Effects of Oral Doxofylline and Procaterol on Chronic Obstructive Pulmonary Disease: A Randomized Crossover Study
Abstract
1. Introduction
2. Materials and Methods
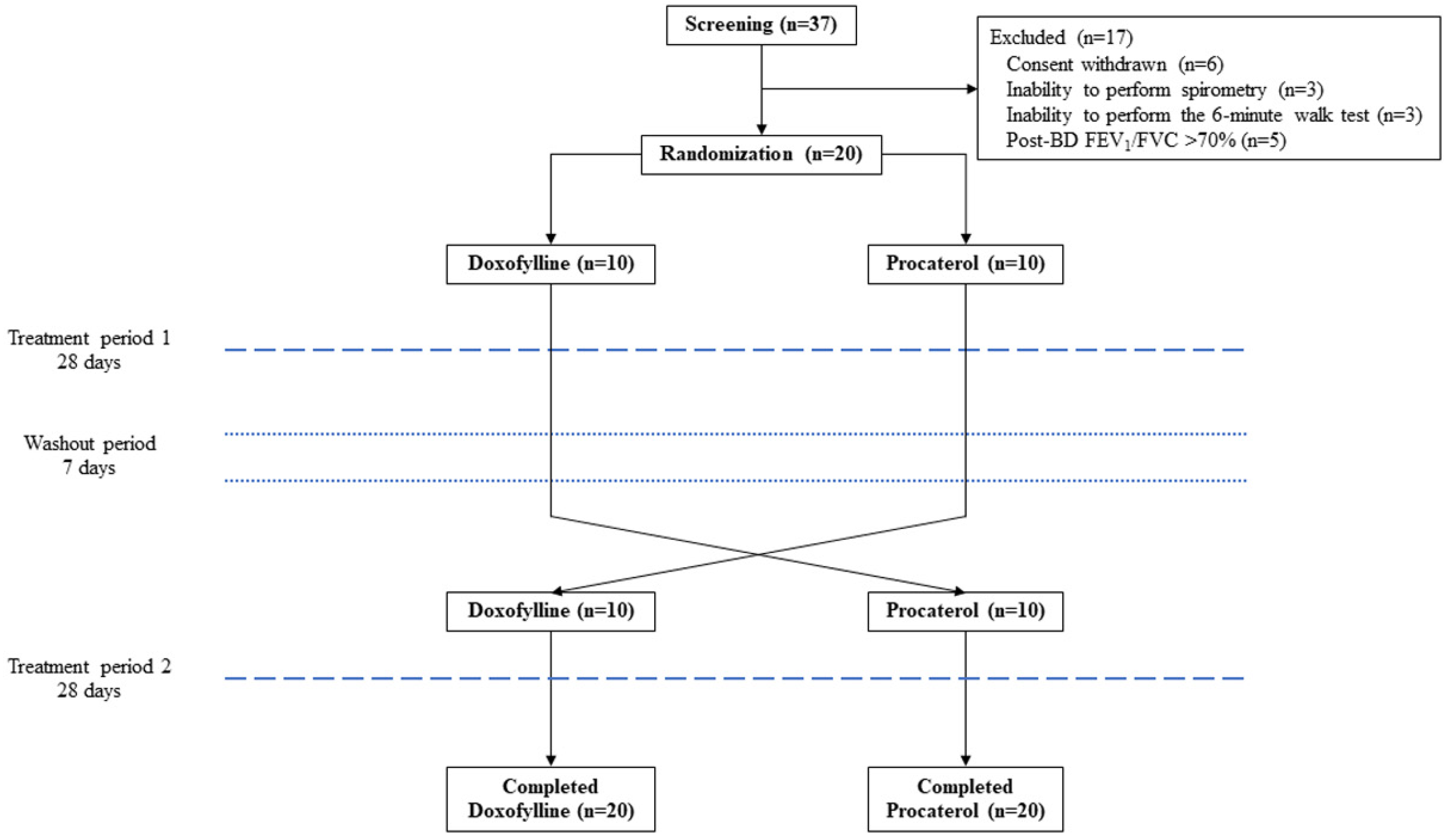
2.1. Study Design and Participants
2.2. Randomization and Intervention
2.3. Outcome Assessment
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Primary Outcome
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATS | American Thoracic Society |
| BD | bronchodilator |
| CAT | COPD assessment test |
| COPD | chronic obstructive pulmonary disease |
| ERS | European Respiratory Society |
| FEF25–75 | forced expiration flow rate at 25–75% of FVC |
| FEV1 | forced expiratory volume in 1 second |
| FVC | forced vital capacity |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| HDAC | histone deacetylase |
| HFL-1 | human fetal lung fibroblasts-1 |
| ICS | inhaled corticosteroids |
| LABA | long-acting beta-2 agonists |
| LAMA | long-acting muscarinic antagonists |
| MCID | minimal clinically important difference |
| mMRC | modified Medical Research Council |
| PDE | phosphodiesterase |
| PEF | peak expiratory flow |
| 6MWD | 6-minute walking distance |
| 6MWT | 6-minute walk test |
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2024 Report. Available online: https://goldcopd.org/ (accessed on 3 January 2025).
