | N/S |
| Dreyer et al. [22] | EnVisor | N/S | End of menses−14th day of the cycle | ExEm-gel® | 10 mL/10 mL | 10 mL, 1.67 mL/min | Cervical balloon-less catheter | HSG | AXIOM Iconos R 200 | Telebrix Hystero, Lipiodol Ultra | N/A | 10 mL, 1.67 mL/min | Hysterophore | 6–8 |
| Lim et al. [23] | Voluson E8 | 6–12 MHz | 6–12th day of the cycle | ExEm-gel® | 10 mL/10 mL | 20 mL, NS | Cervical balloon-less catheter | HyFoSy | Same as HyFosy | Saline solution | N/S | 20 mL, N/S | No. 5 pediatric Foley catheter | N/A |
| Piccioni et al. [24] | Accuvix A30 | 5–9 MHz | 7–13th day of the cycle | ExEm-gel® | 10 mL/10 mL | 20 mL | cervical balloon-less applicator | HyCoSy | Same as HyFosy | Saline solution | 15 mL/5 mL | 20 mL, N/S | 5-French balloon catheter | N/A |
| Ludwin et al. [25] | Voluson E8 Expert | 5–9 MHz | 5–12th day of the cycle | ExEm-gel® | 10 mL/10 mL | 20 mL | 5-French balloon catheter | HyCoSy | Same as HyFosy | Saline solution | 10 mL/10 mL | 20–40 mL, N/S | 5-French balloon catheter | N/A |
| Ramos et al. [26] | Voluson 730 proV | 4–9 MHz | N/S | ExEm-gel® | 10 mL/10 mL | 5 mL | GIS cervical balloon-less catheter * | HSG | N/S | N/S | N/A | N/S | N/S | N/S |
| Van Welie et al. [27] | Varied per center | Varied per center | 2–14 day of the cycle | ExEm-gel® | 5 mL/5 mL | 5–10 mL | GIS cervical balloon-less catheter | HSG | N/S | Varied per center | N/A | 5–10 mL, N/S | vacuum cervical cup, hysterophore, balloon catheter | 6–8 |
| Serrano Gonzalez et al. [28] | Voluson 730 Pro | 6–12 MHz | 6–12 day of the cycle | ExEm-gel® | N/S | 3–10 mL | Unomedical CH6, 6-Fr Kitazato balloon-less, Foerster/Pozzi clamp for assistance | HSG | Siemens C-shaped arc | Visipaque TM 270 mg/mL | N/A | Max 10 mL, N/S | 5-French balloon catheter | N/S |
| Study | Year | Risk of Bias (QUADAS-2) | Applicability (QUADAS-2) | Risk of Bias (QUADAS-2C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P.S. | I.T. | R.S. | F.T. | P.S. | I.T. | R.S. | P.S. | I.T. | R.S. | F.T. | ||
| Emanuel et al., 2012 [20] | HyFoSy |  |  |  |  |  |  |  |  |  |  |  |
| HSG |  |  |  |  |  |  |  | |||||
| van Schoubroeck et al., 2013 [21] | HyFoSy |  |  |  |  |  |  |  |  |  |  |  |
| HSG |  |  |  |  |  |  |  | |||||
| Lim et al., 2015 [23] | HyFoSy |  |  |  |  |  |  |  |  |  |  |  |
| HyCoSy |  |  |  |  |  |  |  | |||||
| Piccioni et al., 2016 [24] | HyFoSy |  |  |  |  |  |  |  |  |  |  |  |
| HyCoSy |  |  |  |  |  |  |  | |||||
| Ludwin et al., 2017 [25] | HyFoSy |  |  |  |  |  |  |  |  |  |  |  |
| HyCoSy |  |  |  |  |  |  |  | |||||
| Ramos et al., 2021 [26] | HyFoSy |  |  |  |  |  |  |  |  |  |  |  |
| HSG |  |  |  |  |  |  |  | |||||
| van Welie et al., 2022 [27] | HyFoSy |  |  |  |  |  |  |  |  |  |  |  |
| HSG |  |  |  |  |  |  |  | |||||
| HyFoSy | HyCoSy | HSG | ||
|---|---|---|---|---|
| Characteristics | Sensitivity |
| Same as HyFoSy (poorer performance than HyFoSy based on the study by Lim et al. 2015 [23]) | Low agreement with HyFoSy and low concordance for true positive cases |
| Specificity |
| Lower than HyCoSy | Low agreement with HyFoSy, but high concordance for true negative cases | |
| Procedure pain |
| Same as HyFoSy | Higher than HyFoSy | |
| Practicality |
| Same as HyFoSy | Lower than HyFoSy | |
| Summary of findings | Advantages |
|
|
|
| Disadvantages |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xydias, E.M.; Emmanouil, V.; Koutini, M.; Ntanika, A.; Tsakos, E.; Prior, M.; Sarris, I.; Thanasas, I.; Daponte, A.; Ziogas, A.C. Comparison of HyFoSy, HyCoSy and X-Ray Hysterosalpingography in the Assessment of Tubal Patency in Women with Infertility: A Systematic Review and Meta-Analysis. Med. Sci. 2025, 13, 168. https://doi.org/10.3390/medsci13030168
Xydias EM, Emmanouil V, Koutini M, Ntanika A, Tsakos E, Prior M, Sarris I, Thanasas I, Daponte A, Ziogas AC. Comparison of HyFoSy, HyCoSy and X-Ray Hysterosalpingography in the Assessment of Tubal Patency in Women with Infertility: A Systematic Review and Meta-Analysis. Medical Sciences. 2025; 13(3):168. https://doi.org/10.3390/medsci13030168
Chicago/Turabian StyleXydias, Emmanouil M., Vasileios Emmanouil, Maria Koutini, Anna Ntanika, Elias Tsakos, Matthew Prior, Ippokratis Sarris, Ioannis Thanasas, Alexandros Daponte, and Apostolos C. Ziogas. 2025. "Comparison of HyFoSy, HyCoSy and X-Ray Hysterosalpingography in the Assessment of Tubal Patency in Women with Infertility: A Systematic Review and Meta-Analysis" Medical Sciences 13, no. 3: 168. https://doi.org/10.3390/medsci13030168
APA StyleXydias, E. M., Emmanouil, V., Koutini, M., Ntanika, A., Tsakos, E., Prior, M., Sarris, I., Thanasas, I., Daponte, A., & Ziogas, A. C. (2025). Comparison of HyFoSy, HyCoSy and X-Ray Hysterosalpingography in the Assessment of Tubal Patency in Women with Infertility: A Systematic Review and Meta-Analysis. Medical Sciences, 13(3), 168. https://doi.org/10.3390/medsci13030168