Dichloroacetate and Salinomycin as Therapeutic Agents in Cancer
Abstract
1. Introduction
2. Repurposed Anticancer Agents
3. Pharmacology of Dichloroacetate
4. DCA as an Anticancer Agent and Its Synergy with Other Therapeutic Agents
| Drug | Cell Line(s) | Target(s) | Key Findings | Refs. |
|---|---|---|---|---|
| DCA | A549, H1299 | Lactate production/ glucose consumption | DCA decreases lactate production and glucose consumption. | [42] |
| DCA | HCT116 WT, HT29, PC3 | Autophagy (LC3B ll protein), mTOR, lactate excretion, tumor growth | DCA induces autophagy in colorectal cell lines via inducing ROS generation and inhibiting mTOR and lactate excretion. | [47] |
| DCA, paclitaxel | A549, H1975 | Autophagy (LC3 and p62 proteins), tumor growth | DCA inhibits autophagy, decreases cell proliferation and tumor growth, and increases mice survival. | [48] |
| DCA, paclitaxel | A549-R | PDK2 | DCA resensitizes paclitaxel-resistant cells through PDK2 inhibition and siRNA-mediated downregulation. | [52] |
| DCA, cisplatin | HeLa | Glucose metabolism | DCA is able to shift glucose metabolism to sensitize HeLa cells and exert synergy with cisplatin. | [53] |
| DCA, cisplatin, decetaxel | 54 NSCLC cells | - | Under hypoxic conditions, DCA and cisplatin (not docetaxel) combination enhances the sensitivity of a few NSCLC cell lines. | [54] |
| DCA + cisplatin/ gefitinib or erlotinib | A549, LNM35 | Cell viability, angiogenesis, tumor growth | DCA decreases cell viability, disrupts angiogenesis, and reduces tumor growth. | [55] |
| DCA, gefitinib, erlotinib | NCI-H1975, NCI-H1650, A549, NCI-H460 | EGFR, AKT, ERK1/2, PDH signaling | DCA in combination with gefitinib or erlotinib significantly inhibits the cell viability, promotes the apoptosis, and suppresses the activation of EGFR, AKT, and ERK1/2 signaling in EGFR-mutant NSCLC cell lines. | [58] |
| DCA, doxorubicin | MDA-MB-231 | Autophagy | DCA sensitizes doxorubicin-induced cell death via autophagy inhibition, and its mechanism resensitizes doxorubicin-resistant cells and enhances the chemotherapeutic effect. | [59] |
| DCA, capecitabine | A549, B-16 | Caspases/ apoptosis | DCA enhances the cytotoxic effects of capecitabine by promoting the release of caspases 3, 8, and 9. | [60] |
| DCA, metformin | SKOV3, OVCAR3 | Mcl-1, Warburg effect, glycolysis, autophagy | DCA and metformin’s combination synergistically inhibits cell viability via inducing apoptosis, and reduces the growth of tumor xenografts. | [63] |
| DCA, metformin DCA, PX-478 | MCF7, T47D MCF-7, MDA-MB-231, A549, H441, HEK-293, U251, HeLa, HEPG2, HT-29 | PDK1, glycolysis HIF-1α, PDK1 | DCA and metformin’s combination synergistically induces cell death and oxidative damage via PDK1 inhibition. DCA synergistically inhibits cancer cell growth with HIF-1α inhibitor PX-478 via inducing ROS-mediated apoptosis. | [64,65] |
| DCA, ivermectin, omeprazole, tamoxifen | Clinical trial | Cancer progression symptoms | DCA in combination with ivermectin, omeprazole, and tamoxifen relieves metastatic cancer-induced pain in patients. | [66] |
5. Pharmacology of Salinomycin
6. SAL as an Anticancer Agent and Its Synergy with Other Therapeutic Agents
7. DCA and SAL as a Combination Therapy
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSCLC | Non-small-cell lung cancer |
| DCA | Dichloroacetate |
| SAL | Salinomycin |
| SCLC | Small-cell lung carcinoma |
| AMPK | Adenosine monophosphate-activated protein kinase |
| EGFR | Epidermal growth factor receptor |
| TKIs | Tyrosine kinase inhibitors |
| PDK | Pyruvate dehydrogenase kinase |
| CSCs | Cancer stem cells |
| PDC | Pyruvate dehydrogenase complex |
| TCA | Tricarboxylic acid cycle |
| OXPHOS | Oxidative phosphorylation |
| PDHE1α | Pyruvate dehydrogenase E1 subunit 1 alpha |
| HIF1α | Hypoxia-inducible factor 1 alpha |
| MTCs | Mono-carboxylic transporter proteins |
| ROS | Reactive oxygen species |
| HUVEC | Human umbilical vein endothelial cells |
| EMT | Epithelial–mesenchymal transition |
| TGFs | Transforming growth factors |
| TGFβ | Transforming growth factor β |
| Cytc | Cytochrome c |
| ER | Endoplasmic reticulum |
| HNC | Head and neck cancer |
| ERK1/2 | Extracellular signal-regulated kinase ½ |
| HGG | High-grade glioma |
| mTOR | Mammalian targets of rapamycin |
| LC3 | Microtubule-associated protein 1 light chain 3 |
| LC3B | LC3 isoform B |
| p62 | Sequestosome-1 |
| Wnt | Wingless-related integration site pathway |
| AKT-CA | Ak strain transforming protein kinase B (Ca2+) |
| TS | Thymidylate synthase |
| SIRT1 | Sirtuin gene 1 |
| NAG-1 | NSAID-activated gene |
| ATF4 | Activating transcription factor 4 |
| DDIT3 | DNA damage-inducible transcript 3 |
| CHOP | C/EBP homologous protein |
| TRIB3 | Tribbles pseudokinase 3 |
| AKT1 | AKT serine/threonine kinase 1 |
| DADA | Diisopropylamine dichloroacetate |
| TME | Tumor microenvironment |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Cruz, C.S.D. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Smith, R.A.; Glynn, T.J. Epidemiology of lung cancer. Radiol. Clin. N. Am. 2000, 38, 453–470. [Google Scholar] [CrossRef]
- Lung Cancer Statistics|How Common is Lung Cancer? (n.d.). American Cancer Society. Available online: https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html (accessed on 1 April 2025).
