Pirfenidone in Skin Fibrosis and Scarring: From Bench Insights to Clinical Data
Abstract
1. Introduction
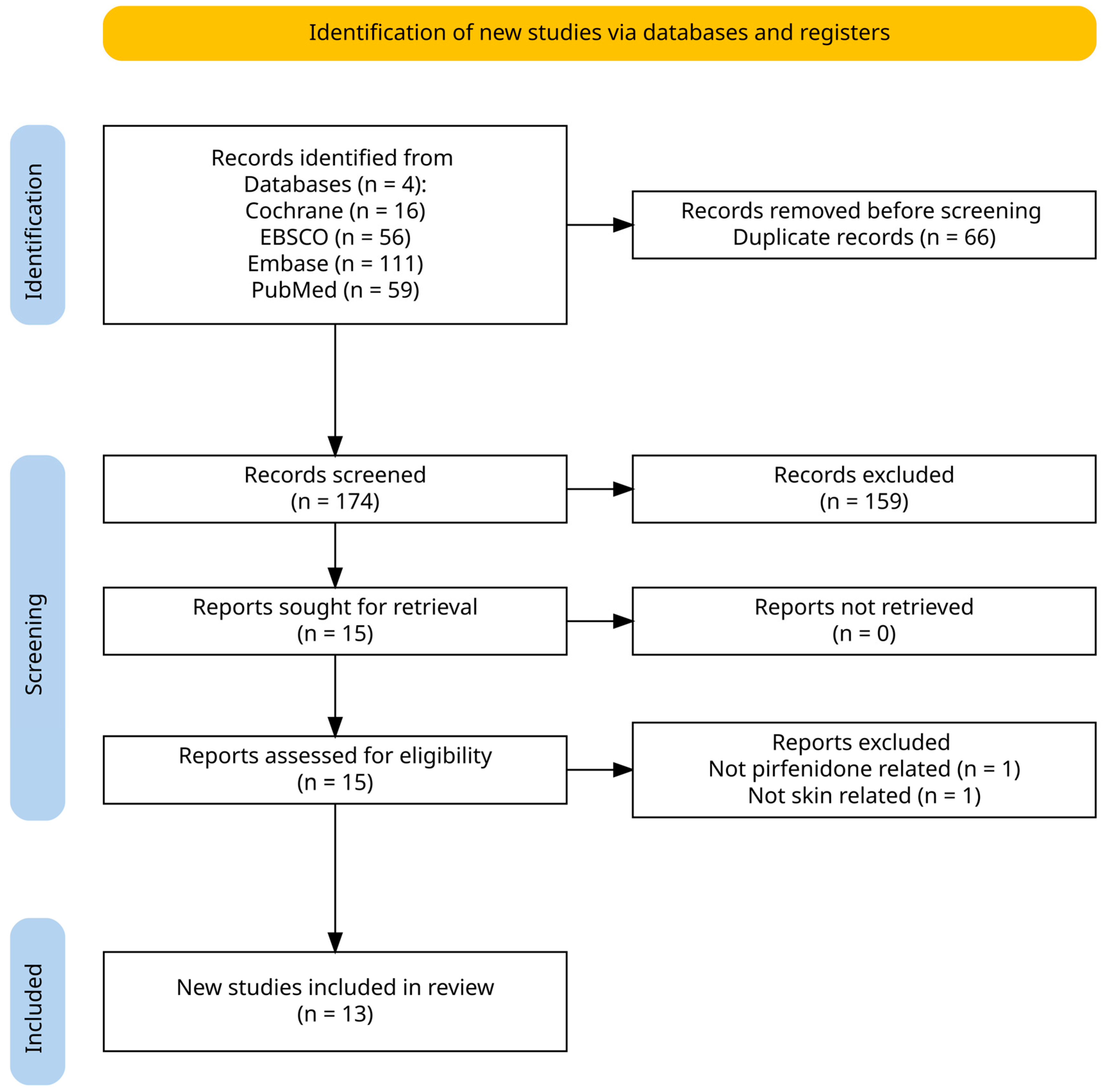
2. Materials and Methods
3. Results
3.1. Mechanism
3.2. Clinical Evidence
3.2.1. A Controlled Clinical Trial with Pirfenidone in the Treatment of Pathological Skin Scarring Caused by Burns in Pediatric Patients
3.2.2. Pirfenidone Gel in Patients with Localized Scleroderma: A Phase II Study
3.2.3. Pirfenidone Increases the Epithelialization Rate of Skin Graft Donor Sites
3.2.4. Efficacy and Safety of Pirfenidone in Patients with Second-Degree Burns: A Proof-of-Concept Randomized Controlled Trial
3.3. Adverse Reactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFD | Pirfenidone |
| IPF | Idiopathic Pulmonary Fibrosis |
| TGF-β | Transforming Growth Factor-Beta |
References
- Fertala, J.; Wang, M.L.; Rivlin, M.; Beredjiklian, P.K.; Abboud, J.; Arnold, W.V.; Fertala, A. Extracellular Targets to Reduce Excessive Scarring in Response to Tissue Injury. Biomolecules 2023, 13, 758. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef] [PubMed]
- Amici, J.M.; Taïeb, C.; LeFloc’h, C.; Demessant-Flavigny, A.-L.; Seité, S.; Cogrel, O. Prevalence of Scars: An International Epidemiological Survey in Adults. Acad. Dermatol. Venereol. 2022, 36, e799–e800. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ogawa, R. The Vascular Involvement in Soft Tissue Fibrosis-Lessons Learned from Pathological Scarring. Int. J. Mol. Sci. 2020, 21, 2542. [Google Scholar] [CrossRef]
- Elsaie, M.L. Update on Management of Keloid and Hypertrophic Scars: A Systemic Review. J. Cosmet. Dermatol. 2021, 20, 2729–2738. [Google Scholar] [CrossRef]
- Azuma, A. Pirfenidone Treatment of Idiopathic Pulmonary Fibrosis. Ther. Adv. Respir. Dis. 2012, 6, 107–114. [Google Scholar] [CrossRef]
- Margolin, S. Compositions and Methods for Reparation and Prevention of Fibrotic Lesions. U.S. Patent 5,716,632, 10 February 1998. [Google Scholar]
- Shetlar, M.R.; Shetlar, D.J.; Bloom, R.F.; Shetlar, C.L.; Margolin, S.B. Involution of Keloid Implants in Athymic Mice Treated with Pirfenidone or with Triamcinolone. J. Lab. Clin. Med. 1998, 132, 491–496. [Google Scholar] [CrossRef]
- Schelegle, E.S.; Mansoor, J.K.; Giri, S. Pirfenidone Attenuates Bleomycin-Induced Changes in Pulmonary Functions in Hamsters. Proc. Soc. Exp. Biol. Med. 1997, 216, 392–397. [Google Scholar] [CrossRef]
