Automated Single-Sperm Selection Software (SiD) during ICSI: A Prospective Sibling Oocyte Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Settings
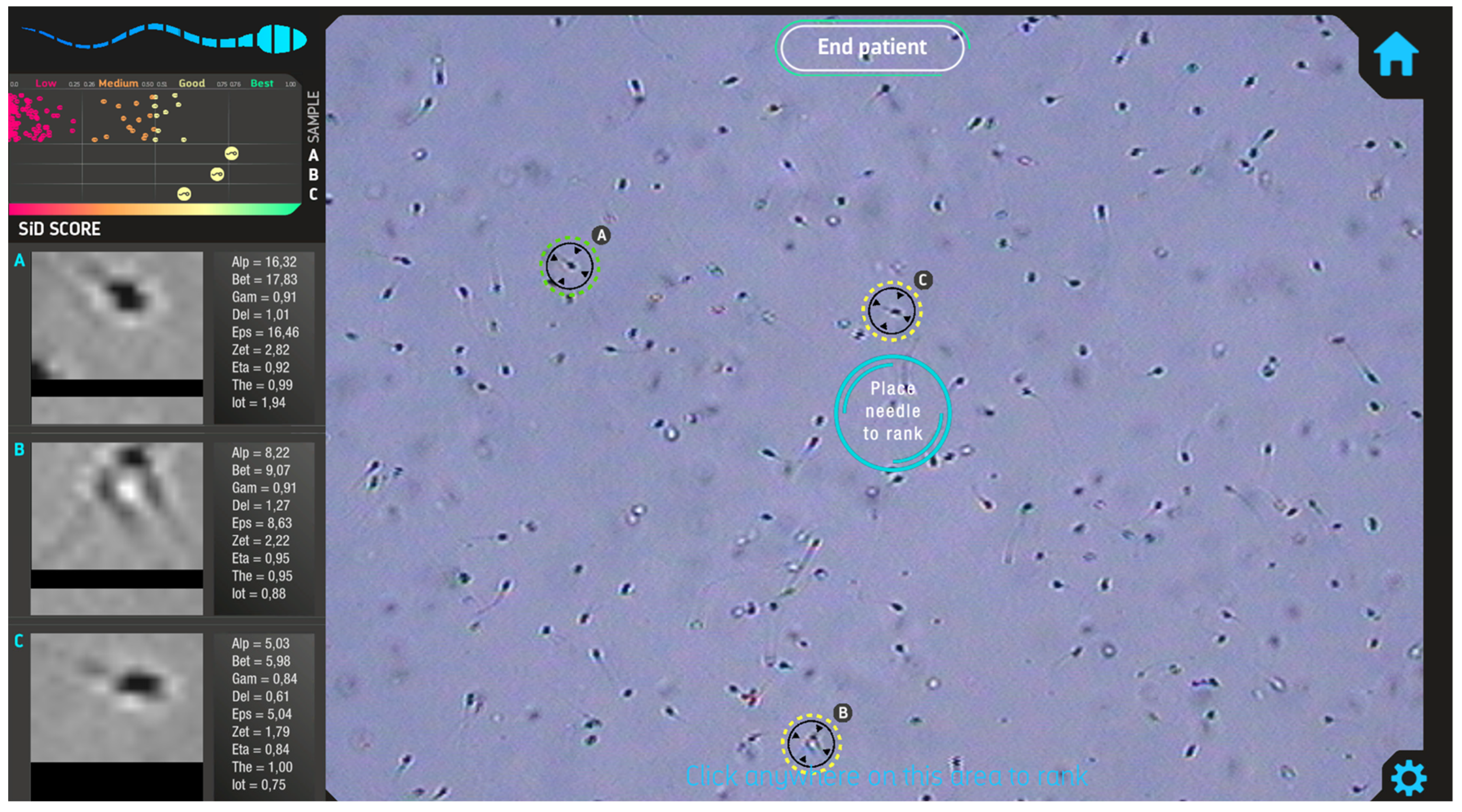
2.2. Automated Sperm Selection Software
2.3. Embryo Vitrification and Warming
2.4. Outcome Definitions
2.5. Statistics
3. Results
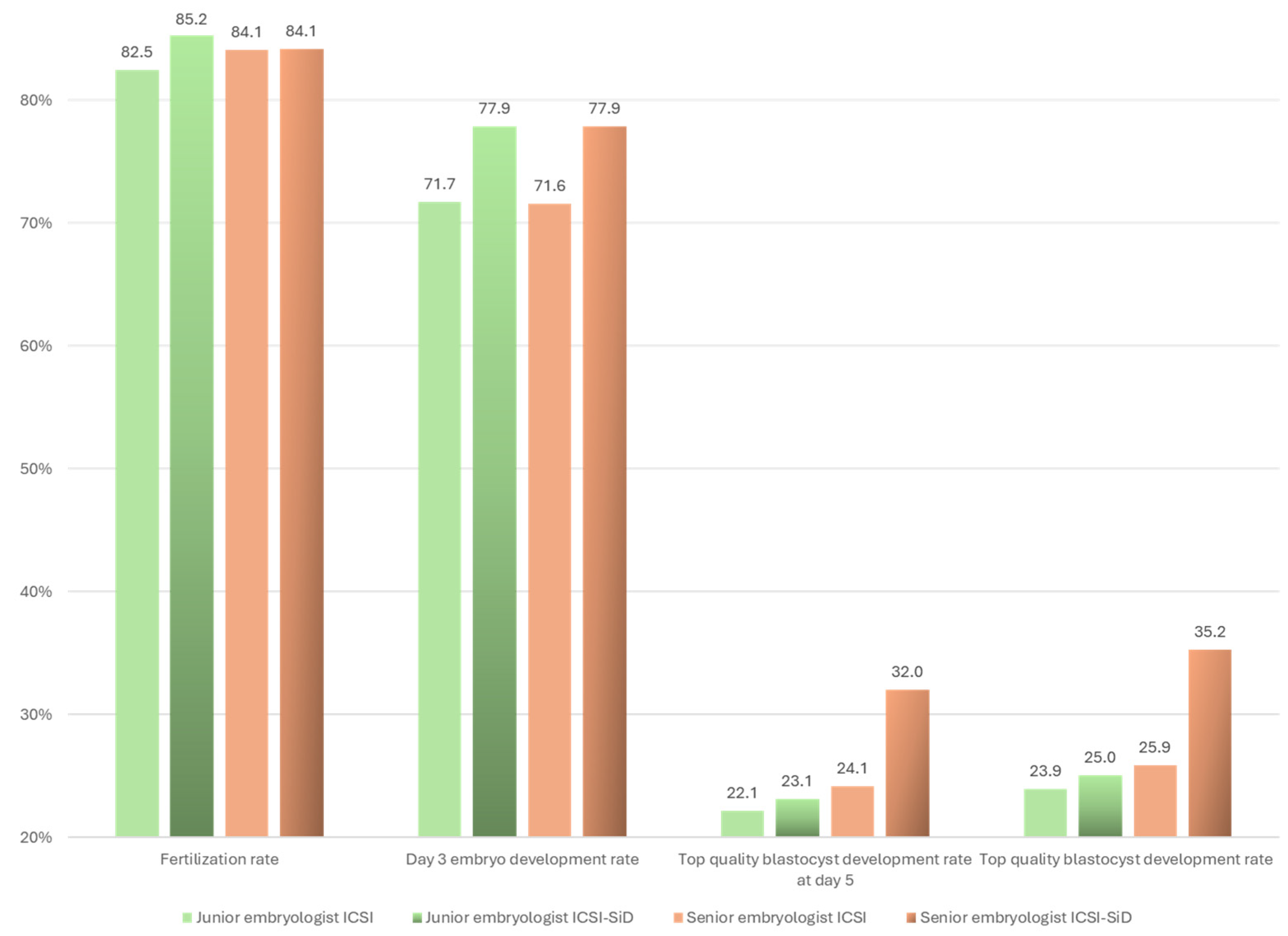
3.1. Laboratory Outcomes
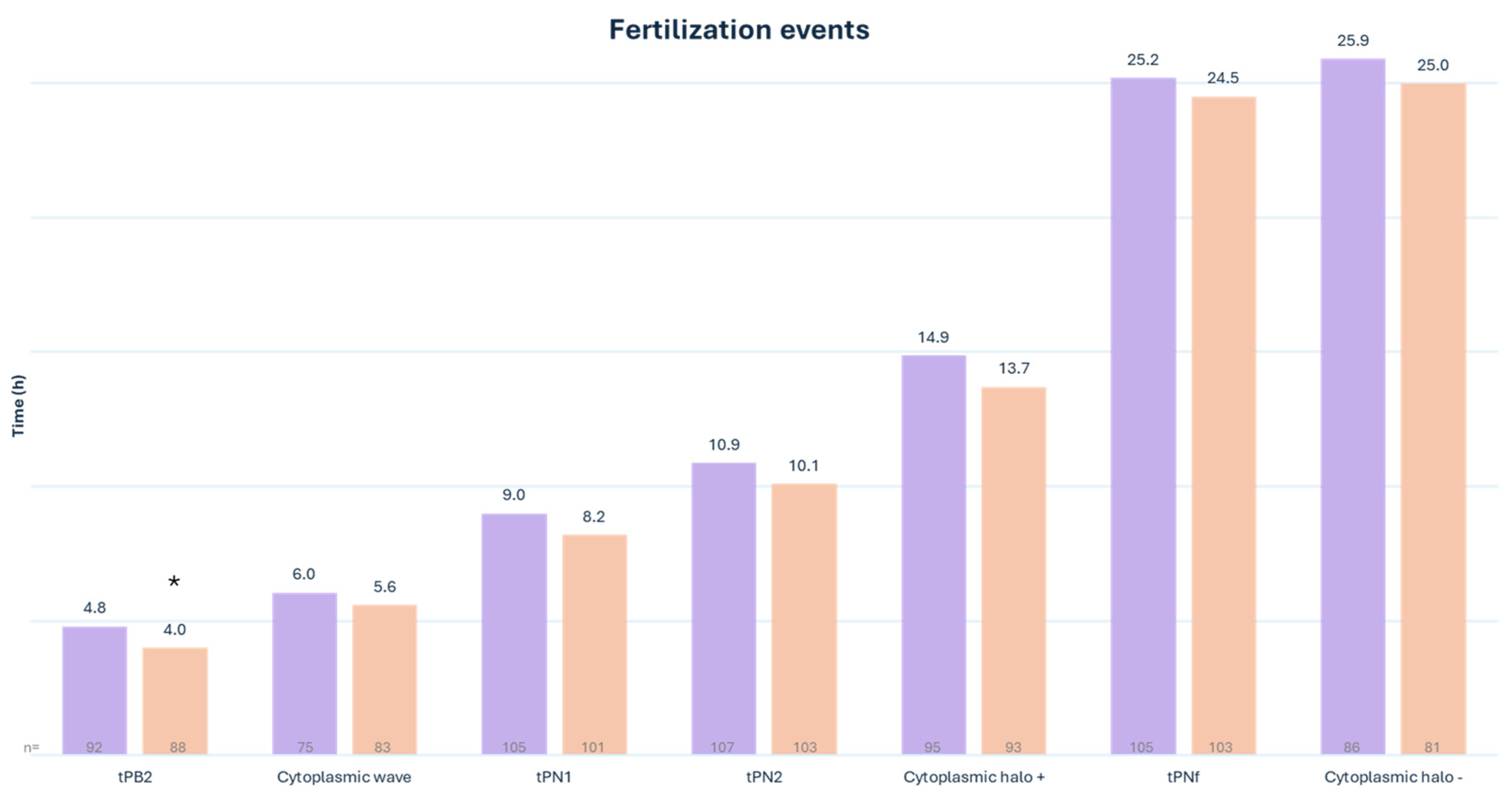
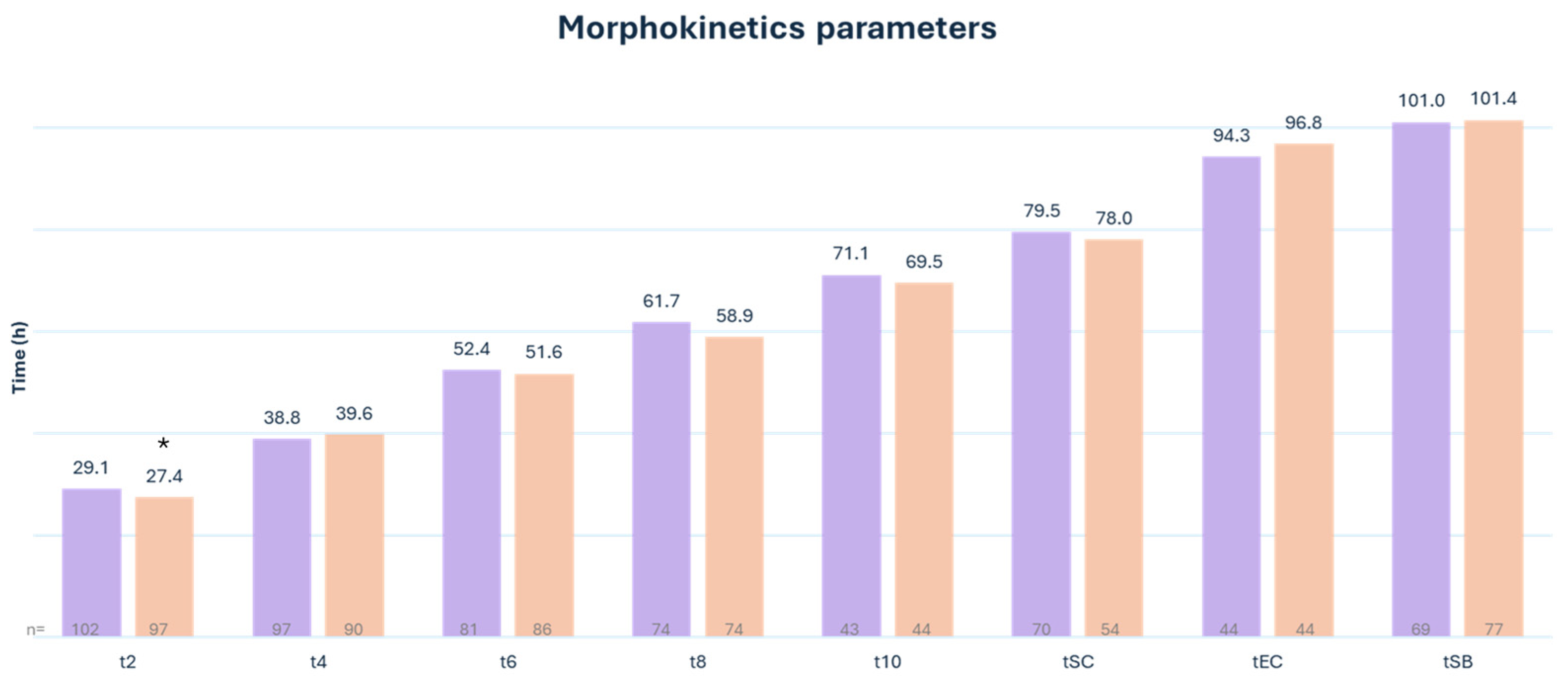
3.2. Embryo Morphokinetics
3.3. Genetic and Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies After Intracytoplasmic Injection of Single Spermatozoon into an Oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Adamson, G.D.; de Mouzon, J.; Chambers, G.M.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Dyer, S. International Committee for Monitoring Assisted Reproductive Technology: World Report on Assisted Reproductive Technology, 2011. Fertil. Steril. 2018, 110, 1067–1080. [Google Scholar] [CrossRef]
- Haddad, M.; Stewart, J.; Xie, P.; Cheung, S.; Trout, A.; Keating, D.; Parrella, A.; Lawrence, S.; Rosenwaks, Z.; Palermo, G.D. Thoughts on the Popularity of ICSI. J. Assist. Reprod. Genet. 2020, 38, 101–123. [Google Scholar] [CrossRef]
- Loutradi, K.E.; Tarlatzis, B.C.; Goulis, D.G.; Zepiridis, L.; Pagou, T.; Chatziioannou, E.; Grimbizis, G.F.; Papadimas, I.; Bontis, I. The Effects of Sperm Quality on Embryo Development after Intracytoplasmic Sperm Injection. J. Assist. Reprod. Genet. 2006, 23, 69–74. [Google Scholar] [CrossRef]