- Molina, J.R.; Yang, P. Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Walker, S. Updates in non-small cell lung cancer. Clin. J. Oncol. Nurs. 2008, 12, 587–596. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, C.H.; Park, Y.S. Location of stage I-III non-small cell lung cancer and survival rate: Systematic review and meta-analysis. Thorac. Cancer 2018, 9, 1614–1622. [Google Scholar] [CrossRef]
- Li, Y.; Yan, B.; He, S. Advances and challenges in the treatment of lung cancer. Biomed. Pharmacother. 2023, 169, 115891. [Google Scholar] [CrossRef]
- Wu, J.; Lin, Z. Non-Small Cell Lung Cancer Targeted Therapy: Drugs and Mechanisms of Drug Resistance. Int. J. Mol. Sci. 2022, 23, 15056. [Google Scholar] [CrossRef]
- Kumar, M.; Sarkar, A. Current therapeutic strategies and challenges in nsclc treatment: A comprehensive review. Exp. Oncol. 2022, 44, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.F.; Zhu, M.L.; Liu, M.M.; Xu, Y.T.; Yuan, L.L.; Bian, J.; Xia, Y.Z.; Kong, L.Y. EGFR mutation mediates resistance to EGFR tyrosine kinase inhibitors in NSCLC: From molecular mechanisms to clinical research. Pharmacol. Res. 2021, 167, 105583. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, E.; Feld, E.; Horn, L. Driven by Mutations: The Predictive Value of Mutation Subtype in EGFR-Mutated Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 612–623. [Google Scholar] [CrossRef]
- Yoneda, K.; Imanishi, N.; Ichiki, Y.; Tanaka, F. Treatment of Non-small Cell Lung Cancer with EGFR-mutations. J. UOEH 2019, 41, 153–163. [Google Scholar] [CrossRef]
- Thomas, A.; Rajan, A.; Giaccone, G. Tyrosine kinase inhibitors in lung cancer. Hematol. Oncol. Clin. N. Am. 2012, 26, 589–605. [Google Scholar] [CrossRef]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y.S. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Tibes, R.; Trent, J.; Kurzrock, R. Tyrosine kinase inhibitors and the dawn of molecular cancer therapeutics. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 357–384. [Google Scholar] [CrossRef]
- Kang, H.; Kim, B.; Park, J.; Youn, H.; Youn, B. The Warburg effect on radioresistance: Survival beyond growth. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188988. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Schwartz, L.; Supuran, C.T.; Alfarouk, K.O. The Warburg Effect and the Hallmarks of Cancer. Anticancer. Agents Med. Chem. 2017, 17, 164–170. [Google Scholar] [CrossRef]
- Icard, P.; Shulman, S.; Farhat, D.; Steyaert, J.M.; Alifano, M.; Lincet, H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist. Updat. 2018, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Kankotia, S.; Stacpoole, P.W. Dichloroacetate and cancer: New home for an orphan drug? Biochem. Biophys. Acta 2014, 1846, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T.; Fliegel, L. Dichloroacetate for Cancer Treatment: Some Facts and Many Doubts. Pharmaceuticals 2024, 17, 744. [Google Scholar] [CrossRef]
- Naujokat, C.; Fuchs, D.; Opelz, G. Salinomycin in cancer: A new mission for an old agent. Mol. Med. Rep. 2010, 3, 555–559. [Google Scholar] [CrossRef]
- Naujokat, C.; Steinhart, R. Salinomycin as a drug for targeting human cancer stem cells. J. Biomed. Biotechnol. 2012, 2012, 950658. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, F.; Wong, E.T.; Fonkem, E.; Hsieh, T.C.; Wu, J.M.; Wu, E. Salinomycin: A novel anti-cancer agent with known anti-coccidial activities. Curr. Med. Chem. 2013, 20, 4095–4101. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Zhu, Y.; Wu, Z.; Cui, C.; Cai, F. Anticancer Mechanisms of Salinomycin in Breast Cancer and Its Clinical Applications. Front. Oncol. 2021, 11, 654428. [Google Scholar] [CrossRef]
- Huczynski, A. Salinomycin: A new cancer drug candidate. Chem. Biol. Drug Des. 2012, 79, 235–238. [Google Scholar] [CrossRef]
- Stacpoole, P.W.; Greene, Y.J. Dichloroacetate. Diabetes Care 1992, 15, 785–791. [Google Scholar] [CrossRef]
- Stacpoole, P.W. The pharmacology of dichloroacetate. Metabolism 1989, 38, 1124–1144. [Google Scholar] [CrossRef] [PubMed]