- Suga, H.; Teraoka, S.; Ota, K.; Komemushi, S.; Furutani, S.; Yamauchi, S.; Margolin, S. Preventive Effect of Pirfenidone against Experimental Sclerosing Peritonitis in Rats. Exp. Toxicol. Pathol. 1995, 47, 287–291. [Google Scholar] [CrossRef]
- Shimizu, T.; Fukagawa, M.; Kuroda, T.; Hata, S.; Iwasaki, Y.; Nemoto, M.; Shirai, K.; Yamauchi, S.; Margolin, S.B.; Shimizu, F.; et al. Pirfenidone Prevents Collagen Accumulation in the Remnant Kidney in Rats with Partial Nephrectomy. Kidney Int. Suppl. 1997, 63, S239–S243. [Google Scholar]
- Lee, B.S.; Margolin, S.B.; Nowak, R.A. Pirfenidone: A Novel Pharmacological Agent That Inhibits Leiomyoma Cell Proliferation and Collagen Production. J. Clin. Endocrinol. Metab. 1998, 83, 219–223. [Google Scholar] [CrossRef]
- Kehrer, J.P.; Margolin, S.B. Pirfenidone Diminishes Cyclophosphamide-Induced Lung Fibrosis in Mice. Toxicol. Lett. 1997, 90, 125–132. [Google Scholar] [CrossRef]
- Iyer, S.N.; Wild, J.S.; Schiedt, M.J.; Hyde, D.M.; Margolin, S.B.; Giri, S.N. Dietary Intake of Pirfenidone Ameliorates Bleomycin-Induced Lung Fibrosis in Hamsters. J. Lab. Clin. Med. 1995, 125, 779–785. [Google Scholar]
- Bruss, M.L.; Stanley, S.D.; Margolin, S.B.; Giri, S.N. Pharmacokinetics and Metabolism of Intravenous Pirfenidone in Sheep. Biopharm. Drug Disp. 2008, 29, 119–126. [Google Scholar] [CrossRef]
- Shi, S.; Wu, J.; Chen, H.; Chen, H.; Wu, J.; Zeng, F. Single-and Multiple-Dose Pharmacokinetics of Pirfenidone, an Antifibrotic Agent, in Healthy Chinese Volunteers. J. Clin. Pharma 2007, 47, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.-Y.; Ding, L.; Wang, J.; Zhang, Q.-Y.; Liu, X.; Lin, H.-D.; Hua, W.-Y. Pharmacokinetics, Safety and Tolerability of Pirfenidone and Its Major Metabolite after Single and Multiple Oral Doses in Healthy Chinese Subjects under Fed Conditions. Drug Res. 2013, 63, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Gili, E.; Fagone, E.; Fruciano, M.; Iemmolo, M.; Vancheri, C. Effect of Pirfenidone on Proliferation, TGF-β-Induced Myofibroblast Differentiation and Fibrogenic Activity of Primary Human Lung Fibroblasts. Eur. J. Pharm. Sci. 2014, 58, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, E.; Fujimori, M.; Kodama, H.; Felsen, D.; Chen, J.; Durack, J.C.; Solomon, S.B.; Coleman, J.A.; Srimathveeravalli, G. Macrophage-Secreted TGF-Β1 Contributes to Fibroblast Activation and Ureteral Stricture after Ablation Injury. Am. J. Physiol. Ren. Physiol. 2019, 317, F52–F64. [Google Scholar] [CrossRef]