- Mazzilli, R.; Cimadomo, D.; Vaiarelli, A.; Capalbo, A.; Dovere, L.; Alviggi, E.; Dusi, L.; Foresta, C.; Lombardo, F.; Lenzi, A.; et al. Effect of the Male Factor on the Clinical Outcome of Intracytoplasmic Sperm Injection Combined with Preimplantation Aneuploidy Testing: Observational Longitudinal Cohort Study of 1219 Consecutive Cycles. Fertil. Steril. 2017, 108, 961–972.e3. [Google Scholar] [CrossRef] [PubMed]
- Ron-El, R.; Nachum, H.; Herman, A.; Golan, A.; Caspi, E.; Soffer, Y. Delayed Fertilization and Poor Embryonic Development Associated with Impaired Semen Quality. Fertil. Steril. 1991, 55, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Janny, L.; Menezo, Y.J.R. Evidence for a Strong Paternal Effect on Human Preimplantation Embryo Development and Blastocyst Formation. Mol. Reprod. Dev. 1994, 38, 36–42. [Google Scholar] [CrossRef]
- Bartoov, B.; Berkovitz, A.; Eltes, F.; Kogosowski, A.; Menezo, Y.; Barak, Y. Real-Time Fine Morphology of Motile Human Sperm Cells Is Associated with IVF-ICSI Outcome. J. Androl. 2002, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anbari, F.; Khalili, M.A.; Ahamed, A.M.S.; Mangoli, E.; Nabi, A.; Dehghanpour, F.; Sabour, M. Microfluidic Sperm Selection Yields Higher Sperm Quality Compared to Conventional Method in ICSI Program: A Pilot Study. Syst. Biol. Reprod. Med. 2021, 67, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Asali, A.; Miller, N.; Pasternak, Y.; Freger, V.; Belenky, M.; Berkovitz, A. The Possibility of Integrating Motile Sperm Organelle Morphology Examination (MSOME) with Intracytoplasmic Morphologically-Selected Sperm Injection (IMSI) When Treating Couples with Unexplained Infertility. PLoS ONE 2020, 15, e0232156. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.A.; Sakkas, D. Sperm Selection Methods in the 21st Century. Biol. Reprod. 2019, 101, 1076–1082. [Google Scholar] [CrossRef]