- Tataranni, T.; Piccoli, C. Dichloroacetate (DCA) and Cancer: An Overview towards Clinical Applications. Oxid. Med. Cell Longev. 2019, 2019, 8201079. [Google Scholar] [CrossRef]
- Romero, Y.; Castillejos-López, M.; Romero-García, S.; Aguayo, A.S.; Herrera, I.; Garcia-Martin, M.O.; Torres-Espíndola, L.M.; Negrete-García, M.C.; Olvera, A.C.; Huerta-Cruz, J.C.; et al. Antitumor Therapy under Hypoxic Microenvironment by the Combination of 2-Methoxyestradiol and Sodium Dichloroacetate on Human Non-Small-Cell Lung Cancer. Oxid. Med. Cell Longev. 2020, 2020, 3176375. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Webster, L.; Mackey, J.R. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer 2008, 99, 989–994. [Google Scholar] [CrossRef]
- Sutendra, G.; Dromparis, P.; Kinnaird, A.; Stenson, T.H.; Haromy, A.; Parker, J.M.; McMurtry, M.S.; Michelakis, E.D. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene 2013, 32, 1638–1650. [Google Scholar] [CrossRef]
- Dyrstad, S.E.; Lotsberg, M.L.; Tan, T.Z.; Pettersen, I.K.N.; Hjellbrekke, S.; Tusubira, D.; Engelsen, A.S.T.; Daubon, T.; Mourier, A.; Thiery, J.P.; et al. Blocking Aerobic Glycolysis by Targeting Pyruvate Dehydrogenase Kinase in Combination with EGFR TKI and Ionizing Radiation Increases Therapeutic Effect in Non-Small Cell Lung Cancer Cells. Cancers 2021, 13, 941. [Google Scholar] [CrossRef] [PubMed]
- Škorja Milić, N.; Dolinar, K.; Miš, K.; Matkovič, U.; Bizjak, M.; Pavlin, M.; Podbregar, M.; Pirkmajer, S. Suppression of Pyruvate Dehydrogenase Kinase by Dichloroacetate in Cancer and Skeletal Muscle Cells Is Isoform Specific and Partially Independent of HIF-1α. Int. J. Mol. Sci. 2021, 22, 8610. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.F.; Mahmoud, W.; Al-Harizy, R.M. Targeting glucose metabolism to suppress cancer progression: Prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019, 150, 104511. [Google Scholar] [CrossRef]
- Sun, R.C.; Fadia, M.; Dahlstrom, J.E.; Parish, C.R.; Board, P.G.; Blackburn, A.C. Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Res. Treat. 2010, 120, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Shavit, R.; Ilouze, M.; Feinberg, T.; Lawrence, Y.R.; Tzur, Y.; Peled, N. Mitochondrial induction as a potential radio-sensitizer in lung cancer cells—A short report. Cell. Oncol. 2015, 38, 247–252. [Google Scholar] [CrossRef]
- Allen, K.T.; Chin-Sinex, H.; DeLuca, T.; Pomerening, J.R.; Sherer, J.; Watkins, J.B., 3rd; Foley, J.; Jesseph, J.M.; Mendonca, M.S. Dichloroacetate alters Warburg metabolism, inhibits cell growth, and increases the X-ray sensitivity of human A549 and H1299 NSC lung cancer cells. Free Radic. Biol. Med. 2015, 89, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.; Lee, H.; Park, J.; Kim, S.H.; Park, J. Targeting Cancer Metabolism—Revisiting the Warburg Effects. Toxicol. Res. 2016, 32, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Lei, Y.H.; Yao, N.; Wang, C.R.; Hu, N.; Ye, W.C.; Zhang, D.M.; Chen, Z.S. Autophagy and multidrug resistance in cancer. Chin. J. Cancer 2017, 36, 52. [Google Scholar] [CrossRef]
- Vempati, R.K.; Malla, R.R. Autophagy-Induced Drug Resistance in Liver Cancer. Crit. Rev. Oncog. 2020, 25, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Lin, G.; Hill, D.K.; Andrejeva, G.; Boult, J.K.; Troy, H.; Fong, A.C.; Orton, M.R.; Panek, R.; Parkes, H.G.; Jafar, M.; et al. Dichloroacetate induces autophagy in colorectal cancer cells and tumours. Br. J. Cancer 2014, 111, 375–385. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, D.; Hou, B.; Liu, Q.X.; Chen, Q.; Deng, X.F.; Yu, Z.B.; Dai, J.G.; Zheng, H. Dichloroacetate enhances the antitumor efficacy of chemotherapeutic agents via inhibiting autophagy in non-small-cell lung cancer. Cancer Manag. Res. 2018, 10, 1231–1241. [Google Scholar] [CrossRef]
- Stacpoole, P.W. The dichloroacetate dilemma: Environmental hazard versus therapeutic goldmine--both or neither? Environ. Health Perspect. 2011, 119, 155–158. [Google Scholar] [CrossRef]
- Stacpoole, P.W.; Martyniuk, C.J.; James, M.O.; Calcutt, N.A. Dichloroacetate-induced peripheral neuropathy. Int. Rev. Neurobiol. 2019, 145, 211–238. [Google Scholar] [CrossRef]