- Celli, B.; Fabbri, L.; Criner, G.; Martinez, F.J.; Mannino, D.; Vogelmeier, C.; Montes de Oca, M.; Papi, A.; Sin, D.D.; Han, M.K.; et al. Definition and nomenclature of chronic obstructive pulmonary disease: Time for its revision. Am. J. Respir. Crit. Care Med. 2022, 206, 1317–1325. [Google Scholar] [CrossRef]
- Rabe, K.F.; Magnussen, H.; Dent, G. Theophylline and selective PDE inhibitors as bronchodilators and smooth muscle relaxants. Eur. Respir. J. 1995, 8, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Lim, S.; Caramori, G.; Cosio, B.; Chung, K.F.; Adcock, I.M.; Barnes, P.J. A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression. Proc. Natl. Acad. Sci. USA 2002, 99, 8921–8926. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Theophylline. Pharmaceuticals 2010, 3, 725–747. [Google Scholar] [CrossRef] [PubMed]
- Scurek, M.; Brat, K. A narrative review of theophylline: Is there still a place for an old friend? J. Thorac. Dis. 2024, 16, 3450–3460. [Google Scholar] [CrossRef]
- Weinberger, M.; Hendeles, L. Therapeutic effect and dosing strategies for theophylline in the treatment of chronic asthma. J. Allergy Clin. Immunol. 1986, 78, 762–768. [Google Scholar] [CrossRef]
- Matera, M.G.; Page, C.; Cazzola, M. Doxofylline is not just another theophylline! Int. J. Chron. Obstr. Pulm. Dis. 2017, 12, 3487–3493. [Google Scholar] [CrossRef]
- Cazzola, M.; Matera, M.G. The effect of doxofylline in asthma and COPD. Respir. Med. 2020, 164, 105904. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Rogliani, P.; Page, C.; Matera, M.G. Impact of doxofylline in COPD: A pairwise meta-analysis. Pulm. Pharmacol. Ther. 2018, 51, 1–9. [Google Scholar] [CrossRef]
- Tashimo, H.; Yamashita, N.; Ishida, H.; Nagase, H.; Adachi, T.; Nakano, J.; Yamamura, K.; Yano, T.; Yoshihara, H.; Ohta, K. Effect of procaterol, a beta(2) selective adrenergic receptor agonist, on airway inflammation and hyperresponsiveness. Allergol. Int. 2007, 56, 241–247. [Google Scholar] [CrossRef]
- Kohyama, T.; Yamauchi, Y.; Takizawa, H.; Itakura, S.; Kamitani, S.; Desaki, M.; Kawasaki, S.; Nagase, T. Procaterol inhibits lung fibroblast migration. Inflammation 2009, 32, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.D. Effect of beta-adrenergic agonists on mucociliary clearance. J. Allergy Clin. Immunol. 2002, 110, S291–S297. [Google Scholar] [CrossRef] [PubMed]
- Mahler, D.A.; Wells, C.K. Evaluation of clinical methods for rating dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Bologna, E.; Lagana, A.; Terracino, D.; Bolignari, P.; Biffignandi, P. Oral and intravenous pharmacokinetic profiles of doxofylline in patients with chronic bronchitis. J. Int. Med. Res. 1990, 18, 282–288. [Google Scholar] [CrossRef]