- Parrella, A.; Keating, D.; Cheung, S.; Xie, P.; Stewart, J.D.; Rosenwaks, Z.; Palermo, G.D. A Treatment Approach for Couples with Disrupted Sperm DNA Integrity and Recurrent ART Failure. J. Assist. Reprod. Genet. 2019, 36, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Keating, D.; Tavares, D.R.; Rosenwaks, Z.; Palermo, G.D. Utilizing Spermatozoa with the Highest Genomic Integrity Enhances Icsi Outcome. Fertil. Steril. 2021, 116, e67–e68. [Google Scholar] [CrossRef]
- Yetkinel, S.; Kilicdag, E.B.; Aytac, P.C.; Haydardedeoglu, B.; Simsek, E.; Cok, T. Effects of the Microfluidic Chip Technique in Sperm Selection for Intracytoplasmic Sperm Injection for Unexplained Infertility: A Prospective, Randomized Controlled Trial. J. Assist. Reprod. Genet. 2018, 36, 403–409. [Google Scholar] [CrossRef]
- Gode, F.; Bodur, T.; Gunturkun, F.; Gurbuz, A.S.; Tamer, B.; Pala, I.; Isik, A.Z. Comparison of Microfluid Sperm Sorting Chip and Density Gradient Methods for Use in Intrauterine Insemination Cycles. Fertil. Steril. 2019, 112, 842–848.e1. [Google Scholar] [CrossRef] [PubMed]
- Jiang, V.S.; Pavlovic, Z.J.; Hariton, E. The Role of Artificial Intelligence and Machine Learning in Assisted Reproductive Technologies. Obstet. Gynecol. Clin. N. Am. 2023, 50, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Zaninovic, N.; Rosenwaks, Z. Artificial Intelligence in Human In Vitro Fertilization and Embryology. Fertil. Steril. 2020, 114, 914–920. [Google Scholar] [CrossRef]
