Tumour-Derived, Extracellular Microvesicles in the Treatment of Acute Renal Failure: An Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Generation of Bone Marrow-Derived MSCs
2.3. L929 Cell Line
2.4. Separation of Peripheral Blood Mononuclear Cells (PBMCs)
2.5. Obtaining and Characterization of Apoptotic Extracellular MVs
2.6. Induction of AKI
2.7. Treatment of AKI Mice with MVs
- Intact mice
- Control mice with AKI, no treatment
- AKI mice treated with MSC-derived MVs (MSC-MVs)
- AKI mice treated with MVs derived from L929 cells (L929-MVs)
- AKI mice treated with MVs derived from PBMCs (PBMC-MVs)
2.8. Biochemical Analysis
2.9. Histological Examinations
2.10. Flow Cytometry
2.11. Statistics
3. Results
3.1. Size Distribution of Isolated EVs
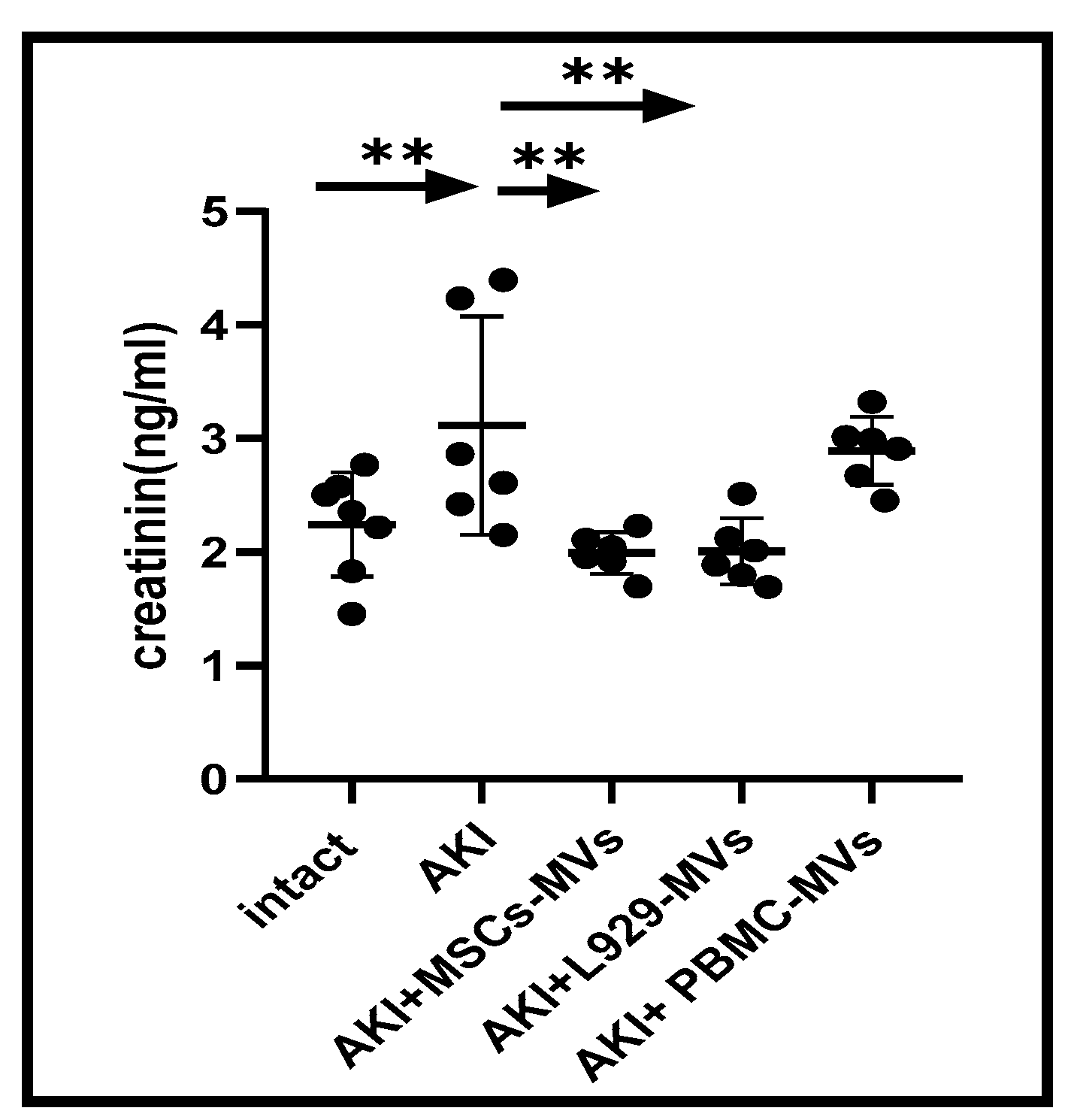
3.2. The Effects of MVs on Functionality and Morphological Structure of the Damaged Kidney
3.3. Both L929-MVs and MSC-MVs Promote Cytoprotective Immunomodulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; De Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug delivery with extracellular vesicles: From imagination to innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.T.; Liu, B.C. Extracellular vesicles: Opportunities and challenges for the treatment of renal fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 693–709. [Google Scholar] [CrossRef]
- Bonsergent, E.; Lavieu, G. Content release of extracellular vesicles in a cell-free extract. FEBS Lett. 2019, 593, 1983–1992. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal stem cells for regenerative medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, T.; Jiang, Y.; Xu, S.; Cheuk, Y.C.; Wang, J.; Yang, C.; Rong, R. Poly(I:C)-induced mesenchymal stem cells protect the kidney against ischemia/reperfusion injury via the TLR3/PI3K pathway. Front. Med. 2021, 8, 755849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kadono, M.; Nakashima, A.; Ishiuchi, N.; Sasaki, K.; Miura, Y.; Maeda, S.; Fujita, A.; Sasaki, A.; Nagamatsu, S.; Masaki, T. Adipose-derived mesenchymal stem cells cultured in serum-free medium attenuate acute contrast-induced nephropathy by exerting anti-apoptotic effects. Stem Cell Res. Ther. 2023, 14, 337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, B.; Tian, X.; Hao, J.; Xu, G.; Zhang, W. Mesenchymal stem cell-derived extracellular vesicles in tissue regeneration. Cell Transplant. 2020, 29, 963689720908500. [Google Scholar] [CrossRef]
- Bruno, S.; Porta, S.; Bussolati, B. Extracellular vesicles in renal tissue damage and regeneration. Eur. J. Pharmacol. 2016, 790, 83–91. [Google Scholar] [CrossRef]