- Ruwanpura, S.M.; Thomas, B.J.; Bardin, P.G. Pirfenidone: Molecular Mechanisms and Potential Clinical Applications in Lung Disease. Am. J. Respir. Cell Mol. Biol. 2020, 62, 413–422. [Google Scholar] [CrossRef]
- Velez, M.I.; Nambiar, A.M. Combination Pirfenidone and Inhaled N-acetylcysteine Therapy for IPF: Does It Take These Two to Tango? Respirology 2015, 20, 359–360. [Google Scholar] [CrossRef]
- Finnerty, J.P.; Ponnuswamy, A.; Dutta, P.; Abdelaziz, A.; Kamil, H. Efficacy of Antifibrotic Drugs, Nintedanib and Pirfenidone, in Treatment of Progressive Pulmonary Fibrosis in Both Idiopathic Pulmonary Fibrosis (IPF) and Non-IPF: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2021, 21, 411. [Google Scholar] [CrossRef]
- Aggarwal, K.; Arora, S.; Nagpal, K. Pulmonary Fibrosis: Unveiling the Pathogenesis, Exploring Therapeutic Targets, and Advancements in Drug Delivery Strategies. AAPS PharmSciTech 2023, 24, 152. [Google Scholar] [CrossRef]
- Torre, A.; Martínez-Sánchez, F.D.; Narvaez-Chávez, S.M.; Herrera-Islas, M.A.; Aguilar-Salinas, C.A.; Córdova-Gallardo, J. Pirfenidone Use in Fibrotic Diseases: What Do We Know so Far? Immun. Inflamm. Dis. 2024, 12, e1335. [Google Scholar] [CrossRef] [PubMed]
- Knighton, K.; Babun, A.; Evans, B.; Turney, J.; Sehgal, I. Pirfenidone: A Review of its Dermatologic Uses. In Proceedings of the 2nd Annual Dermatology Therapeutics Symposium, Austin, TX, USA, 8 November 2024. [Google Scholar]
- Costabel, U.; Bendstrup, E.; Cottin, V.; Dewint, P.; Egan, J.J.J.; Ferguson, J.; Groves, R.; Hellström, P.M.; Kreuter, M.; Maher, T.M.; et al. Pirfenidone in Idiopathic Pulmonary Fibrosis: Expert Panel Discussion on the Management of Drug-Related Adverse Events. Adv. Ther. 2014, 31, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Noble, P.W.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glassberg, M.K.; Kardatzke, D.; King, T.E.; Lancaster, L.; Sahn, S.A.; Szwarcberg, J.; et al. Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis (CAPACITY): Two Randomised Trials. Lancet 2011, 377, 1760–1769. [Google Scholar] [CrossRef]
- Dorati, R.; Medina, J.L.; DeLuca, P.P.; Leung, K.P. Development of a Topical 48-H Release Formulation as an Anti-Scarring Treatment for Deep Partial-Thickness Burns. AAPS PharmSciTech 2018, 19, 2264–2275. [Google Scholar] [CrossRef]