- Guahmich, N.L.; Borini, E.; Zaninovic, N. Improving Outcomes of Assisted Reproductive Technologies Using Artificial Intelligence for Sperm Selection. Fertil. Steril. 2023, 120, 729–734. [Google Scholar] [CrossRef]
- Dimitriadis, I.; Zaninovic, N.; Badiola, A.C.; Bormann, C.L. Artificial Intelligence in the Embryology Laboratory: A Review. Reprod. Biomed. Online 2022, 44, 435–448. [Google Scholar] [CrossRef]
- Ghayda, R.A.; Cannarella, R.; Calogero, A.E.; Shah, R.; Rambhatla, A.; Zohdy, W.; Kavoussi, P.; Avidor-Reiss, T.; Boitrelle, F.; Mostafa, T.; et al. Artificial Intelligence in Andrology: From Semen Analysis to Image Diagnostics. World J. Men’s Health 2024, 42, 39–61. [Google Scholar] [CrossRef]
- Mendizabal-Ruiz, G.; Chavez-Badiola, A.; Figueroa, I.A.; Nuño, V.M.; Farias, A.F.-S.; Valencia-Murilloa, R.; Drakeley, A.; Garcia-Sandoval, J.P.; Cohen, J. Computer Software (SiD) Assisted Real-Time Single Sperm Selection Associated with Fertilization and Blastocyst Formation. Reprod. Biomed. Online 2022, 45, 703–711. [Google Scholar] [CrossRef]
- ESHRE Special Interest Group Of Embryology; Alpha Scientists in Reproductive Medicine the Vienna Consensus: Report of an Expert Meeting on the Development of Art Laboratory Performance Indicators†‡. Hum. Reprod. Open 2017, 2017, hox011. [CrossRef]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst Score Affects Implantation and Pregnancy Outcome: Towards a Single Blastocyst Transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef]