- Kobayashi, H.; Masuda, M.; Kashiyama, E.; Koga, N.; Yasuda, Y.; Shibutani, T.; Endo, K.; Funaki, T.; Miyamoto, T. Pharmacokinetic study of the oral administration of procaterol hydrochloride hydrate 50 microg in healthy adult Japanese men. Int. J. Clin. Pharmacol. Ther. 2010, 48, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Dhariwal, K.; Jackson, A. Effect of length of sampling schedule and washout interval on magnitude of drug carryover from period 1 to period 2 in two-period, two-treatment bioequivalence studies and its attendant effects on determination of bioequivalence. Biopharm. Drug Dispos. 2003, 24, 219–228. [Google Scholar] [CrossRef]
- Wang, T.; Luo, G.; Hu, Y.; Li, F.; Ma, J.; Wang, J.; Zuo, P.; Xiong, W.; Liu, X.; Zhao, J.; et al. Comparative study on the efficacy of tiotropium bromide inhalation and oral doxofylline treatment of moderate to severe stable chronic obstructive pulmonary disease. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 614. [Google Scholar] [CrossRef]
- Crowe, M.J.; Counihan, H.E.; O’Malley, K. A comparative study of a new selective beta 2-adrenoceptor agonist, procaterol and salbutamol in asthma. Br. J. Clin. Pharmacol. 1985, 19, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Cho-Reyes, S.; Celli, B.R.; Dembek, C.; Yeh, K.; Navaie, M. Inhalation technique errors with metered-dose inhalers among patients with obstructive lung diseases: A systematic review and meta-analysis of U.S. studies. Chronic Obstr. Pulm. Dis. 2019, 6, 267–280. [Google Scholar] [CrossRef]
- Kocks, J.W.H.; Chrystyn, H.; van der Palen, J.; Thomas, M.; Yates, L.; Landis, S.H.; Driessen, M.T.; Gokhale, M.; Sharma, R.; Molimard, M. Systematic review of association between critical errors in inhalation and health outcomes in asthma and COPD. NPJ Prim. Care Respir. Med. 2018, 28, 43. [Google Scholar] [CrossRef] [PubMed]
- Leving, M.T.; van Boven, J.F.M.; Bosnic-Anticevich, S.Z.; van Cooten, J.; Correia de Sousa, J.; Cvetkovski, B.; Dekhuijzen, R.; Dijk, L.; Garcia Pardo, M.; Gardev, A.; et al. Suboptimal peak inspiratory flow and critical inhalation errors are associated with higher COPD-related healthcare costs. Int. J. Chron. Obstr. Pulm. Dis. 2022, 17, 2401–2415. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline in chronic obstructive pulmonary disease: New horizons. Proc. Am. Thorac. Soc. 2005, 2, 334–339. [Google Scholar] [CrossRef]
- Murciano, D.; Auclair, M.H.; Pariente, R.; Aubier, M. A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. N. Engl. J. Med. 1989, 320, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, X.; Zeng, X.; Qiu, R.; Xie, J.; Liu, S.; Zheng, J.; Zhong, N.; Ran, P. Positive benefits of theophylline in a randomized, double-blind, parallel-group, placebo-controlled study of low-dose, slow-release theophylline in the treatment of COPD for 1 year. Respirology 2006, 11, 603–610. [Google Scholar] [CrossRef]
- Bellia, V.; Foresi, A.; Bianco, S.; Grassi, V.; Olivieri, D.; Bensi, G.; Volonte, M.; Group, B.I.S. Efficacy and safety of oxitropium bromide, theophylline and their combination in COPD patients: A double-blind, randomized, multicentre study (BREATH Trial). Respir. Med. 2002, 96, 881–889. [Google Scholar] [CrossRef]
