Memory T Cells in Respiratory Virus Infections: Protective Potential and Persistent Vulnerabilities
Abstract
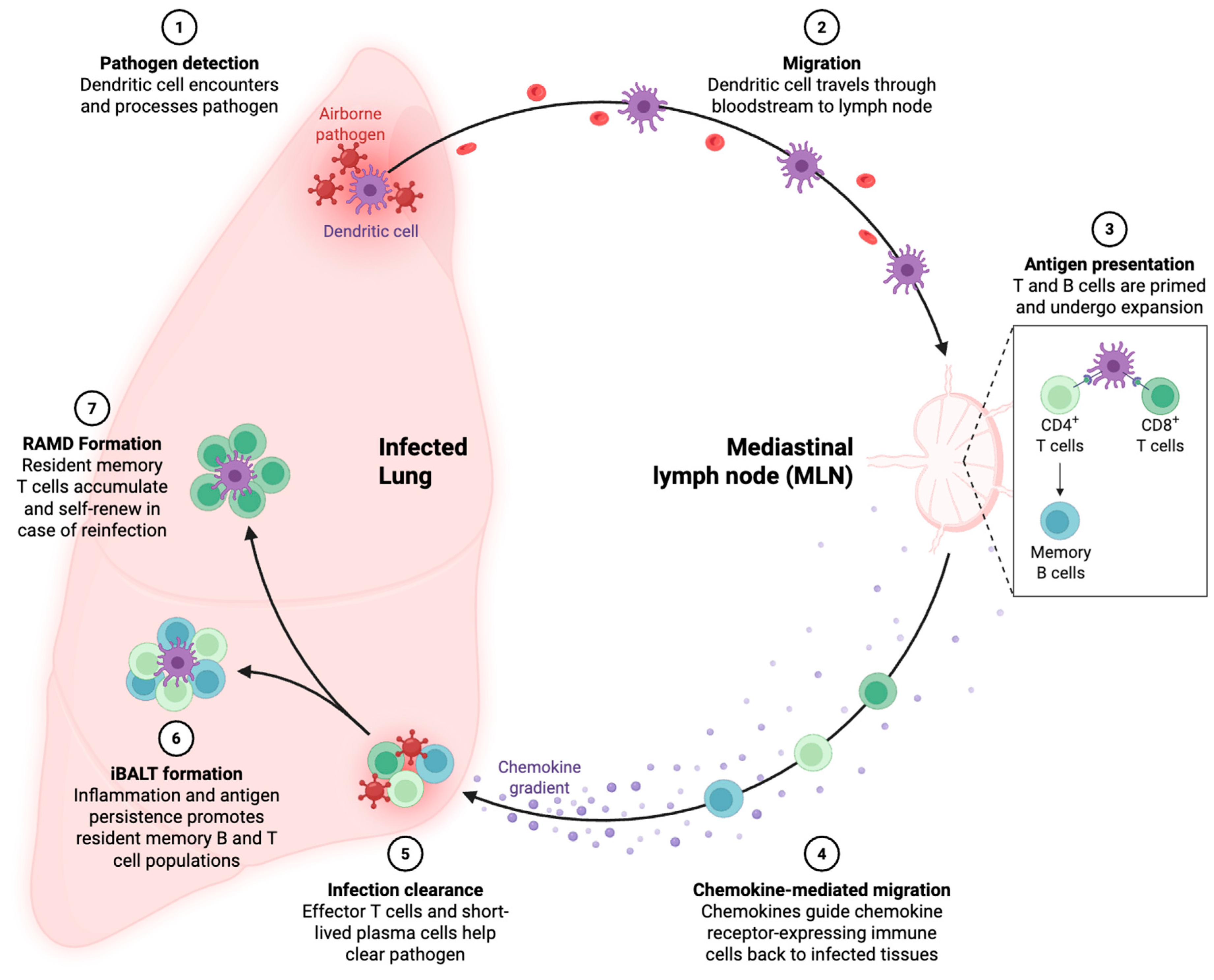
1. Introduction
2. Memory T Cells: Overview
2.1. Definition and Types of Memory T Cells
2.2. Mechanisms of Memory T Cell Generation During Viral Infections
2.3. Lifespan and Maintenance of Memory T Cells
3. Protective Role of Memory T Cells Against Respiratory Viruses
4. Challenges to Effective Immunity by Memory T Cells
4.1. Viral Evasion Mechanisms
4.2. Anatomical Barriers
4.3. Waning Immunity over Time
5. Why Do People Still Get Sick?
5.1. Gaps in Memory T Cell Responses
5.2. Impact of Host Factors
5.3. Emerging Infections and Lack of Pre-Existing Memory
6. Enhancing Memory T Cell Responses
6.1. Vaccine Strategies to Boost Memory T Cells
6.2. Therapeutics Targeting T Cell Function
6.3. Potential Roles of Exercise and Nutrition
7. Concerns Regarding the Safety of Memory T Cell Modulation
7.1. Immune Dysregulation and Cytokine Storm
7.2. Heterologous Immunity: Pros and Cons
7.3. Specific Risks with Immune Checkpoint Inhibitors
7.4. Other Concerns
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woodland, D.L.; Hogan, R.J.; Zhong, W. Cellular Immunity and Memory to Respiratory Virus Infections. IR 2001, 24, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Kream, R.M. Convalescent Memory T Cell Immunity in Individuals with Mild or Asymptomatic SARS-CoV-2 Infection May Result from an Evolutionarily Adapted Immune Response to Coronavirus and the ‘Common Cold’. Med. Sci. Monit 2020, 26, e929789. [Google Scholar] [CrossRef]
- Sutanto, H.; Soegiarto, G. Risk of Thrombosis during and after a SARS-CoV-2 Infection: Pathogenesis, Diagnostic Approach, and Management. Hematol. Rep. 2023, 15, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Shin, H. Formation and Function of Tissue-Resident Memory T Cells during Viral Infection. Curr. Opin. Virol. 2018, 28, 61–67. [Google Scholar] [CrossRef]
- Richter, B.W.M.; Onuska, J.M.; Niewiesk, S.; Prince, G.A.; Eichelberger, M.C. Antigen-Dependent Proliferation and Cytokine Induction in Respiratory Syncytial Virus-Infected Cotton Rats Reflect the Presence of Effector-Memory T Cells. Virology 2005, 337, 102–110. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Knudson, C.J.; Hartwig, S.M.; Pewe, L.L.; Meyerholz, D.K.; Langlois, R.A.; Harty, J.T.; Varga, S.M. Memory CD8 T Cells Mediate Severe Immunopathology Following Respiratory Syncytial Virus Infection. PLoS Pathog. 2018, 14, e1006810. [Google Scholar] [CrossRef]
- Allie, S.R.; Randall, T.D. Pulmonary Immunity to Viruses. Clin. Sci. 2017, 131, 1737–1762. [Google Scholar] [CrossRef] [PubMed]
- Rahe, M.; Murtaugh, M. Mechanisms of Adaptive Immunity to Porcine Reproductive and Respiratory Syndrome Virus. Viruses 2017, 9, 148. [Google Scholar] [CrossRef]
- Mangare, C.; Tischer-Zimmermann, S.; Riese, S.B.; Dragon, A.C.; Prinz, I.; Blasczyk, R.; Maecker-Kolhoff, B.; Eiz-Vesper, B. Robust Identification of Suitable T-Cell Subsets for Personalized CMV-Specific T-Cell Immunotherapy Using CD45RA and CD62L Microbeads. Int. J. Mol. Sci. 2019, 20, 1415. [Google Scholar] [CrossRef]
- Kwiecień, I.; Rutkowska, E.; Sokołowski, R.; Bednarek, J.; Raniszewska, A.; Jahnz-Różyk, K.; Rzepecki, P.; Domagała-Kulawik, J. Effector Memory T Cells and CD45RO+ Regulatory T Cells in Metastatic vs. Non-Metastatic Lymph Nodes in Lung Cancer Patients. Front. Immunol. 2022, 13, 864497. [Google Scholar] [CrossRef]