- Coticchio, G.; Renzini, M.M.; Novara, P.V.; Lain, M.; De Ponti, E.; Turchi, D.; Fadini, R.; Canto, M.D. Focused Time-Lapse Analysis Reveals Novel Aspects of Human Fertilization and Suggests New Parameters of Embryo Viability. Hum. Reprod. 2017, 33, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Apter, S.; Ebner, T.; Freour, T.; Guns, Y.; Kovacic, B.; Le Clef, N.; Marques, M.; Meseguer, M.; Montjean, D.; Sfontouris, I.; et al. Good Practice Recommendations for the Use of Time-Lapse Technology†. Hum. Reprod. Open 2020, 2020, hoaa008. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.; Motato, Y.; Escribá, M.J.; Ojeda, M.; Muñoz, E.; Meseguer, M. The Human First Cell Cycle: Impact on Implantation. Reprod. Biomed. Online 2014, 28, 475–484. [Google Scholar] [CrossRef]
- Mizobe, Y.; Oya, N.; Iwakiri, R.; Yoshida, N.; Sato, Y.; Miyoshi, K.; Tokunaga, M.; Ezono, Y. Effects of Early Cleavage Patterns of Human Embryos on Subsequent In Vitro Development and Implantation. Fertil. Steril. 2016, 106, 348–353.e2. [Google Scholar] [CrossRef]
- Mizobe, Y.; Tokunaga, M.; Oya, N.; Iwakiri, R.; Yoshida, N.; Sato, Y.; Onoue, N.; Ezono, Y. Synchrony of the First Division as an Index of the Blastocyst Formation Rate during Embryonic Development. Reprod. Med. Biol. 2017, 17, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, M.; Herrero, J.; Tejera, A.; Hilligsøe, K.M.; Ramsing, N.B.; Remohí, J. The Use of Morphokinetics as a Predictor of Embryo Implantation. Hum. Reprod. 2011, 26, 2658–2671. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Han, W.; Zhang, X.; Wang, J.; Liu, W.; Xiong, S.; Huang, G. A Retrospective Analysis of Morphokinetic Parameters According to the Implantation Outcome of IVF Treatment. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 186–190. [Google Scholar] [CrossRef]