- Riffo-Vasquez, Y.; Venkatasamy, R.; Page, C.P. Steroid sparing effects of doxofylline. Pulm. Pharmacol. Ther. 2018, 48, 1–4. [Google Scholar] [CrossRef]
- Lal, D.; Manocha, S.; Ray, A.; Vijayan, V.K.; Kumar, R. Comparative study of the efficacy and safety of theophylline and doxofylline in patients with bronchial asthma and chronic obstructive pulmonary disease. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 443–451. [Google Scholar] [CrossRef]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; van der Grinten, C.P.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Donohue, J.F. Minimal clinically important differences in COPD lung function. COPD 2005, 2, 111–124. [Google Scholar] [CrossRef]
- Jones, P.W.; Beeh, K.M.; Chapman, K.R.; Decramer, M.; Mahler, D.A.; Wedzicha, J.A. Minimal clinically important differences in pharmacological trials. Am. J. Respir. Crit. Care Med. 2014, 189, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Kon, S.S.; Canavan, J.L.; Jones, S.E.; Nolan, C.M.; Clark, A.L.; Dickson, M.J.; Haselden, B.M.; Polkey, M.I.; Man, W.D. Minimum clinically important difference for the COPD Assessment Test: A prospective analysis. Lancet Respir. Med. 2014, 2, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Liippo, K.; Silvasti, M.; Tukiainen, H. Inhaled procaterol versus salbutamol in bronchial asthma. Eur. J. Clin. Pharmacol. 1991, 40, 417–418. [Google Scholar] [CrossRef]
- Sukisaki, T.; Senjyu, H.; Oishi, K.; Rikitomi, N.; Ariyoshi, K. Single dose of inhaled procaterol has a prolonged effect on exercise performance of patients with COPD. Physiother. Theory Pract. 2008, 24, 255–263. [Google Scholar] [CrossRef]
- Shioya, T.; Satake, M.; Sato, K.; Sano, M.A.; Sugawara, K.; Takahashi, H.; Honma, M. Long-term effect of the beta2-receptor agonist procaterol on daily life performance and exercise capacity in patients with stable chronic obstructive pulmonary disease. Clinical study with special reference to health-related quality of life and activities of daily living. Arzneimittelforschung 2008, 58, 24–28. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 20) | Doxofylline (n = 10) | Procaterol (n = 10) | p-Value |
|---|---|---|---|---|
| Age, years | 71.7 ± 9.4 | 74.1 ± 9.4 | 69.3 ± 9.1 | 0.263 |
| Male/female | 19 (95)/1 (5) | 9 (90)/1 (10) | 10 (100)/0 (0) | 1.000 |
| Body mass index, kg/m2 | 23.2 ± 4.2 | 22.5 ± 4.0 | 23.8 ± 4.5 | 0.487 |
| Formerly smoking | 16 (80) | 9 (90) | 7 (70) | 0.453 |
| Smoking, pack-years | 38.0 ± 22.8 | 34.6 ± 23.0 | 41.4 ± 23.2 | 0.519 |
| Comorbidity | ||||
| Dyslipidemia | 16 (80) | 9 (90) | 7 (70) | 0.582 |
| Hypertension | 13 (65) | 8 (80) | 5 (50) | 0.350 |
| Coronary artery disease | 8 (40) | 5 (50) | 3 (30) | 0.980 |
| Diabetes mellitus | 7 (35) | 4 (40) | 3 (30) | 1.000 |
| Obstructive sleep apnea | 4 (20) | 2 (20) | 2 (20) | 1.000 |
| Chronic kidney disease | 4 (20) | 3 (30) | 1 (10) | 0.582 |
| Allergic rhinitis | 7 (35) | 4 (40) | 3 (30) | 1.000 |
| COPD grade | 0.478 | |||
| 1 | 1 (5) | 1 (10.0) | 0 (0) | |
| 2 | 12 (60) | 5 (50) | 7 (70) | |
| 3 | 7 (35) | 4 (40) | 3 (30) | |