- Kilpinen, L.; Impola, U.; Sankkila, L.; Ritamo, I.; Aatonen, M.; Kilpinen, S.; Tuimala, J.; Valmu, L.; Levijoki, J.; Finckenberg, P.; et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J. Extracell. Vesicles 2013, 2, 21927. [Google Scholar] [CrossRef] [PubMed]
- She, Z.; Xie, M.; Hun, M.; Abdirahman, A.S.; Li, C.; Wu, F.; Luo, S.; Wan, W.; Wen, C.; Tian, J. Immunoregulatory effects of mitochondria transferred by extracellular vesicles. Front. Immunol. 2021, 11, 628576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizk, S.; Abdel Moneim, A.E.; Abdel-Gaber, R.A.; Alquraishi, M.I.; Santourlidis, S.; Dkhil, M.A. Nephroprotective efficacy of Echinops spinosus against a glycerol-induced acute kidney injury model. ACS Omega 2023, 8, 41865–41875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, F.; Zhao, B.; Zhang, L.; Chen, G.Q.; Zhu, L.; Feng, X.L.; Gong, M.J.; Hu, C.C.; Zhang, Y.Y.; Li, M.; et al. Therapeutic potential of urine-derived stem cells in renal regeneration following acute kidney injury: A comparative analysis with mesenchymal stem cells. World J. Stem Cells 2024, 16, 525–537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szajnik, M.; Czystowska, M.; Szczepanski, M.J.; Mandapathil, M.; Whiteside, T.L. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS ONE 2010, 5, e11469. [Google Scholar] [CrossRef] [PubMed]
- Seledtsov, V.I.; Darinskas, A.; Von Delwig, A.; Seledtsova, G.V. Inflammation control and immunotherapeutic strategies in comprehensive cancer treatment. Metabolites 2023, 13, 123. [Google Scholar] [CrossRef]
- Johnson, P.; Ruffell, B. CD44 and its role in inflammation and inflammatory diseases. Inflamm. Allergy Drug Targets 2009, 8, 208–220. [Google Scholar] [CrossRef]
- Seledtsov, V.I.; von Delwig, A. Clinically feasible and prospective immunotherapeutic interventions in multidirectional comprehensive treatment of cancer. Expert. Opin. Biol. Ther. 2021, 21, 323–342. [Google Scholar] [CrossRef]
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Souza, A.; Burch, A.; Dave, K.M.; Sreeram, A.; Reynolds, M.J.; Dobbins, D.X.; Kamte, Y.S.; Zhao, W.; Sabatelle, C.; Joy, G.M.; et al. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J. Control. Release 2021, 338, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, G.; Santoso, M.R.; Tada, Y.; Li, A.M.; Vaskova, E.; Jung, J.-H.; O’brien, C.; Egan, E.; Ye, J.; Yang, P.C. Mitochondria-rich extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic mocardium. J. Am. Coll. Cardiol. 2021, 77, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Seledtsova, G.V.; Seledtsov, V.I.; Dorzhieva, A.B.; Ivanova, I.P.; Khabalova, T.S.; Blinova, E.A.; Darinskas, A.; von Delwig, A. Utilizing Tumor-Derived Extracellular Microvesicles for Kidney Regeneration. A Non-Peer Reviewed Study from the Laboratory/Preprint. Available online: https://www.researchsquare.com/article/rs-3594528/v1 (accessed on 14 November 2023).