- Pool, T.B.; Schoolfield, J.; Han, D.K. Human Embryo Culture Media Comparisons. Methods Mol. Biol. 2012, 912, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Takahashi, T.; Nagase, S. Oocyte Aging Underlies Female Reproductive Aging: Biological Mechanisms and Therapeutic Strategies. Reprod. Med. Biol. 2015, 14, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, O.; Michaeli, M.; Aslih, N.; Poltov, D.; Estrada, D.; Atzmon, Y.; Shalom-Paz, E. Embryonic Development in Relation to Maternal Age and Conception Probability. Reprod. Sci. 2021, 28, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
| Outcome (%) | ICSI-SiD | ICSI | OR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Fertilization rate | 83.1 | 82.4 | 1.1 | 0.7–1.6 | ns |
| Cleavage rate | 97.6 | 97.2 | 1.2 | 0.4–3.7 | ns |
| Day 2 embryo development rate | 70.6 | 74.6 | 0.8 | 0.5–1.2 | ns |
| Top-quality development rate on day 2 | 48.6 | 52.8 | 0.9 | 0.6–1.2 | ns |
| Day 3 embryo development rate | 72.9 | 70.6 | 1.1 | 0.8–1.7 | ns |
| Top-quality embryo development rate on day 3 | 51.4 | 51.6 | 1.0 | 0.7–1.4 | ns |
| Blastocyst development rate on day 5 | 49.0 | 44.8 | 1.2 | 0.8–1.7 | ns |
| Good-quality blastocyst development rate on day 5 | 45.1 | 41.5 | 1.2 | 0.8–1.7 | ns |
| Top-quality blastocyst development rate on day 5 | 25.9 | 22.2 | 1.2 | 0.8–1.9 | ns |
| Blastocyst development rate * | 70.2 | 62.5 | 1.4 | 1.0–2.0 | ns |
| Good-quality blastocyst development rate * | 57.3 | 53.6 | 1.1 | 0.8–1.7 | ns |