| COPD group | 0.549 | |||
| A | 14 (70) | 7 (70) | 7 (70) | |
| B | 5 (25) | 3 (30) | 2 (20) | |
| E | 1 (5) | 0 (0) | 1 (10) | |
| Medication | 0.736 | |||
| LAMA | 1 (5) | 1 (10) | 0 (0) | |
| LABA/LAMA | 8 (40) | 4 (40) | 4 (40) | |
| ICS/LABA | 2 (10) | 1 (10) | 1 (10) | |
| ICS/LABA/LAMA | 9 (45) | 4 (40) | 5 (40) | |
| Functional capacity | ||||
| mMRC scores | 1.1 ± 0.9 | 1.3 ± 0.9 | 0.6 ± 1.0 | 0.119 |
| CAT scores | 6.3 ± 5.6 | 6.2 ± 4.4 | 5.0 ± 8.3 | 0.689 |
| 6MWD, m | 391.9 ± 107.7 | 357.0 ± 102.7 | 432.9 ± 101.5 | 0.114 |
| Laboratory data | ||||
| Blood eosinophils, % | 3.57 ± 2.70 | 4.40 ± 3.51 | 2.73 ± 1.25 | 0.183 |
| Blood eosinophil counts, cells/mm3 | 250.20 ± 178.09 | 276.9 ± 224.8 | 223.5 ± 121.9 | 0.518 |
| Blood eosinophil counts ≥ 300 cells/mm3 | 14 (35) | 7 (70) | 7 (70) | 1.000 |
| Spirometry data | ||||
| Pre-BD FVC, L | 2.68 ± 0.69 | 2.42 ± 0.80 | 2.98 ± 0.51 | 0.078 |
| Pre-BD FVC, % predicted | 86.11 ± 16.77 | 82.62 ± 20.62 | 91.63 ± 16.67 | 0.297 |
| Post-BD FVC, L | 2.84 ± 0.73 | 2.50 ± 0.82 | 3.07 ± 0.53 | 0.084 |
| Post-BD FVC, % predicted | 91.33 ± 17.45 | 85.61 ± 20.70 | 94.35 ± 18.09 | 0.328 |
| FVC change after BD test, % | 3.37 ± 4.54 | 3.92 ± 4.53 | 2.82 ± 4.72 | 0.601 |
| Pre-BD FEV1, L | 1.35 ± 0.39 | 1.36 ± 0.46 | 1.34 ± 0.34 | 0.886 |
| Pre-BD FEV1, % predicted | 55.56 ± 14.06 | 59.74 ± 14.80 | 52.40 ± 14.20 | 0.273 |
| Post-BD FEV1, L | 1.41 ± 0.36 | 1.42 ± 0.46 | 1.38 ± 0.33 | 0.837 |
| Post-BD FEV1, % predicted | 59.34 ± 13.92 | 61.74 ± 14.18 | 51.11 ± 18.37 | 0.165 |
| FEV1 change after BD test, % | 3.72 ± 5.75 | 3.77 ± 6.35 | 3.66 ± 5.42 | 0.967 |
| Pre-BD FEV1/FVC, % | 50.39 ± 11.39 | 57.78 ± 11.21 | 45.31 ± 10.57 | 0.020 |
| Post-BD FEV1/FVC, % | 51.17 ± 12.49 | 57.55 ± 10.61 | 45.76 ± 11.08 | 0.026 |
| FEV1/FVC change after BD test, % | 0.39 ± 4.41 | −0.08 ± 4.85 | 0.86 ± 4.11 | 0.646 |
| Pre-BD PEF, L/s | 4.37 ± 1.26 | 4.93 ± 1.63 | 4.21 ± 0.99 | 0.249 |
| Pre-BD PEF, % predicted | 59.96 ± 15.86 | 70.88 ± 19.77 | 56.87 ± 13.79 | 0.083 |
| Post-BD PEF, L/s | 4.61 ± 1.25 | 4.99 ± 1.67 | 4.32 ± 0.87 | 0.276 |
| Post-BD PEF, % predicted | 63.46 ± 15.37 | 71.15 ± 19.83 | 58.43 ± 12.81 | 0.106 |
| PEF change after BD test, % | 2.15 ± 12.76 | 0.24 ± 10.88 | 4.05 ± 14.75 | 0.519 |
| Pre-BD FEF25–75, L/s | 0.49 ± 0.25 | 0.58 ± 0.29 | 0.39 ± 0.17 | 0.115 |
| Pre-BD FEF25–75, % predicted | 20.60 ± 10.25 | 29.90 ± 14.76 | 18.43 ± 8.46 | 0.047 |
| Post-BD FEF25–75, L/s | 0.51 ± 0.24 | 0.52 ± 0.23 | 0.44 ± 0.18 | 0.397 |
| Post-BD FEF25–75, % predicted | 22.83 ± 10.37 | 25.94 ± 9.71 | 20.50 ± 9.59 | 0.223 |
| FEF25–75 change after BD test, % | 2.08 ± 20.31 | −7.08 ± 22.54 | 11.23 ± 13.27 | 0.040 |
| Data | Doxofylline | Procaterol | Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|
| Spirometry data change from baseline | ||||
| Pre-BD FVC, L | −0.021 ± 0.224 | −0.021 ± 0.196 | 0.000 (−0.137, 0.137) | 1.000 |
| Pre-BD FVC, % predicted | −0.365 ± 8.995 | −1.011 ± 6.858 | 0.645 (−4.565, 5.856) | 0.803 |