- Chung, E.P.; Nguyen, J.Q.; Tellkamp-Schehr, T.; Goebel, K.; Ollek, A.; Krein, C.; Wells, A.R.; Sebastian, E.A.; Goebel, A.; Niese, S.; et al. A Soft Skin Adhesive (SSA) Patch for Extended Release of Pirfenidone in Burn Wounds. Pharmaceutics 2023, 15, 1842. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Mei, L.; Wang, B.; Huang, Y.; Quan, G.; Lu, C.; Peng, T.; Pan, X.; Wu, C. A Pirfenidone Loaded Spray Dressing Based on Lyotropic Liquid Crystals for Deep Partial Thickness Burn Treatment: Healing Promotion and Scar Prophylaxis. J. Mater. Chem. B 2020, 8, 2573–2588. [Google Scholar] [CrossRef]
- Ma, Z.; Song, W.; He, Y.; Li, H. Multilayer Injectable Hydrogel System Sequentially Delivers Bioactive Substances for Each Wound Healing Stage. ACS Appl. Mater. Interfaces 2020, 12, 29787–29806. [Google Scholar] [CrossRef]
- Armendariz-Borunda, J.; Lyra-Gonzalez, I.; Medina-Preciado, D.; Gonzalez-García, I.; Martinez-Fong, D.; Miranda, R.A.; Magaña-Castro, R.; Peña-Santoyo, P.; Garcia-Rocha, S.; Bautista, C.A.; et al. A Controlled Clinical Trial with Pirfenidone in the Treatment of Pathological Skin Scarring Caused by Burns in Pediatric Patients. Ann. Plast. Surg. 2012, 68, 22–28. [Google Scholar] [CrossRef]
- Mandapalli, P.K.; Labala, S.; Bojja, J.; Venuganti, V.V.K. Effect of Pirfenidone Delivered Using Layer-by-Layer Thin Film on Excisional Wound Healing. Eur. J. Pharm. Sci. 2016, 83, 166–174. [Google Scholar] [CrossRef]
- Medina, J.D.; Nuñez, V. Efficacy of Pirfenidone in Post-Dermal Burns Hypertrophic Scars: A Multicenter Experience. J. Burn Care Res. 2012, 33, S170. [Google Scholar]
- He, J.; Meng, X.; Meng, C.; Zhao, J.; Chen, Y.; Zhang, Z.; Zhang, Y. Layer-by-Layer Pirfenidone/Cerium Oxide Nanocapsule Dressing Promotes Wound Repair and Prevents Scar Formation. Molecules 2022, 27, 1830. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castellanos, M.; Tlacuilo-Parra, A.; Sánchez-Enríquez, S.; Vélez-Gómez, E.; Guevara-Gutiérrez, E. Pirfenidone Gel in Patients with Localized Scleroderma: A Phase II Study. Arthritis Res. Ther. 2015, 16, 510. [Google Scholar] [CrossRef] [PubMed]
- P.R. Vademecum. Kitoscell: Cell Pharma. Available online: https://mx.prvademecum.com/medicamento/kitoscell-13244/ (accessed on 2 November 2024).