- Maggioli, M.F.; Palmer, M.V.; Thacker, T.C.; Vordermeier, H.M.; Waters, W.R. Characterization of Effector and Memory T Cell Subsets in the Immune Response to Bovine Tuberculosis in Cattle. PLoS ONE 2015, 10, e0122571. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Ma, W.; Miron, M.; Granot, T.; Guyer, R.S.; Carpenter, D.J.; Senda, T.; Sun, X.; Ho, S.-H.; Lerner, H.; et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017, 20, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Bromley, S.K.; Akbaba, H.; Mani, V.; Mora-Buch, R.; Chasse, A.Y.; Sama, A.; Luster, A.D. CD49a Regulates Cutaneous Resident Memory CD8+ T Cell Persistence and Response. Cell Rep. 2020, 32, 108085. [Google Scholar] [CrossRef]
- Reilly, E.C.; Sportiello, M.; Emo, K.L.; Amitrano, A.M.; Jha, R.; Kumar, A.B.R.; Laniewski, N.G.; Yang, H.; Kim, M.; Topham, D.J. CD49a Identifies Polyfunctional Memory CD8 T Cell Subsets That Persist in the Lungs After Influenza Infection. Front. Immunol. 2021, 12, 728669. [Google Scholar] [CrossRef]
- Gerlach, C.; Moseman, A.; Loughhead, S.; Alvarez, D.; Von Andrian, U.H. CX3CR1 Distinguishes Three Antigen-Experienced CD8 T Cell Subsets with Distinct Migratory, Functional and Homeostatic Properties. J. Immunol. 2016, 196, 64.3. [Google Scholar] [CrossRef]
- Shin, M.S.; Kim, D.; Yim, K.; Park, H.-J.; You, S.; Dong, X.; Koumpouras, F.; Shaw, A.C.; Fan, R.; Krishnaswamy, S.; et al. IL-7 Receptor Alpha Defines Heterogeneity and Signature of Human Effector Memory CD8+ T Cells in High Dimensional Analysis. Cell Immunol 2020, 355, 104155. [Google Scholar] [CrossRef]
- Kim, T.S.; Hufford, M.M.; Sun, J.; Fu, Y.-X.; Braciale, T.J. Antigen Persistence and the Control of Local T Cell Memory by Migrant Respiratory Dendritic Cells after Acute Virus Infection. J. Exp. Med. 2010, 207, 1161–1172. [Google Scholar] [CrossRef]
- Fritsch, R.D.; Shen, X.; Sims, G.P.; Hathcock, K.S.; Hodes, R.J.; Lipsky, P.E. Stepwise Differentiation of CD4 Memory T Cells Defined by Expression of CCR7 and CD27. J. Immunol. 2005, 175, 6489–6497. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Effector and Memory T-Cell Differentiation: Implications for Vaccine Development. Nat. Rev. Immunol. 2002, 2, 251–262. [Google Scholar] [CrossRef]
- Kalia, V.; Sarkar, S.; Gourley, T.S.; Rouse, B.T.; Ahmed, R. Differentiation of Memory B and T Cells. Curr. Opin. Immunol. 2006, 18, 255–264. [Google Scholar] [CrossRef]
- Kaech, S.M.; Cui, W. Transcriptional Control of Effector and Memory CD8+ T Cell Differentiation. Nat Rev Immunol 2012, 12, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, S.T. Modeling Memory Differentiation in T Cells. Science 2018, 360, 1198.9–1200. [Google Scholar] [CrossRef]
- Chen, H.D.; Fraire, A.E.; Joris, I.; Brehm, M.A.; Welsh, R.M.; Selin, L.K. Memory CD8+ T Cells in Heterologous Antiviral Immunity and Immunopathology in the Lung. Nat Immunol 2001, 2, 1067–1076. [Google Scholar] [CrossRef]
- Reijnders, T.D.Y.; Schuurman, A.R.; Van Der Poll, T. The Immune Response to Respiratory Viruses: From Start to Memory. Semin Respir Crit Care Med 2021, 42, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, J.E.; Miller, S.C.; Smith, J.; Lu, B.; Gerard, C.; Cookenham, T.; Roberts, A.D.; Woodland, D.L. The Chemokine Receptor CCR5 Plays a Key Role in the Early Memory CD8+ T Cell Response to Respiratory Virus Infections. Immunity 2008, 29, 101–113. [Google Scholar] [CrossRef]