| Post-BD FVC, L | −0.195 ± 0.254 | −0.073 ± 0.222 | 0.054 (−0.101, 0.209) | 0.488 |
| Post-BD FVC, % predicted | −0.580 ± 10.126 | −2.874 ± 7.490 | 2.294 (−3.510, 8.098) | 0.428 |
| FVC change after BD test, % | −0.435 ± 6.551 | −2.258 ± 9.440 | 1.823 (−3.426, 7.072) | 0.486 |
| Pre-BD FEV1, L | −0.001 ± 0.128 | −0.015 ± 0.112 | 0.724 (−0.065, 0.092) | 0.724 |
| Pre-BD FEV1, % predicted | 0.160 ± 6.623 | −0.847 ± 4.758 | 1.007 (−2.752, 4.766) | 0.590 |
| Post-BD FEV1, L | 0.004 ± 0.116 | −0.031 ± 0.150 | 0.035 (−0.052, 0.121) | 0.426 |
| Post-BD FEV1, % predicted | 1.750 ± 7.643 | −1.642 ± 5.762 | 3.392 (−1.018, 7.802) | 0.128 |
| FEV1 change after BD test, % | 0.190 ± 5.624 | −1.421 ± 8.088 | 1.611 (−2.889, 6.111) | 0.473 |
| Pre-BD FEV1/FVC, % | 0.207 ± 3.074 | −0.343 ± 2.927 | 0.550 (−1.399, 2.500) | 0.571 |
| Post-BD FEV1/FVC, % | 0.565 ± 2.594 | 0.381 ± 3.754 | 0.183 (−1.901, 2.268) | 0.859 |
| FEV1/FVC change after BD test, % | 0.710 ± 4.670 | 1.089 ± 9.490 | −0.379 (−5.194, 4.435) | 0.874 |
| Pre-BD PEF, L/s | 0.127 ± 0.495 | −0.070 ± 0.563 | 0.197 (−0.147, 0.541) | 0.253 |
| Pre-BD PEF, % predicted | 2.125 ± 7.948 | −4.979 ± 20.830 | 7.104 (−3.026, 17.233) | 0.164 |
| Post-BD PEF, L/s | 0.103 ± 0.436 | −0.352 ± 0.568 | 0.455 (0.127, 0.782) | 0.008 |
| Post-BD PEF, % predicted | −0.215 ± 12.637 | −4.663 ± 7.949 | 4.448 (−2.444, 11.340) | 0.199 |
| PEF change after BD test, % | −1.330 ± 15.503 | −5.884 ± 11.900 | 4.554 (−4.448, 13.556) | 0.312 |
| Pre-BD FEF25–75, L/s | 0.011 ± 0.144 | 0.001 ± 0.072 | 0.010 (−0.064, 0.085) | 0.778 |
| Pre-BD FEF25–75, % predicted | 0.670 ± 8.266 | 0.274 ± 3.573 | 0.396 (−3.775, 4.568) | 0.848 |
| Post-BD FEF25–75, L/s | 0.041 ± 0.082 | −0.426 ± 0.102 | 0.083 (0.023, 0.143) | 0.008 |
| Post-BD FEF25–75, % predicted | 2.310 ± 4.268 | −1.805 ± 5.538 | 4.115 (0.917, 7.313) | 0.013 |
| FEF25–75 change after BD test, % | 2.840 ± 22.101 | −9.021 ± 31.336 | 11.861 (−5.659, 29.381) | 0.178 |
| Functional capacity | ||||
| mMRC scores | −0.100 ± 0.447 | −0.105 ± 0.315 | 0.005 (−0.247, 0.258) | 0.967 |
| CAT scores | −1.000 ± 4.316 | −0.473 ± 2.458 | −0.526 (−2.822, 1.769) | 0.645 |
| 6MWD, m | 7.450 ± 30.705 | 9.053 ± 28.905 | −1.603 (−20.974, 17.769) | 0.868 |
| Data | Doxofylline | Procaterol | ||||||
|---|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | Mean Change (95% CI) | p-Value | Before Treatment | After Treatment | Mean Change (95% CI) | p-Value | |
| Pre-BD FVC, L | 2.699 ± 0.717 | 2.679 ± 0.645 | −0.021 ± 0.224 (−0.125, 0.084) | 0.687 | 2.678 ± 0.643 | 2.658 ± 0.636 | −0.021 ± 0.196 (−0.115, 0.074) | 0.654 |
| Pre-BD FVC, % predicted | 87.125 ± 18.828 | 86.760 ± 17.225 | −0.365 ± 8.995 (−4.575, 3.845) | 0.858 | 84.858 ± 20.159 | 83.847 ± 19.200 | −1.011 ± 6.858 (−4.316, 2.295) | 0.529 |
| Post-BD FVC, L | 2.784 ± 0.733 | 2.764 ± 0.714 | −0.195 ± 0.254 (−0.138, 0.099) | 0.735 | 2.806 ± 0.649 | 2.733 ± 0.659 | −0.073 ± 0.222 (−0.180, 0.034) | 0.168 |