- DailyMed-ESBRIET-Pirfenidone Capsule ESBRIET-Pirfenidone Tablet, Coated. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e8c3537-36d7-4de5-9b5c-7a624b9a9e6e (accessed on 27 August 2024).
- Giri, S.N.; Leonard, S.; Shi, X.; Margolin, S.B.; Vallyathan, V. Effects of Pirfenidone on the Generation of Reactive Oxygen Species in Vitro. J. Environ. Pathol. Toxicol. Oncol. 1999, 18, 169–177. [Google Scholar] [PubMed]
- Yang, B.; Qiao, Y.; Yan, D.; Meng, Q. Targeting Interactions between Fibroblasts and Macrophages to Treat Cardiac Fibrosis. Cells 2024, 13, 764. [Google Scholar] [CrossRef]
- Evani, S.J.; Karna, S.R.; Seshu, J.; Leung, K.P. Pirfenidone Regulates LPS Mediated Activation of Neutrophils. Sci. Rep. 2020, 10, 19936. [Google Scholar] [CrossRef]
- Ali, M.F.; Egan, A.M.; Shaughnessy, G.F.; Anderson, D.K.; Kottom, T.J.; Dasari, H.; Van Keulen, V.P.; Aubry, M.-C.; Yi, E.S.; Limper, A.H.; et al. Antifibrotics Modify B-Cell-Induced Fibroblast Migration and Activation in Patients with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2021, 64, 722–733. [Google Scholar] [CrossRef]
- Shi, K.; Wang, F.; Xia, J.; Zuo, B.; Wang, Z.; Cao, X. Pirfenidone Inhibits Epidural Scar Fibroblast Proliferation and Differentiation by Regulating TGF-Β1-Induced Smad-Dependent and -Independent Pathways. Am. J. Transl. Res. 2019, 11, 1593–1604. [Google Scholar]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef]
- Saito, M.; Yamazaki, M.; Maeda, T.; Matsumura, H.; Setoguchi, Y.; Tsuboi, R. Pirfenidone Suppresses Keloid Fibroblast-Embedded Collagen Gel Contraction. Arch. Dermatol. Res. 2012, 304, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Evdokiou, A.; Hahn, J.M.; Supp, D.M.; Satish, L. Pirfenidone Inhibits Epithelial-Mesenchymal Transition (EMT) Genes in Keloid Keratinocytes. J. Burn Care Res. 2019, 40, S239. [Google Scholar] [CrossRef]
- Hall, C.L.; Wells, A.R.; Leung, K.P. Pirfenidone Reduces Profibrotic Responses in Human Dermal Myofibroblasts, in Vitro. Lab. Investig. 2018, 98, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.R.; Leung, K.P. Pirfenidone Attenuates the Profibrotic Contractile Phenotype of Differentiated Human Dermal Myofibroblasts. Biochem. Biophys. Res. Commun. 2020, 521, 646–651. [Google Scholar] [CrossRef]
- Huo, D.; Bi, X.-Y.; Zeng, J.-L.; Dai, D.-M.; Dong, X.-L. Drugs Targeting TGF-β/Notch Interaction Attenuate Hypertrophic Scar Formation by Optic Atrophy 1-Mediated Mitochondrial Fusion. Mol. Cell Biochem. 2023, 479, 3049–3061. [Google Scholar] [CrossRef]
- Dai, Z.H.; Jiang, Y.W.; Guo, H.; Lu, Y.T.; Chen, W.G.; Liang, T. Pirfenidone Ameliorates Hypertrophic Scar Through Inhibiting Proliferation and Migration of Fibroblasts by Regulating the Wnt/GSK-3β/β-Catenin Signaling Pathway. J. Burn Care Res. 2025, iraf040. [Google Scholar] [CrossRef]