- Nguyen, T.H.; McAuley, J.L.; Kim, Y.; Zheng, M.Z.; Gherardin, N.A.; Godfrey, D.I.; Purcell, D.F.; Sullivan, L.C.; Westall, G.P.; Reading, P.C.; et al. Influenza, but Not SARS-CoV-2, Infection Induces a Rapid Interferon Response That Wanes with Age and Diminished Tissue-Resident Memory CD8+ T Cells. Clin Transl Immunol. 2021, 10, e1242. [Google Scholar] [CrossRef] [PubMed]
- Cusi, M.G.; Martorelli, B.; Di Genova, G.; Terrosi, C.; Campoccia, G.; Correale, P. Age Related Changes in T Cell Mediated Immune Response and Effector Memory to Respiratory Syncytial Virus (RSV) in Healthy Subjects. Immun. Ageing 2010, 7, 14. [Google Scholar] [CrossRef]
- Tong, M.Z.W.; Hulme, K.D.; Law, S.C.; Noye, E.; Dorey, E.S.; Chew, K.Y.; Rowntree, L.C.; van de Sandt, C.E.; Kedzierska, K.; Goeijenbier, M.; et al. High Glycemic Variability Is Associated with a Reduced T Cell Cytokine Response to Influenza A Virus. iScience 2024, 27, 111166. [Google Scholar] [CrossRef]
- McKendry, R.T.; Spalluto, C.M.; Burke, H.; Nicholas, B.; Cellura, D.; Al-Shamkhani, A.; Staples, K.J.; Wilkinson, T.M.A. Dysregulation of Antiviral Function of CD8+ T Cells in the Chronic Obstructive Pulmonary Disease Lung. Role of the PD-1–PD-L1 Axis. Am. J. Respir. Crit. Care Med. 2016, 193, 642–651. [Google Scholar] [CrossRef]
- De Bree, G.J.; Heidema, J.; Van Leeuwen, E.M.M.; Van Bleek, G.M.; Jonkers, R.E.; Jansen, H.M.; Van Lier, R.A.W.; Out, T.A. Respiratory Syncytial Virus–Specific CD8+ Memory T Cell Responses in Elderly Persons. J. Infect. Dis. 2005, 191, 1710–1718. [Google Scholar] [CrossRef]
- Goldman, J.D.; Robinson, P.C.; Uldrick, T.S.; Ljungman, P. COVID-19 in Immunocompromised Populations: Implications for Prognosis and Repurposing of Immunotherapies. J. Immunother Cancer 2021, 9, e002630. [Google Scholar] [CrossRef]
- McMaster, S.R.; Gabbard, J.D.; Koutsonanos, D.G.; Compans, R.W.; Tripp, R.A.; Tompkins, S.M.; Kohlmeier, J.E. Memory T Cells Generated by Prior Exposure to Influenza Cross React with the Novel H7N9 Influenza Virus and Confer Protective Heterosubtypic Immunity. PLoS ONE 2015, 10, e0115725. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.L.; Li, Z.T.; Lobby, J.L.; Eggert, J.O.P.; Kohlmeier, J.E. Unrelated Respiratory Infections Compromise Established Cellular Immunity by Promoting Apoptosis of Pre-Existing Lung-Resident Memory CD8 T Cells. J. Immunol. 2019, 202, 66.17. [Google Scholar] [CrossRef]
- Sutanto, H.; Waitupu, A.; Adytia, G.J.; Fetarayani, D. Fighting the Flu in the Tropics: The Role of Influenza Vaccination in Southeast Asia. Asia Pac. Allergy 2025, 10–5415. [Google Scholar] [CrossRef]
- Liu, Z.; Kabir, M.T.; Chen, S.; Zhang, H.; Wakim, L.M.; Rehm, B.H.A. Intranasal Epitope-Polymer Vaccine Lodges Resident Memory T Cells Protecting Against Influenza Virus. Adv Health Mater 2024, 13, 2304188. [Google Scholar] [CrossRef]
- Ko, K.H.; Bae, H.S.; Park, J.W.; Lee, J.-S.; Park, S.; Heo, J.; Park, H.; Choi, J.; Bae, E.; Na, W.; et al. A Vaccine Platform Targeting Lung-Resident Memory CD4+ T-Cells Provides Protection against Heterosubtypic Influenza Infections in Mice and Ferrets. Nat. Commun. 2024, 15, 10368. [Google Scholar] [CrossRef]