| Post-BD FVC, % predicted | 89.980 ± 19.447 | 89.400 ± 18.636 | −0.580 ± 10.126 (−5.319, 4.159) | 0.801 | 88.779 ± 19.603 | 85.905 ± 18.647 | −2.874 ± 7.490 (−6.484, 0.737) | 0.112 |
| FVC change after BD test, % | 3.370 ± 4.536 | 2.935 ± 5.318 | −0.435 ± 6.551 (−3.501, 2.631) | 0.770 | 5.268 ± 8.125 | 3.011 ± 8.398 | −2.258 ± 9.440 (−6.808, 2.292) | 0.311 |
| Pre-BD FEV1, L | 1.351 ± 0.390 | 1.350 ± 0.362 | −0.001 ± 0.128 (−0.061, 0.059) | 0.973 | 1.336 ± 0.381 | 1.322 ± 0.393 | −0.015 ± 0.112 (−0.069, 0.039) | 0.572 |
| Pre-BD FEV1, % predicted | 56.070 ± 14.611 | 56.230 ± 14.643 | 0.160 ± 6.623 (−2.939, 3.259) | 0.915 | 54.068 ± 15.817 | 53.221 ± 15.756 | −0.847 ± 4.758 (−3.141, 1.446) | 0.448 |
| Post-BD FEV1, L | 1.397 ± 0.386 | 1.401 ± 0.374 | 0.004 ± 0.116 (−0.050, 0.058) | 0.879 | 1.435 ± 0.356 | 1.404 ± 0.366 | −0.031 ± 0.150 (−0.103, 0.042) | 0.387 |
| Post-BD FEV1, % predicted | 56.425 ± 16.875 | 58.175 ± 14.151 | 1.750 ± 7.643 (−1.827, 5.327) | 0.319 | 58.116 ± 14.782 | 56.474 ± 14.211 | −1.642 ± 5.762 (−4.419,1.135) | 0.230 |
| FEV1 change after BD test, % | 3.715 ± 5.748 | 3.905 ± 4.069 | 0.190 ± 5.624 (−2.442, 2.822) | 0.881 | 8.900 ± 9.453 | 7.479 ± 9.161 | −1.421 ± 8.088 (−5.320, 2.477) | 0.454 |
| Pre-BD FEV1/FVC, % | 51.546 ± 12.386 | 51.753 ± 12.487 | 0.207 ± 3.074 (−1.231, 1.645) | 0.767 | 51.181 ± 13.692 | 50.837 ± 12.927 | −0.343 ± 2.927 (−1.754, 1.068) | 0.616 |
| Post-BD FEV1/FVC, % | 51.650 ± 12.167 | 52.215 ± 12.427 | 0.565 ± 2.594 (−0.650, 1.779) | 0.343 | 52.658 ± 13.026 | 53.040 ± 13.700 | 0.381 ± 3.754 (−1.428, 2.191) | 0.663 |
| FEV1/FVC change after BD test, % | 0.390 ± 4.406 | 1.100 ± 4.752 | 0.710 ± 4.670 (−1.476, 2.896) | 0.505 | 3.542 ± 5.847 | 4.632 ± 8.147 | 1.089 ± 9.490 (−3.485, 5.663) | 0.623 |
| Pre-BD PEF, L/s | 4.573 ± 1.367 | 4.700 ± 1.113 | 0.127 ± 0.495 (−0.105, 0.359) | 0.266 | 4.620 ± 1.182 | 4.550 ± 1.180 | −0.070 ± 0.563 (−0.342, 0.202) | 0.595 |
| Pre-BD PEF, % predicted | 63.875 ± 18.079 | 66.000 ± 14.486 | 2.125 ± 7.948 (−1.595, 5.845) | 0.247 | 63.274 ± 15.999 | 58.295 ± 18.752 | −4.979 ± 20.830 (−15.019, 5.061) | 0.311 |
| Post-BD PEF, L/s | 4.652 ± 1.343 | 4.7545 ± 1.258 | 0.103 ± 0.436 (−0.102, 0.307) | 0.307 | 4.897 ± 1.080 | 4.545 ± 0.958 | −0.352 ± 0.568 (−0.626, −0.078) | 0.015 |
| Post-BD PEF, % predicted | 64.790 ± 17.511 | 64.575 ± 15.294 | −0.215 ± 12.637 (−6.129, 5.699) | 0.940 | 67.137 ± 14.740 | 62.474 ± 12.719 | −4.663 ± 7.949 (−8.495, −0.832) | 0.020 |
| PEF change after BD test, % | 2.145 ± 12.762 | 0.815 ± 9.176 | −1.330 ± 15.503 (−8.586, 5.926) | 0.705 | 7.321 ± 11.070 | 1.437 ± 9.632 | −5.884 ± 11.900 (−11.620, −0.149) | 0.045 |
| Pre-BD FEF25–75, L/s | 0.486 ± 0.250 | 0.497 ± 0.223 | 0.011 ± 0.144 (−0.057, 0.079) | 0.074 | 0.466 ± 0.237 | 0.466 ± 0.252 | 0.001 ± 0.072 (−0.034, 0.035) | 0.975 |
| Pre-BD FEF25–75, % predicted | 24.160 ± 13.105 | 24.830 ± 11.553 | 0.670 ± 8.266 (−3.199, 4.539) | 0.721 | 22.268 ± 12.059 | 22.542 ± 13.056 | 0.274 ± 3.573 (−1.448, 1.996) | 0.742 |
| Post-BD FEF25–75, L/s | 0.476 ± 0.205 | 0.517 ± 0.226 | 0.041 ± 0.082 (0.002, 0.079) | 0.040 | 0.532 ± 0.233 | 0.490 ± 0.238 | −0.426 ± 0.102 (−0.092, 0.007) | 0.086 |