- Coentro, J.Q.; May, U.; Prince, S.; Zwaagstra, J.; Ritvos, O.; Järvinen, T.A.; Zeugolis, D.I. Adapting the Scar-in-a-Jar to Skin Fibrosis and Screening Traditional and Contemporary Anti-Fibrotic Therapies. Front. Bioeng. Biotechnol. 2021, 9, 756399. [Google Scholar] [CrossRef]
- Mecott-Rivera, G.Á.; Aguilar-Baqueiro, J.A.; Bracho, S.; Miranda-Maldonado, I.; Franco-Márquez, R.; Castro-Govea, Y.; Dorsey-Treviño, E.G.; García-Pérez, M.M. Pirfenidone Increases the Epithelialization Rate of Skin Graft Donor Sites. Burns 2018, 44, 2051–2058. [Google Scholar] [CrossRef]
- Mecott, G.A.; González-Cantú, I.; Dorsey-Treviño, E.G.; Matta-Yee-Chig, D.; Saucedo-Cárdenas, O.; de Oca-Luna, R.M.; Pérez-Porras, S.; García-Pérez, M.M. Efficacy and Safety of Pirfenidone in Patients with Second-Degree Burns: A Proof-of-Concept Randomized Controlled Trial. Adv. Ski. Wound Care 2020, 33, 1–7. [Google Scholar] [CrossRef]
- Satish, L.; Evdokiou, A.; Geletu, E.; Hahn, J.M.; Supp, D.M. Pirfenidone Inhibits Epithelial-Mesenchymal Transition in Keloid Keratinocytes. Burn. Trauma 2020, 8, tkz007. [Google Scholar] [CrossRef]
- David, M.P.J.; Ariel, M.A.; Juan, A.B. Kitoscell r in the Treatment of Scars after Burn Injuries, Long-Term Follow-Up. J. Wound Care 2020, 29, 192. [Google Scholar]
- Cantú-Cantú, M.Z.; Lyra-González, I.; Armendáriz-Borunda, J. Coadjuvant Treatment with Surgery and Pirfenidone in Severe Facial Trauma Due to Dog Bite. J. Craniofac. Surg. 2013, 24, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Arkachaisri, T.; Vilaiyuk, S.; Li, S.; O’Neil, K.M.; Pope, E.; Higgins, G.C.; Punaro, M.; Rabinovich, E.C.; Rosenkranz, M.; Kietz, D.A.; et al. The Localized Scleroderma Skin Severity Index and Physician Global Assessment of Disease Activity: A Work in Progress toward Development of Localized Scleroderma Outcome Measures. J. Rheumatol. 2009, 36, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Chipp, E.; Charles, L.; Thomas, C.; Whiting, K.; Moiemen, N.; Wilson, Y. A Prospective Study of Time to Healing and Hypertrophic Scarring in Paediatric Burns: Every Day Counts. Burn. Trauma 2017, 5. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, M.; Su, J.; Cai, B.; Li, J.; Zhang, K. New Insights into Balancing Wound Healing and Scarless Skin Repair. J. Tissue Eng. 2023, 14, 20417314231185848. [Google Scholar] [CrossRef]
- Jeong, S.H.; Hong, E.H.; Park, E.J.; Kim, K.J.; Kim, K.H. A Case of Pirfenidone Induced Photosensitive Drug Eruption. Ann. Dermatol. 2023, 35, e41. [Google Scholar] [CrossRef]
- Parmar, V.K.; Desai, S.B.; Vaja, T. RP-HPLC and UV Spectrophotometric Methods for Estimation of Pirfenidone in Pharmaceutical Formulations. Indian J. Pharm. Sci. 2014, 76, 225. [Google Scholar]