- Schmidt, A.; Fuchs, J.; Dedden, M.; Kocher, K.; Schülein, C.; Hübner, J.; Vieira Antão, A.; Irrgang, P.; Oltmanns, F.; Viherlehto, V.; et al. Inflammatory Conditions Shape Phenotypic and Functional Characteristics of Lung-Resident Memory T Cells in Mice. Nat. Commun. 2025, 16, 3612. [Google Scholar] [CrossRef]
- Bošnjak, B.; Odak, I.; Barros-Martins, J.; Sandrock, I.; Hammerschmidt, S.I.; Permanyer, M.; Patzer, G.E.; Greorgiev, H.; Gutierrez Jauregui, R.; Tscherne, A.; et al. Intranasal Delivery of MVA Vector Vaccine Induces Effective Pulmonary Immunity Against SARS-CoV-2 in Rodents. Front. Immunol. 2021, 12, 772240. [Google Scholar] [CrossRef]
- Lei, H.; Alu, A.; Yang, J.; He, C.; Shi, J.; Hong, W.; Peng, D.; Zhang, Y.; Liu, J.; Qin, F.; et al. Intranasal Inoculation of Cationic Crosslinked Carbon Dots-Adjuvanted Respiratory Syncytial Virus F Subunit Vaccine Elicits Mucosal and Systemic Humoral and Cellular Immunity. MedComm 2025, 6, e70146. [Google Scholar] [CrossRef]
- Xu, R.; Hong, H.A.; Khandaker, S.; Baltazar, M.; Allehyani, N.; Beentjes, D.; Prince, T.; Ho, Y.-L.; Nguyen, L.H.; Hynes, D.; et al. Nasal Delivery of Killed Bacillus Subtilis Spores Protects against Influenza, RSV and SARS-CoV-2. Front. Immunol. 2025, 16, 1501907. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Hu, L.; Huang, Q.; Qi, J.; Shen, L.; Wang, G.; Yu, W.; Hu, T. A SARS-CoV-2 Mucosal Nanovaccine Based on Assembly of Maltodextrin, STING Agonist and Polyethyleneimine. Int. J. Biol. Macromol. 2025, 294, 139395. [Google Scholar] [CrossRef]
- Tan, A.C.L.; Deliyannis, G.; Bharadwaj, M.; Brown, L.E.; Zeng, W.; Jackson, D.C. The Design and Proof of Concept for a CD8+ T Cell-Based Vaccine Inducing Cross-Subtype Protection against Influenza A Virus. Immunol. Cell Biol. 2013, 91, 96–104. [Google Scholar] [CrossRef]
- Matyushenko, V.; Kotomina, T.; Kudryavtsev, I.; Mezhenskaya, D.; Prokopenko, P.; Matushkina, A.; Sivak, K.; Muzhikyan, A.; Rudenko, L.; Isakova-Sivak, I. Conserved T-Cell Epitopes of Respiratory Syncytial Virus (RSV) Delivered by Recombinant Live Attenuated Influenza Vaccine Viruses Efficiently Induce RSV-Specific Lung-Localized Memory T Cells and Augment Influenza-Specific Resident Memory T-Cell Responses. Antivir. Res. 2020, 182, 104864. [Google Scholar] [CrossRef]
- Davis-Porada, J.; George, A.B.; Lam, N.; Caron, D.P.; Gray, J.I.; Huang, J.; Hwu, J.; Wells, S.B.; Matsumoto, R.; Kubota, M.; et al. Maintenance and Functional Regulation of Immune Memory to COVID-19 Vaccines in Tissues. Immunity 2024, 57, 2895–2913.e8. [Google Scholar] [CrossRef]
- Fisicaro, P.; Barili, V.; Rossi, M.; Montali, I.; Vecchi, A.; Acerbi, G.; Laccabue, D.; Zecca, A.; Penna, A.; Missale, G.; et al. Pathogenetic Mechanisms of T Cell Dysfunction in Chronic HBV Infection and Related Therapeutic Approaches. Front. Immunol. 2020, 11, 849. [Google Scholar] [CrossRef]
- Sutanto, H.; Safira, A.; Fetarayani, D. From Tumor to Tolerance: A Comprehensive Review of Immune Checkpoint Inhibitors and Immune-Related Adverse Events. Asia Pac. Allergy 2024, 14, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Grabmeier-Pfistershammer, K.; Stecher, C.; Zettl, M.; Rosskopf, S.; Rieger, A.; Zlabinger, G.J.; Steinberger, P. Antibodies Targeting BTLA or TIM-3 Enhance HIV-1 Specific T Cell Responses in Combination with PD-1 Blockade. Clin. Immunol. 2017, 183, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous Delivery of Oncolytic Reovirus to Brain Tumor Patients Immunologically Primes for Subsequent Checkpoint Blockade. Sci. Transl. Med. 2018, 10, eaam7577. [Google Scholar] [CrossRef]