| Top-quality blastocyst development rate * | 29.0 | 24.2 | 1.3 | 0.9–1.9 | ns |
| Outcome (%) | ICSI-SiD | ICSI | OR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Number of embryos transferred | 51 | 47 | |||
| Euploid rate # | 53.3 | 44.4 | 2.3 | 0.4–12.8 | ns |
| Cumulative * early pregnancy rate | 43.1 | 34.0 | 1.5 | 0.7–3.3 | ns |
| Cumulative * clinical pregnancy rate | 27.4 | 21.3 | 1.4 | 0.6–3.6 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montjean, D.; Godin Pagé, M.-H.; Pacios, C.; Calvé, A.; Hamiche, G.; Benkhalifa, M.; Miron, P. Automated Single-Sperm Selection Software (SiD) during ICSI: A Prospective Sibling Oocyte Evaluation. Med. Sci. 2024, 12, 19. https://doi.org/10.3390/medsci12020019
Montjean D, Godin Pagé M-H, Pacios C, Calvé A, Hamiche G, Benkhalifa M, Miron P. Automated Single-Sperm Selection Software (SiD) during ICSI: A Prospective Sibling Oocyte Evaluation. Medical Sciences. 2024; 12(2):19. https://doi.org/10.3390/medsci12020019
Chicago/Turabian StyleMontjean, Debbie, Marie-Hélène Godin Pagé, Carmen Pacios, Annabelle Calvé, Ghenima Hamiche, Moncef Benkhalifa, and Pierre Miron. 2024. "Automated Single-Sperm Selection Software (SiD) during ICSI: A Prospective Sibling Oocyte Evaluation" Medical Sciences 12, no. 2: 19. https://doi.org/10.3390/medsci12020019
APA StyleMontjean, D., Godin Pagé, M.-H., Pacios, C., Calvé, A., Hamiche, G., Benkhalifa, M., & Miron, P. (2024). Automated Single-Sperm Selection Software (SiD) during ICSI: A Prospective Sibling Oocyte Evaluation. Medical Sciences, 12(2), 19. https://doi.org/10.3390/medsci12020019