- Deng, C.-C.; Hu, Y.-F.; Zhu, D.-H.; Cheng, Q.; Gu, J.-J.; Feng, Q.-L.; Zhang, L.-X.; Xu, Y.-P.; Wang, D.; Rong, Z.; et al. Single-Cell RNA-Seq Reveals Fibroblast Heterogeneity and Increased Mesenchymal Fibroblasts in Human Fibrotic Skin Diseases. Nat. Commun. 2021, 12, 3709. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, Z.; Shan, S.; Shen, H.; Zheng, H.; Jin, L.; Li, Q.; Zhou, J. Single Cell Transcriptomics Reveals the Cellular Heterogeneity of Keloids and the Mechanism of Their Aggressiveness. Commun. Biol. 2024, 7, 1647. [Google Scholar] [CrossRef]
- Liu, Z.; Bian, X.; Luo, L.; Björklund, Å.K.; Li, L.; Zhang, L.; Chen, Y.; Guo, L.; Gao, J.; Cao, C.; et al. Spatiotemporal Single-Cell Roadmap of Human Skin Wound Healing. Cell Stem Cell 2025, 32, 479–498.e8. [Google Scholar] [CrossRef]
- Shi, X.; Yu, Z.; Zhu, C.; Jiang, L.; Geng, N.; Fan, X.; Guan, Z.; Lu, X. Synthesis and Structure–Activity Relationships of Pirfenidone Derivatives as Anti-Fibrosis Agents in Vitro. RSC Med. Chem. 2022, 13, 610–621. [Google Scholar] [CrossRef]
- Cai, X.; Liu, X.; Xie, W.; Ma, A.; Tan, Y.; Shang, J.; Zhang, J.; Chen, C.; Yu, Y.; Qu, Y.; et al. Hydronidone for the Treatment of Liver Fibrosis Related to Chronic Hepatitis B: A Phase 2 Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2023, 21, 1893–1901.e7. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, C.; Gao, Y.; Yu, Z.; Yan, Q.; Liu, Y.; Luo, M.; Shi, X. Design, Synthesis, and Evaluation of Pirfenidone-NSAIDs Conjugates for the Treatment of Idiopathic Pulmonary Fibrosis. Bioorg. Chem. 2024, 143, 107018. [Google Scholar] [CrossRef]
- Roth, G.J.; Binder, R.; Colbatzky, F.; Dallinger, C.; Schlenker-Herceg, R.; Hilberg, F.; Wollin, S.-L.; Kaiser, R. Nintedanib: From Discovery to the Clinic. J. Med. Chem. 2015, 58, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-S.; Li, J.Z.-H.; Chen, S.; Chan, J.Y.-W.; Gao, W. The Efficacy of Triamcinolone Acetonide in Keloid Treatment: A Systematic Review and Meta-Analysis. Front. Med. 2016, 3, 71. [Google Scholar] [CrossRef]
- Morelli Coppola, M.; Salzillo, R.; Segreto, F.; Persichetti, P. Triamcinolone Acetonide Intralesional Injection for the Treatment of Keloid Scars: Patient Selection and Perspectives. Clin. Cosmet. Investig. Dermatol. 2018, 11, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, X.; Wang, X.; Jiang, X. Efficacy of Topical Silicone Gel in Scar Management: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. Wound J. 2020, 17, 765–773. [Google Scholar] [CrossRef]
- Cho, J.; Lee, J.; Park, J. Increased Patient Compliance with Silicone Gel Sheeting and Topical Silicone Gel for Hypertrophic Scar Improves Scar Outcomes. J. Wound Manag. Res. 2024, 20, 128–136. [Google Scholar] [CrossRef]
- Won, P.; Cooper, M.; Gillenwater, T.J.; Yenikomshian, H.A. Treatment of Hypertrophic Burn Scars With Laser Therapy: A Review of Adverse Events. Ann. Plast. Surg. 2023, 91, 715–719. [Google Scholar] [CrossRef]