- Niimi, N.; Takaku, S.; Yako, H.; Sango, K. Drug-Induced Demyelinating Neuropathies. Adv. Exp. Med. Biol. 2019, 1190, 357–369. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, A.; Zhou, X.; Wang, F. Suppression of pyruvate dehydrogenase kinase-2 re-sensitizes paclitaxel-resistant human lung cancer cells to paclitaxel. Oncotarget 2017, 8, 52642–52650. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, B.S.; Yu, D.H.; Lu, Q.; Ma, J.; Qi, H.; Fang, C.; Chen, H.Z. Dichloroacetate shifts the metabolism from glycolysis to glucose oxidation and exhibits synergistic growth inhibition with cisplatin in HeLa cells. Int. J. Oncol. 2011, 38, 409–417. [Google Scholar] [CrossRef]
- Garon, E.B.; Christofk, H.R.; Hosmer, W.; Britten, C.D.; Bahng, A.; Crabtree, M.J.; Hong, C.S.; Kamranpour, N.; Pitts, S.; Kabbinavar, F.; et al. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 443–452. [Google Scholar] [CrossRef]
- Al-Azawi, A.; Sulaiman, S.; Arafat, K.; Yasin, J.; Nemmar, A.; Attoub, S. Impact of Sodium Dichloroacetate Alone and in Combination Therapies on Lung Tumor Growth and Metastasis. Int. J. Mol. Sci. 2021, 22, 12553. [Google Scholar] [CrossRef] [PubMed]
- Schoenmann, N.; Tannenbaum, N.; Hodgeman, R.M.; Raju, R.P. Regulating mitochondrial metabolism by targeting pyruvate dehydrogenase with dichloroacetate, a metabolic messenger. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166769. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, N.; Villalobos Solis, M.I.; Kara Murdoch, F.; Murdoch, R.W.; Xie, Y.; Swift, C.M.; Hettich, R.L.; Löffler, F.E. Anaerobic Microbial Metabolism of Dichloroacetate. Mbio 2021, 12, e00537-21. [Google Scholar] [CrossRef]
- Yang, Z.; Tam, K.Y. Anti-cancer synergy of dichloroacetate and EGFR tyrosine kinase inhibitors in NSCLC cell lines. Eur. J. Pharmacol. 2016, 789, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liao, C.; Hu, Y.; Qinwen, P.; Jiang, J. Sensitization of breast cancer cells to paclitaxel by dichloroacetate through inhibiting autophagy. Biochem. Biophys. Res. Commun. 2017, 489, 103–108. [Google Scholar] [CrossRef]
- Zheng, M.F.; Shen, S.Y.; Huang, W.D. DCA increases the antitumor effects of capecitabine in a mouse B16 melanoma allograft and a human non-small cell lung cancer A549 xenograft. Cancer Chemother. Pharmacol. 2013, 72, 1031–1041. [Google Scholar] [CrossRef]
- De Mey, S.; Dufait, I.; Jiang, H.; Corbet, C.; Wang, H.; Van De Gucht, M.; Kerkhove, L.; Law, K.L.; Vandenplas, H.; Gevaert, T.; et al. Dichloroacetate Radiosensitizes Hypoxic Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9367. [Google Scholar] [CrossRef]
- Cook, K.M.; Shen, H.; McKelvey, K.J.; Gee, H.E.; Hau, E. Targeting Glucose Metabolism of Cancer Cells with Dichloroacetate to Radiosensitize High-Grade Gliomas. Int. J. Mol. Sci. 2021, 22, 7265. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, X.; Ni, Z.; Zhang, Y.; Zeng, Y.; Yan, X.; Huang, Y.; He, J.; Lyu, X.; Wu, Y.; et al. Dichloroacetate and metformin synergistically suppress the growth of ovarian cancer cells. Oncotarget 2016, 7, 59458–59470. [Google Scholar] [CrossRef] [PubMed]
- Haugrud, A.B.; Zhuang, Y.; Coppock, J.D.; Miskimins, W.K. Dichloroacetate enhances apoptotic cell death via oxidative damage and attenuates lactate production in metformin-treated breast cancer cells. Breast Cancer Res. Treat. 2014, 147, 539–550. [Google Scholar] [CrossRef]
- Parczyk, J.; Ruhnau, J.; Pelz, C.; Schilling, M.; Wu, H.; Piaskowski, N.N.; Eickholt, B.; Kühn, H.; Danker, K.; Klein, A. Dichloroacetate and PX-478 exhibit strong synergistic effects in a various number of cancer cell lines. BMC Cancer 2021, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, T.; Ishiguro, R.H.; Ishiguro, M.; Toki, A.; Terunuma, H. Synergistic Anti-Tumor Effect of Dichloroacetate and Ivermectin. Cureus 2022, 14, e21884. [Google Scholar] [CrossRef]
- Salinomycin. Nih.gov; PubChem. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Salinomycin (accessed on 1 April 2025).
- SALINOMYCIN MYCELIA-Salinomycin Powder. DailyMed 2020. Nih.gov. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=76ecb04c-f8c3-4607-9681-1909a3ffb22e (accessed on 1 April 2025).