- Chang, J.; Choi, S.Y.; Jin, H.T.; Sung, Y.C.; Braciale, T.J. Improved Effector Activity and Memory CD8 T Cell Development by IL-2 Expression during Experimental Respiratory Syncytial Virus Infection. J. Immunol. 2004, 172, 503–508. [Google Scholar] [CrossRef]
- Lee, S.; Moore, M. Enhancement of Vaccine-Induced Respiratory Syncytial Virus-Specific Memory CD8 T Cell Responses by Targeting 41BB (P6158). J. Immunol. 2013, 190, 66.12. [Google Scholar] [CrossRef]
- Salek-Ardakani, S.; Moutaftsi, M.; Sette, A.; Croft, M. Targeting OX40 Promotes Lung-Resident Memory CD8 T Cell Populations That Protect against Respiratory Poxvirus Infection. J. Virol. 2011, 85, 9051–9059. [Google Scholar] [CrossRef]
- Zhang, W.; Tripp, R.A. RNA Interference Inhibits Respiratory Syncytial Virus Replication and Disease Pathogenesis without Inhibiting Priming of the Memory Immune Response. J. Virol. 2008, 82, 12221–12231. [Google Scholar] [CrossRef]
- Brandão, I.; Suzuki, T.; Alsharairi, N.A. Editorial: Influence of Dietary Factors, Nutrients, and the Gut-Lung Axis on Respiratory Health. Front. Nutr. 2025, 12, 1556801. [Google Scholar] [CrossRef]
- Warren, K.; Thompson, N.; Wannemuehler, M.; Kohut, M. Antibody and CD8+ T Cell Memory Response to Influenza A/PR/8/34 Infection Is Reduced in Treadmill-Exercised Mice, yet Still Protective. J. Appl. Physiol. 2013, 114, 1413–1420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bradley, M.C.; Idzikowski, E.; La Carpia, F.; Gray, J.I.; Rybkina, K.; Guyer, R.S.; Pethe, K.; Hod, E.A.; Connors, T.J. Dietary Iron Deficiency Impairs the Functionality but Not Generation or Establishment of Memory T Cells Following Influenza Infection. J. Immunol. 2022, 208, 57.03. [Google Scholar] [CrossRef]
- Govers, C.; Calder, P.C.; Savelkoul, H.F.J.; Albers, R.; Van Neerven, R.J.J. Ingestion, Immunity, and Infection: Nutrition and Viral Respiratory Tract Infections. Front. Immunol. 2022, 13, 841532. [Google Scholar] [CrossRef]
- Antunes, K.H.; Becker, A.; Franceschina, C.; De Freitas, D.D.N.; Lape, I.; Da Cunha, M.D.; Leitão, L.; Rigo, M.M.; Pinto, L.A.; Stein, R.T.; et al. Respiratory Syncytial Virus Reduces STAT3 Phosphorylation in Human Memory CD8 T Cells Stimulated with IL-21. Sci. Rep. 2019, 9, 17766. [Google Scholar] [CrossRef]
- Wlodarczyk, M.F.; Kraft, A.; Chen, H.; Selin, L.K. Protection or Immunopathology upon Heterologous Virus Infection: A Decision of Memory Cells (43.15). J. Immunol. 2009, 182, 43.15. [Google Scholar] [CrossRef]
- Ostler, T.; Pircher, H.; Ehl, S. “Bystander” Recruitment of Systemic Memory T Cells Delays the Immune Response to Respiratory Virus Infection. Eur. J. Immunol. 2003, 33, 1839–1848. [Google Scholar] [CrossRef]
- Selin, L.K.; Lin, M.Y.; Kraemer, K.A.; Pardoll, D.M.; Schneck, J.P.; Varga, S.M.; Santolucito, P.A.; Pinto, A.K.; Welsh, R.M. Attrition of T Cell Memory. Immunity 1999, 11, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.; Karniadakis, I.; Mazonakis, N.; Akinosoglou, K.; Tsioutis, C.; Spernovasilis, N. Immune Checkpoint Inhibitors and Infection: What Is the Interplay? In Vivo 2023, 37, 2409–2420. [Google Scholar] [CrossRef]