- Leszczynski, R.; da Silva, C.; Pinto, A.C.P.N.; Kuczynski, U.; da Silva, E. Laser Therapy for Treating Hypertrophic and Keloid Scars. Cochrane Database Syst. Rev. 2022, 9, CD011642. [Google Scholar] [CrossRef]
- UpToDate. Pirfenidone: Drug Information (Pricing). In UpToDate Lexidrug; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2024. [Google Scholar]
| Reference | Population | Main Outcomes | Safety |
|---|---|---|---|
| Armendariz-Borunda et al. (2012) [32] | A total of 63 pediatric patients with hypertrophic burn scars were assigned to topical pirfenidone (n = 33) or pressure therapy (n = 30). | The pirfenidone group showed a 43% improvement on the VSS, compared to a 16% improvement in the control group after six months. | No adverse events were reported. |
| Rodriguez-Castellanos et al. (2014) [36] | A total of 12 adult patients with active localized scleroderma. | The study showed a decrease in the mLoSSI score (from a mean of 5.83 to 0.83) and histologic improvement after six months of treatment. | A slight, temporary burning sensation after application was reported by 92% of patients. No systemic or laboratory adverse effects were found. |
| Mecott et al. (2018) [52] | In total, 24 adult patients requiring split-thickness skin grafts received either topical pirfenidone (n = 19) or usual care (n = 5). | At day 10, the pirfenidone group had a higher rate of epithelialization (99.5% vs. 88.6%) and greater mean epithelial thickness (108 µm vs. 75.1 µm) compared to the control group. | The initial application caused moderate pain that became well-tolerated over time. No systemic or laboratory adverse effects were found. |
| Mecott et al. (2020) [53] | A total of 8 patients with second-degree burns were randomized to oral pirfenidone (n = 5) or usual care (n = 3). | At day 7, the pirfenidone group had a thicker re-epithelialized epidermis (148.98 µm vs. 119.27 µm) and less observed fibrosis. | No alterations in liver or renal function panels were found. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knighton, K.; Babun, A.; Turney, J.; Evans, B.; Sehgal, I. Pirfenidone in Skin Fibrosis and Scarring: From Bench Insights to Clinical Data. Med. Sci. 2025, 13, 148. https://doi.org/10.3390/medsci13030148
Knighton K, Babun A, Turney J, Evans B, Sehgal I. Pirfenidone in Skin Fibrosis and Scarring: From Bench Insights to Clinical Data. Medical Sciences. 2025; 13(3):148. https://doi.org/10.3390/medsci13030148
Chicago/Turabian StyleKnighton, Kelson, Asis Babun, James Turney, Brehyn Evans, and Inder Sehgal. 2025. "Pirfenidone in Skin Fibrosis and Scarring: From Bench Insights to Clinical Data" Medical Sciences 13, no. 3: 148. https://doi.org/10.3390/medsci13030148
APA StyleKnighton, K., Babun, A., Turney, J., Evans, B., & Sehgal, I. (2025). Pirfenidone in Skin Fibrosis and Scarring: From Bench Insights to Clinical Data. Medical Sciences, 13(3), 148. https://doi.org/10.3390/medsci13030148