| Post-BD FEF25–75, % predicted | 23.220 ± 9.795 | 25.530 ± 11.218 | 2.310 ± 4.268 (0.313, 4.307) | 0.026 | 25.374 ± 11.638 | 23.568 ± 11.563 | −1.805 ± 5.538 (−4.474, 0.864) | 0.172 |
| FEF25–75 change after BD test, % | 2.075 ± 20.309 | 4.915 ± 14.577 | 2.840 ± 22.101 (−7.504, 13.184) | 0.572 | 18.700 ± 18.752 | 9.679 ± 23.757 | −9.021 ± 31.336 (−24.124, 6.082) | 0.226 |
| mMRC scores | 0.950 ± 0.999 | 0.850 ± 0.875 | −0.100 ± 0.447 (−0.309, 0.109) | 0.330 | 1.000 ± 0.882 | 0.890 ± 0.937 | −0.105 ± 0.315 (−0.257, 0.047) | 0.163 |
| CAT scores | 5.600 ± 6.460 | 4.600 ± 5.605 | −1.000 ± 4.316 (−3.020, 1.020) | 0.313 | 5.320 ± 5.239 | 4.840 ± 6.423 | −0.474 ± 2.458 (−1.658, 0.711) | 0.412 |
| 6MWD, m | 394.950 ± 106.729 | 402.400 ± 96.960 | 7.450 ± 30.705 (−6.920, 21.820) | 0.291 | 408.737 ± 108.588 | 417.790 ± 112.749 | 9.053 ± 28.905 (−4.879, 22.984) | 0.189 |
| Adverse Events | Doxofylline (n = 20) | Procaterol (n = 20) | p-Value |
|---|---|---|---|
| Serious adverse event | 0 | 1 (5) | 1.000 |
| COPD exacerbation | 1 (5) | 2 (10) | 1.000 |
| Stopping medication | 4 (20) | 0 | 0.106 |
| Cardiovascular adverse events | 3 (15) | 1 (5) | 0.605 |
| Palpitation | 3 (15) | 1 (5) | 0.605 |
| Tachycardia | 0 | 0 | NA |
| Chest pain | 1 (5) | 0 | 1.000 |
| Gastrointestinal adverse events | 5 (25) | 1 (5) | 0.182 |
| Nausea | 5 (25) | 1 (5) | 0.182 |
| Diarrhea | 1 (5) | 0 | 1.000 |
| Abdominal pain | 1 (5) | 0 | 1.000 |
| Anorexia | 3 (15) | 0 | 0.231 |
| Neurological adverse events | 7 (35) | 1 (5) | 0.044 |
| Headache | 0 | 0 | NA |
| Dizziness | 5 (25) | 0 | 0.047 |
| Insomnia | 3 (15) | 1 (5) | 0.605 |
| Respiratory adverse events | 2 (10) | 3 (15) | 1.000 |
| Rhinitis | 0 | 1 (5) | 1.000 |
| Pharyngitis | 0 | 0 | NA |
| Increased cough | 0 | 0 | NA |
| Dry mouth | 2 (10) | 0 | 0.487 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiphoklang, N.; Panichaporn, S.; Siriyothipun, T.; Ruchiwit, P. Effects of Oral Doxofylline and Procaterol on Chronic Obstructive Pulmonary Disease: A Randomized Crossover Study. Med. Sci. 2025, 13, 49. https://doi.org/10.3390/medsci13020049
Saiphoklang N, Panichaporn S, Siriyothipun T, Ruchiwit P. Effects of Oral Doxofylline and Procaterol on Chronic Obstructive Pulmonary Disease: A Randomized Crossover Study. Medical Sciences. 2025; 13(2):49. https://doi.org/10.3390/medsci13020049
Chicago/Turabian StyleSaiphoklang, Narongkorn, Sarawut Panichaporn, Thiravit Siriyothipun, and Pitchayapa Ruchiwit. 2025. "Effects of Oral Doxofylline and Procaterol on Chronic Obstructive Pulmonary Disease: A Randomized Crossover Study" Medical Sciences 13, no. 2: 49. https://doi.org/10.3390/medsci13020049
APA StyleSaiphoklang, N., Panichaporn, S., Siriyothipun, T., & Ruchiwit, P. (2025). Effects of Oral Doxofylline and Procaterol on Chronic Obstructive Pulmonary Disease: A Randomized Crossover Study. Medical Sciences, 13(2), 49. https://doi.org/10.3390/medsci13020049