- Redelman-Sidi, G.; Michielin, O.; Cervera, C.; Ribi, C.; Aguado, J.M.; Fernández-Ruiz, M.; Manuel, O. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the Safety of Targeted and Biological Therapies: An Infectious Diseases Perspective (Immune Checkpoint Inhibitors, Cell Adhesion Inhibitors, Sphingosine-1-Phosphate Receptor Modulators and Proteasome Inhibitors). Clin. Microbiol. Infect. 2018, 24, S95–S107. [Google Scholar] [CrossRef]
- Qiu, B.; Xiong, M.; Liu, F.; Zhang, P.; Zhou, R.; Wang, D.; Luo, Y.; Liu, Y.; Guo, J.; Chen, N.; et al. Respiratory Toxicities after Concurrent Chemoradiotherapy and Subsequent Consolidation Immune Checkpoint Inhibitors in Locally Advanced Non-Small Cell Lung Cancer: Infectious and Non-Infectious Subtypes by Pathogen NGS Testing. JCO 2023, 41, e20557. [Google Scholar] [CrossRef]
- Klein, S.; Ghersi, D.; Manns, M.P.; Prinz, I.; Cornberg, M.; Kraft, A.R.M. PD-L1 Checkpoint Inhibition Narrows the Antigen-Specific T Cell Receptor Repertoire in Chronic Lymphocytic Choriomeningitis Virus Infection. J. Virol. 2020, 94, e00795-20. [Google Scholar] [CrossRef]
- Mischke, J.; Klein, S.; Seamann, A.; Prinz, I.; Selin, L.; Ghersi, D.; Cornberg, M.; Kraft, A.R.M. Cross-Reactive T Cell Response Exists in Chronic Lymphocytic Choriomeningitis Virus Infection upon Pichinde Virus Challenge. Viruses 2022, 14, 2293. [Google Scholar] [CrossRef]
- Connor, L.; Ryan, L.; Kohlmeier, J.; Cookenham, T.; Roberts, A.; Quinn, A.; Woodland, D. Virus-Specific T Cell Clonal Expansions Can Limit Recall Responses to Secondary Respiratory Virus Infections (104.4). J. Immunol. 2011, 186, 104.4. [Google Scholar] [CrossRef]
- Shane, H.; Verbist, K.; Klonowski, K. Respiratory Infection Alters Archetypal Anti-Viral CD8 T Cell Differentiation Programs Resulting in Diminished Memory Potential (MUC5P.861). J. Immunol. 2014, 192, 134.4. [Google Scholar] [CrossRef]
- Tripp, R.A.; Tompkins, S.M. Revised Model for Early Memory T-Cell Protection Against Respiratory Virus Challenge. Future Virol. 2008, 3, 533–536. [Google Scholar] [CrossRef]
- Woodland, D.L.; Ely, K.H.; Crowe, S.R.; Tighe, M.; Brennan, J.W.; Harmsen, A.G.; Cauley, L.S. Antiviral Memory T-Cell Responses in the Lung. Microbes Infect. 2002, 4, 1091–1098. [Google Scholar] [CrossRef]
- Luangrath, M.A.; Schmidt, M.E.; Hartwig, S.M.; Varga, S.M. Tissue-Resident Memory T Cells in the Lungs Protect against Acute Respiratory Syncytial Virus Infection. ImmunoHorizons 2021, 5, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Uddback, I.E.M.; Cartwright, E.; Schøller, A.S.; Hayward, S.L.; Lobby, J.; Takamura, S.; Thomsen, A.R.; Kohlmeier, J.E.; Christsensen, J.P. Persistent Antigen Can Maintain Lung Resident Memory CD8 T Cells Long-Term. J. Immunol. 2019, 202, 129.2. [Google Scholar] [CrossRef]
- Olson, J.A.; McDonald-Hyman, C.; Jameson, S.C.; Hamilton, S.E. Effector-like CD8+ T Cells in the Memory Population Mediate Potent Protective Immunity. Immunity 2013, 38, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Selin, L.K.; Varga, S.M.; Wong, I.C.; Welsh, R.M. Protective Heterologous Antiviral Immunity and Enhanced Immunopathogenesis Mediated by Memory T Cell Populations. J. Exp. Med. 1998, 188, 1705–1715. [Google Scholar] [CrossRef]
- Hassert, M.; Brien, J.D.; Pinto, A.K. Mouse Models of Heterologous Flavivirus Immunity: A Role for Cross-Reactive T Cells. Front. Immunol. 2019, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, N.; Bormann, N.L. scRepertoire: An R-Based Toolkit for Single-Cell Immune Receptor Analysis. F1000Res 2020, 9, 47. [Google Scholar] [CrossRef]