- Hackel, A.; Vollmer, S.; Bruderek, K.; Lang, S.; Brandau, S. Immunological priming of mesenchymal stromal/stem cells and their extracellular vesicles augments their therapeutic benefits in experimental graft-versus-host disease via engagement of PD-1 ligands. Front. Immunol. 2023, 14, 1078551. [Google Scholar] [CrossRef]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef] [PubMed]
- Barcena, A.J.R.; Perez, J.V.D.; Damasco, J.A.; Bernardino, M.R.; San Valentin, E.M.D.; Klusman, C.; Martin, B.; Cortes, A.; Canlas, G.M.; Del Mundo, H.C.; et al. Gold nanoparticles for monitoring of mesenchymal stem-cell-loaded bioresorbable polymeric wraps for arteriovenous fistula maturation. Int. J. Mol. Sci. 2023, 24, 11754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gregory, C.D.; Dransfield, I. Apoptotic tumor cell-derived extracellular vesicles as important regulators of the onco-regenerative niche. Front. Immunol. 2018, 9, 1111. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seledtsova, G.V.; Seledtsov, V.I.; Dorzhieva, A.B.; Ivanova, I.P.; Khabalova, T.S.; Darinskas, A.; von Delwig, A.A. Tumour-Derived, Extracellular Microvesicles in the Treatment of Acute Renal Failure: An Experimental Study. Med. Sci. 2025, 13, 35. https://doi.org/10.3390/medsci13020035
Seledtsova GV, Seledtsov VI, Dorzhieva AB, Ivanova IP, Khabalova TS, Darinskas A, von Delwig AA. Tumour-Derived, Extracellular Microvesicles in the Treatment of Acute Renal Failure: An Experimental Study. Medical Sciences. 2025; 13(2):35. https://doi.org/10.3390/medsci13020035
Chicago/Turabian StyleSeledtsova, Galina V., Victor I. Seledtsov, Ayana B. Dorzhieva, Irina P. Ivanova, Tatiana S. Khabalova, Adas Darinskas, and Alexei A. von Delwig. 2025. "Tumour-Derived, Extracellular Microvesicles in the Treatment of Acute Renal Failure: An Experimental Study" Medical Sciences 13, no. 2: 35. https://doi.org/10.3390/medsci13020035
APA StyleSeledtsova, G. V., Seledtsov, V. I., Dorzhieva, A. B., Ivanova, I. P., Khabalova, T. S., Darinskas, A., & von Delwig, A. A. (2025). Tumour-Derived, Extracellular Microvesicles in the Treatment of Acute Renal Failure: An Experimental Study. Medical Sciences, 13(2), 35. https://doi.org/10.3390/medsci13020035