- Aleman, M.; Magdesian, K.G.; Peterson, T.S.; Galey, F.D. Salinomycin toxicosis in horses. J. Am. Vet. Med. Assoc. 2007, 230, 1822–1826. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Ganster, H.J.; Raether, W. Effect of salinomycin-Na on malaria parasites (Plasmodium falciparum and P. berghei). Zentralbl Bakteriol. Mikrobiol. Hyg. A 1984, 256, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Peng, T.T.; Hsu, H.Y.; Lee, Y.Z.; Wu, S.H.; Lin, W.H.; Ke, Y.Y.; Hsu, T.A.; Yeh, T.K.; Huang, W.Z.; et al. Repurposing old drugs as antiviral agents for coronaviruses. Biomed. J. 2020, 43, 368–374. [Google Scholar] [CrossRef]
- Yuan, C.; Huang, X.; Zhai, R.; Ma, Y.; Xu, A.; Zhang, P.; Yang, Q. In Vitro Antiviral Activities of Salinomycin on Porcine Epidemic Diarrhea Virus. Viruses 2021, 13, 580. [Google Scholar] [CrossRef]
- Huczyński, A.; Janczak, J.; Stefańska, J.; Antoszczak, M.; Brzezinski, B. Synthesis and antimicrobial activity of amide derivatives of polyether antibiotic-salinomycin. Bioorg Med. Chem. Lett. 2012, 22, 4697–4702. [Google Scholar] [CrossRef]
- Ghonaim, A.H.; Hopo, M.G.; Ismail, A.K.; AboElnaga, T.R.; Elgawish, R.A.; Abdou, R.H.; Elhady, K.A. Hepatoprotective and renoprotective effects of silymarin against salinomycin-induced toxicity in adult rabbits. Vet. World 2022, 15, 2244–2252. [Google Scholar] [CrossRef]
- Ketola, K.; Hilvo, M.; Hyötyläinen, T.; Vuoristo, A.; Ruskeepää, A.L.; Orešič, M.; Kallioniemi, O.; Iljin, K. Salinomycin inhibits prostate cancer growth and migration via induction of oxidative stress. Br. J. Cancer 2012, 106, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, B.; Zhou, L.; Zhang, Z.; Shen, Y.; Lv, H.; AlQudsy, L.H.H.; Shang, P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020, 483, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Hamaï, A.; Hienzsch, A.; Cañeque, T.; Müller, S.; Wicinski, J.; Cabaud, O.; Leroy, C.; David, A.; Acevedo, V.; et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat. Chem. 2017, 9, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M.; Huczyński, A. Anticancer Activity of Polyether Ionophore-Salinomycin. Anticancer Agents Med. Chem. 2015, 15, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Nassar, D.; Blanpain, C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. 2016, 11, 47–76. [Google Scholar] [CrossRef]
- Lei, X.; He, Q.; Li, Z.; Zou, Q.; Xu, P.; Yu, H.; Ding, Y.; Zhu, W. Cancer stem cells in colorectal cancer and the association with chemotherapy resistance. Med. Oncol. 2021, 38, 43. [Google Scholar] [CrossRef]
- Erkisa, M.; Karakas, D.; Ulukaya, E. Cancer Stem Cells: Root of the Evil. Crit. Rev. Oncog. 2019, 24, 69–87. [Google Scholar] [CrossRef]
- Makena, M.R.; Ranjan, A.; Thirumala, V.; Reddy, A.P. Cancer stem cells: Road to therapeutic resistance and strategies to overcome resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165339. [Google Scholar] [CrossRef]
- Dewangan, J.; Srivastava, S.; Rath, S.K. Salinomycin: A new paradigm in cancer therapy. Tumour Biol. 2017, 39, 1010428317695035. [Google Scholar] [CrossRef]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Y.; Li, J.; Huang, J.H.; Hu, X.; Wu, E. Salinomycin as a potent anticancer stem cell agent: State of the art and future directions. Med. Res. Rev. 2022, 42, 1037–1063. [Google Scholar] [CrossRef]