- Singh, M.; Al-Eryani, G.; Carswell, S.; Ferguson, J.M.; Blackburn, J.; Barton, K.; Roden, D.; Luciani, F.; Giang Phan, T.; Junankar, S.; et al. High-Throughput Targeted Long-Read Single Cell Sequencing Reveals the Clonal and Transcriptional Landscape of Lymphocytes. Nat. Commun. 2019, 10, 3120. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, D.; Wang, X.; Liu, H.; Wang, T. Mapping the Functional Landscape of T Cell Receptor Repertoires by Single-T Cell Transcriptomics. Nat. Methods 2021, 18, 92–99. [Google Scholar] [CrossRef]
- Megas, S.; Wilbrey-Clark, A.; Maartens, A.; Teichmann, S.A.; Meyer, K.B. Spatial Transcriptomics of the Respiratory System. Annu. Rev. Physiol. 2025, 87, 447–470. [Google Scholar] [CrossRef]
- Lau, M.C.; Yi, Y.; Goh, D.; Cheung, C.C.L.; Tan, B.; Lim, J.C.T.; Joseph, C.R.; Wee, F.; Lee, J.N.; Lim, X.; et al. Case Report: Understanding the Impact of Persistent Tissue-Localization of SARS-CoV-2 on Immune Response Activity via Spatial Transcriptomic Analysis of Two Cancer Patients with COVID-19 Co-Morbidity. Front. Immunol. 2022, 13, 978760. [Google Scholar] [CrossRef]
- Tan, X.; Grice, L.F.; Tran, M.; Mulay, O.; Monkman, J.; Blick, T.; Vo, T.; Almeida, A.C.; da Silva Motta Junior, J.; de Moura, K.F.; et al. A Robust Platform for Integrative Spatial Multi-Omics Analysis to Map Immune Responses to SARS-CoV-2 Infection in Lung Tissues. Immunology 2023, 170, 401–418. [Google Scholar] [CrossRef]
- Engblom, C.; Thrane, K.; Lin, Q.; Andersson, A.; Toosi, H.; Chen, X.; Steiner, E.; Lu, C.; Mantovani, G.; Hagemann-Jensen, M.; et al. Spatial Transcriptomics of B Cell and T Cell Receptors Reveals Lymphocyte Clonal Dynamics. Science 2023, 382, eadf8486. [Google Scholar] [CrossRef]
- Mathew, N.R.; Gailleton, R.; Scharf, L.; Schön, K.; Strömberg, A.; Lycke, N.; Bemark, M.; Tang, K.-W.; Angeletti, D. Nasal Tissue-Resident Memory CD4+ T Cells Persist after Influenza A Virus Infection and Provide Heterosubtypic Protection. bioRxiv 2024. [Google Scholar]

| Feature | Central Memory T Cells (TCM) | Effector Memory T Cells (TEM) | Tissue-Resident Memory T Cells (TRM) |
|---|---|---|---|
| Location | Secondary lymphoid organs (e.g., lymph nodes, spleen) | Peripheral tissues, blood | Non-lymphoid tissues (e.g., lungs, skin, gut) |
| Migration Ability | High; recirculates between blood and lymphoid tissues | Intermediate; migrates between blood and tissues | Low; remains in tissue of initial infection |
| Cytokine Production | IL-2 (promotes proliferation) | IFN-γ, TNF-α (rapid effector function) | IFN-γ, TNF-α, and localized cytokines |
| Proliferative Capacity | High; capable of rapid clonal expansion upon reactivation | Moderate; less proliferative than TCM | Low; limited proliferation in situ |
| Longevity | Long-lived | Intermediate longevity | Long-lived but dependent on local environment |
| Role in Immune Response | Provides long-term surveillance; proliferates to generate effector cells upon reinfection | Rapidly provides effector functions upon antigen re-encounter | Immediate localized response to reinfection |
| Protection Scope | Broad, systemic immunity | Rapid protection in peripheral tissues | Site-specific immunity |