- Xiao, Z.; Sperl, B.; Ullrich, A.; Knyazev, P. Metformin and salinomycin as the best combination for the eradication of NSCLC monolayer cells and their alveospheres (cancer stem cells) irrespective of EGFR, KRAS, EML4/ALK and LKB1 status. Oncotarget 2014, 5, 12877–12890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Li, C.X.; Liu, Z.Q.; Ma, S.; Chen, H.B. Cancer stem cell targets—A review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2045–2051. [Google Scholar] [PubMed]
- Tefas, L.R.; Barbălată, C.; Tefas, C.; Tomuță, I. Salinomycin-Based Drug Delivery Systems: Overcoming the Hurdles in Cancer Therapy. Pharmaceutics 2021, 13, 1120. [Google Scholar] [CrossRef] [PubMed]
- Antoszczak, M. A comprehensive review of salinomycin derivatives as potent anticancer and anti-CSCs agents. Eur. J. Med. Chem. 2019, 166, 48–64. [Google Scholar] [CrossRef]
- Zhang, X.; Lou, Y.; Zheng, X.; Wang, H.; Sun, J.; Dong, Q.; Han, B. Wnt blockers inhibit the proliferation of lung cancer stem cells. Drug Des. Devel Ther. 2015, 9, 2399–2407. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, J.; Luo, Q.; Ju, Y.; Song, G. Salinomycin Suppresses Tumorigenicity of Liver Cancer Stem Cells and Wnt/Beta-catenin Signaling. Curr. Stem Cell Res. Ther. 2021, 16, 630–637. [Google Scholar] [CrossRef]
- Koeck, S.; Amann, A.; Huber, J.M.; Gamerith, G.; Hilbe, W.; Zwierzina, H. The impact of metformin and salinomycin on transforming growth factor β-induced epithelial-to-mesenchymal transition in non-small cell lung cancer cell lines. Oncol. Lett. 2016, 11, 2946–2952. [Google Scholar] [CrossRef]
- Hwang, K.E.; Kim, H.J.; Song, I.S.; Park, C.; Jung, J.W.; Park, D.S.; Oh, S.H.; Kim, Y.S.; Kim, H.R. Salinomycin suppresses TGF-β1-induced EMT by down-regulating MMP-2 and MMP-9 via the AMPK/SIRT1 pathway in non-small cell lung cancer. Int. J. Med. Sci. 2021, 18, 715–726. [Google Scholar] [CrossRef]
- Arafat, K.; Iratni, R.; Takahashi, T.; Parekh, K.; Al Dhaheri, Y.; Adrian, T.E.; Attoub, S. Inhibitory Effects of Salinomycin on Cell Survival, Colony Growth, Migration, and Invasion of Human Non-Small Cell Lung Cancer A549 and LNM35: Involvement of NAG-1. PLoS ONE 2013, 8, e66931. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Yu, S.N.; Lee, S.Y.; Chun, S.S.; Choi, Y.L.; Park, Y.M.; Song, C.S.; Chatterjee, B.; Ahn, S.C. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem. Biophys. Res. Commun. 2011, 413, 80–86. [Google Scholar] [CrossRef]
- Li, T.; Su, L.; Zhong, N.; Hao, X.; Zhong, D.; Singhal, S.; Liu, X. Salinomycin induces cell death with autophagy through activation of endoplasmic reticulum stress in human cancer cells. Autophagy 2013, 9, 1057–1068. [Google Scholar] [CrossRef]
- Jangamreddy, J.R.; Ghavami, S.; Grabarek, J.; Kratz, G.; Wiechec, E.; Fredriksson, B.A.; Rao Pariti, R.K.; Cieślar-Pobuda, A.; Panigrahi, S.; Łos, M.J. Salinomycin induces activation of autophagy, mitophagy and affects mitochondrial polarity: Differences between primary and cancer cells. Biochim. Biophys. Acta 2013, 1833, 2057–2069. [Google Scholar] [CrossRef]
- Jangamreddy, J.R.; Panigrahi, S.; Łos, M.J. Monitoring of autophagy is complicated--salinomycin as an example. Biochim. Biophys. Acta 2015, 1853, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, D.; Heinold, A.; Opelz, G.; Daniel, V.; Naujokat, C. Salinomycin induces apoptosis and overcomes apoptosis resistance in human cancer cells. Biochem. Biophys. Res. Commun. 2009, 390, 743–749. [Google Scholar] [CrossRef]
- Boehmerle, W.; Muenzfeld, H.; Springer, A.; Huehnchen, P.; Endres, M. Specific targeting of neurotoxic side effects and pharmacological profile of the novel cancer stem cell drug salinomycin in mice. J. Mol. Med. 2014, 92, 889–900. [Google Scholar] [CrossRef]
- Ojo, O.O.; Bhadauria, S.; Rath, S.K. Correction: Dose-dependent adverse effects of salinomycin on male reproductive organs and fertility in mice. PLoS ONE 2019, 14, e0226872. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.L.; Chen, J.C.; Wu, C.H.; Peng, Y.S.; Chen, W.C.; Zheng, H.Y.; Jian, Y.J.; Wei, C.L.; Cheng, Y.T.; Lin, Y.W. Salinomycin acts through reducing AKT-dependent thymidylate synthase expression to enhance erlotinib-induced cytotoxicity in human lung cancer cells. Exp. Cell Res. 2017, 357, 59–66. [Google Scholar] [CrossRef]
- Ko, J.C.; Zheng, H.Y.; Chen, W.C.; Peng, Y.S.; Wu, C.H.; Wei, C.L.; Chen, J.C.; Lin, Y.W. Salinomycin enhances cisplatin-induced cytotoxicity in human lung cancer cells via down-regulation of AKT-dependent thymidylate synthase expression. Biochem. Pharmacol. 2016, 122, 90–98. [Google Scholar] [CrossRef]
- Abdelgalil, A.A.; Al-Kahtani, H.M.; Al-Jenoobi, F.I. Erlotinib. Profiles Drug Subst. Excip. Relat. Methodol. 2020, 45, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.C.; Kostyanovskaya, E.; Huang, R.S. Pharmacogenomics of cisplatin sensitivity in non-small cell lung cancer. Genom. Proteom. Bioinform. 2014, 12, 198–209. [Google Scholar] [CrossRef]
- Michalak, M.; Lach, M.S.; Antoszczak, M.; Huczyński, A.; Suchorska, W.M. Overcoming Resistance to Platinum-Based Drugs in Ovarian Cancer by Salinomycin and Its Derivatives-An In Vitro Study. Molecules 2020, 25, 537. [Google Scholar] [CrossRef]
- Zou, Z.Z.; Nie, P.P.; Li, Y.W.; Hou, B.X.; Shi, X.P.; Ma, Z.K.; Han, B.W.; Luo, X.Y. Synergistic induction of apoptosis by salinomycin and gefitinib through lysosomal and mitochondrial dependent pathway overcomes gefitinib resistance in colorectal cancer. Oncotarget 2017, 8, 22414–22432. [Google Scholar] [CrossRef][Green Version]
- Skeberdytė, A.; Sarapinienė, I.; Aleksander-Krasko, J.; Stankevičius, V.; Sužiedėlis, K.; Jarmalaitė, S. Dichloroacetate and Salinomycin Exert a Synergistic Cytotoxic Effect in Colorectal Cancer Cell Lines. Sci. Rep. 2018, 8, 17744. [Google Scholar] [CrossRef] [PubMed]
- Skeberdytė, A.; Sarapinienė, I.; Krasko, J.A.; Barakauskienė, A.; Žilionytė, K.; Prokarenkaitė, R.; Sužiedėlis, K.; Bukelskienė, V.; Jarmalaitė, S. Salinomycin and dichloroacetate synergistically inhibit Lewis lung carcinoma cell proliferation, tumor growth and metastasis. Biochem. Biophys. Res. Commun. 2020, 523, 874–879. [Google Scholar] [CrossRef]
- Wei, M.; Shen, X.; Liu, Y.; Chen, X.; Su, S.; Lv, X.; Qian, X.; Yu, L.; Wang, L. The antitumor effect of diisopropylamine dichloroacetate on non-small cell lung cancer and its influence on the tumor immune microenvironment. Front. Oncol. 2024, 29, 1447828. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, W.; Yan, Z.; Jiang, X.; Gao, Y.; Jin, H.; Wu, Y.; Pang, L.; Yu, Z.; Xiao, S.; et al. Modulating autophagy in the tumor microenvironment with a salinomycin-loaded liposome hybrid nanovesicle system for tumor immunotherapy. Nanotoday 2025, 61, 102644. [Google Scholar] [CrossRef]
- Vaghari-Tabari, M.; Hassanpour, P.; Sadeghsoltani, F.; Malakoti, F.; Alemi, F.; Qujeq, D.; Asemi, Z.; Yousefi, B. CRISPR/Cas9 gene editing: A new approach for overcoming drug resistance in cancer. Cell Mol. Biol. Lett. 2022, 27, 49. [Google Scholar] [CrossRef]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef]