| Sensitivity to Antigen | Moderate; requires antigen presentation for activation | High; responds quickly to antigens | Moderate; activation influenced by tissue environment |
| Metabolic Profile | Relies on oxidative phosphorylation | Mix of glycolysis and oxidative phosphorylation | Primarily oxidative phosphorylation |
| Examples of Infections Targeted | Systemic infections (e.g., bloodborne pathogens) | Infections at peripheral sites (e.g., skin, mucosa) | Localized infections (e.g., lung influenza) |
| Surface Marker | TCM | TEM | TRM |
|---|---|---|---|
| CD45RO | + | + | + |
| CCR7 | + | – | – |
| CD62L (L-selectin) | + | – | – |
| CD69 | – | – | + |
| CD103 (αE integrin) | – | – | + (especially CD8+ TRM) |
| S1PR1 | + | + | – |
| CXCR3 | + | + | + |
| CX3CR1 | – | + (especially in cytotoxic TEM) | – |
| CD27 | + | ± | ± |
| CD28 | + | ± | ± |
| Integrins (e.g., VLA-1) | – | ± | + |
| CD49a | – | – | + (in some TRM populations) |
| PD-1 | ± (low) | ± (variable) | + (especially in barrier tissues) |
| IL-7Rα (CD127) | + | + | + |
| Aspect | Pros | Cons |
|---|---|---|
| Enhanced Immunity | Strengthens long-term protection against reinfection [70]. | May lead to excessive inflammation and immunopathology, causing lung damage [6]. |
| Rapid Response | Faster viral clearance due to quicker activation of memory T cells [71]. | Overactivation of memory T cells can contribute to cytokine storms and severe disease [6]. |
| Cross-Protection | Can provide protection against related viral strains through heterologous immunity [23]. | May cause ineffective immune responses if memory T cells are not well-matched to the new virus [60]. |
| Vaccine Enhancement | Boosts vaccine-induced immunity by generating stronger memory T cell populations [51]. | Improper modulation can lead to reduced vaccine efficacy or immune exhaustion [67]. |
| Reduction in Disease Severity | Memory T cells help reduce viral load, leading to milder infections [69]. | Excessive memory T cell responses may worsen lung damage, as seen in RSV infections [6]. |
| Long-Term Immunity | Memory T cells persist for years, providing durable immunity [52]. | Over time, memory T cell responses may weaken or become dysfunctional [67]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutanto, H.; Pradana, F.R.; Adytia, G.J.; Ansharullah, B.A.; Waitupu, A.; Bramantono, B.; Fetarayani, D. Memory T Cells in Respiratory Virus Infections: Protective Potential and Persistent Vulnerabilities. Med. Sci. 2025, 13, 48. https://doi.org/10.3390/medsci13020048
Sutanto H, Pradana FR, Adytia GJ, Ansharullah BA, Waitupu A, Bramantono B, Fetarayani D. Memory T Cells in Respiratory Virus Infections: Protective Potential and Persistent Vulnerabilities. Medical Sciences. 2025; 13(2):48. https://doi.org/10.3390/medsci13020048
Chicago/Turabian StyleSutanto, Henry, Febrian Ramadhan Pradana, Galih Januar Adytia, Bagus Aditya Ansharullah, Alief Waitupu, Bramantono Bramantono, and Deasy Fetarayani. 2025. "Memory T Cells in Respiratory Virus Infections: Protective Potential and Persistent Vulnerabilities" Medical Sciences 13, no. 2: 48. https://doi.org/10.3390/medsci13020048
APA StyleSutanto, H., Pradana, F. R., Adytia, G. J., Ansharullah, B. A., Waitupu, A., Bramantono, B., & Fetarayani, D. (2025). Memory T Cells in Respiratory Virus Infections: Protective Potential and Persistent Vulnerabilities. Medical Sciences, 13(2), 48. https://doi.org/10.3390/medsci13020048