- Slovin, S.; Carissimo, A.; Panariello, F.; Grimaldi, A.; Bouché, V.; Gambardella, G.; Cacchiarelli, D. Single-Cell RNA Sequencing Analysis: A Step-by-Step Overview. Methods Mol. Biol. 2021, 2284, 343–365. [Google Scholar] [CrossRef] [PubMed]

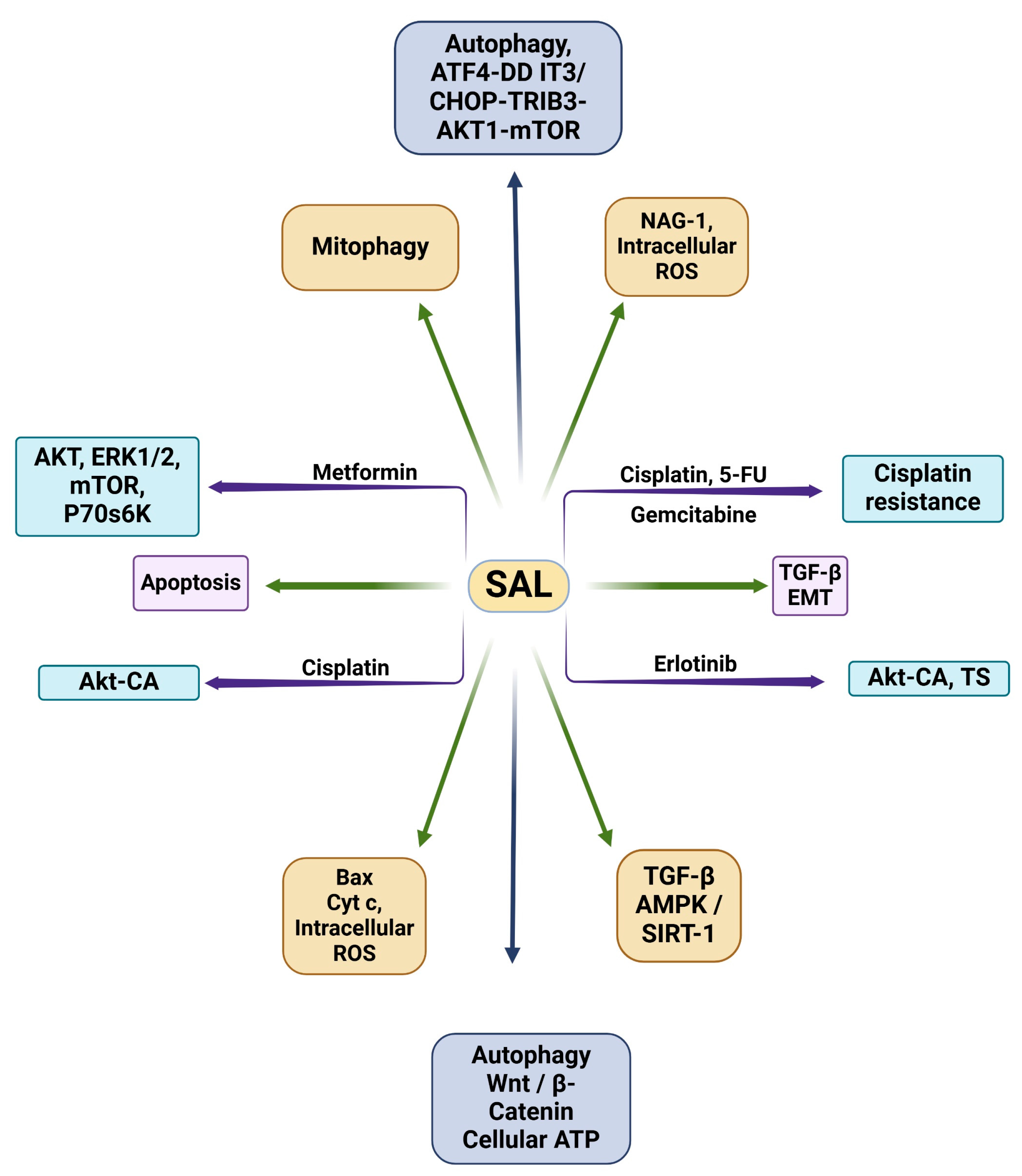
| Drug(s) | Cell Line(s) | Target(s) | Key Findings | Refs. |
|---|---|---|---|---|
| SAL | MHCC97H | Wnt/β-catenin | SAL suppresses the stemness of LCSCs via inhibiting Wnt/β-catenin signaling. | [91] |
| SAL | A549, HCC4006 | TGFβ, EMT process | SAL interrupts EMT by inhibiting TGFβ signaling. | [92] |
| SAL | A549, H460 | TGFβ, AMPK/SIRT1 | SAL inhibits TGFβ through AMPK/SIRT1 signaling pathways. | [93] |
| SAL | A549, LNM35 | NAG-1, intracellular ROS | SAL inhibits growth, migration, and invasion via inducing apoptosis through targeting NAG-1. | [94] |
| SAL | LNCaP, PC-3, DU-145, RWPE-1 | Bax protein, cytochrome-c, intracellular ROS | SAL induces apoptosis by elevating intracellular ROS, cytochrome-c release to cytoplasm, Bax protein translocation to mitochondria, and caspase-3 substrate activation. | [95] |
| SAL | A549, Calu-1, H157 | Autophagy, ATF4-DDIT3/CHOP-TRIB3-AKT1-mTOR | SAL induces apoptosis in lung cancer cells via autophagic flux. | [96] |
| SAL | PC-3, SKBR3, MDAMB468, MEF | Autophagy, mitophagy | SAL decreases cellular ARP, but induced autophagy can counteract its apoptotic mechanisms if not used in combination with autophagy inhibitors. | [97] |
| SAL | Molt-4 CD4+ T-cells | Apoptosis | SAL is able to induce apoptosis in apoptotic-resistant leukemia cells. | [98] |
| SAL, metformin | HCC4006, NCI-H1975, NCI-H2122, HCC95, NCI-H3122 | AKT, ERK1/2, mTOR, p70 s6K | In combination with metformin, SAL induces cell death and decreases cell density/viability. | [86] |
| SAL, erlotinib | H1703, H1975 | AKT-CA, TS | In combination with erlotinib, SAL inhibits TS and AKT signaling. | [102] |
| SAL, cisplatin | A549, H1703 | AKT-CA | SAL and cisplatin synergistically inhibit lung cancer cells via AKT inhibition. | [103] |
| SAL, cisplatin, 5-fluorouracil, gemcitabine | A2780 CDDP, SK-OV-3 CDDP, A2780, SK-OV-3 | Cisplatin resistance | SAL reverses cisplatin resistance in ovarian cancer cells in combination with 5-flurouracil and gemcitabine. | [106] |
| SAL, gefitinib | SW1116, LOVO, HCT-116, SW480, HT-29, NCM460 | AKT | SAL and gefitinib’s combination induces apoptosis in gefitinib-resistant cell lines and overcomes gefitinib resistance in Ras-overexpressing cells. | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunt, S.; Thyagarajan, A.; Sahu, R.P. Dichloroacetate and Salinomycin as Therapeutic Agents in Cancer. Med. Sci. 2025, 13, 47. https://doi.org/10.3390/medsci13020047
Hunt S, Thyagarajan A, Sahu RP. Dichloroacetate and Salinomycin as Therapeutic Agents in Cancer. Medical Sciences. 2025; 13(2):47. https://doi.org/10.3390/medsci13020047
Chicago/Turabian StyleHunt, Sunny, Anita Thyagarajan, and Ravi P. Sahu. 2025. "Dichloroacetate and Salinomycin as Therapeutic Agents in Cancer" Medical Sciences 13, no. 2: 47. https://doi.org/10.3390/medsci13020047
APA StyleHunt, S., Thyagarajan, A., & Sahu, R. P. (2025). Dichloroacetate and Salinomycin as Therapeutic Agents in Cancer. Medical Sciences, 13(2), 47. https://doi.org/10.3390/medsci13020047







