Gender Effects on Left Ventricular Responses and Survival in Patients with Severe Aortic Regurgitation: Results from a Cohort of 756 Patients with up to 22 Years of Follow-Up
Abstract
:1. Background
2. Methods
2.1. Study Population
2.2. Clinical Variables
2.3. Echocardiography
2.4. Pharmacological Data
2.5. Mortality Data
2.6. Statistical Methods
3. Results
3.1. Baseline Characteristics of the Whole Cohort
3.2. Baseline Characteristics as a Function of Gender and Gender Differences in the Remodeling Response
3.3. Gender and Survival
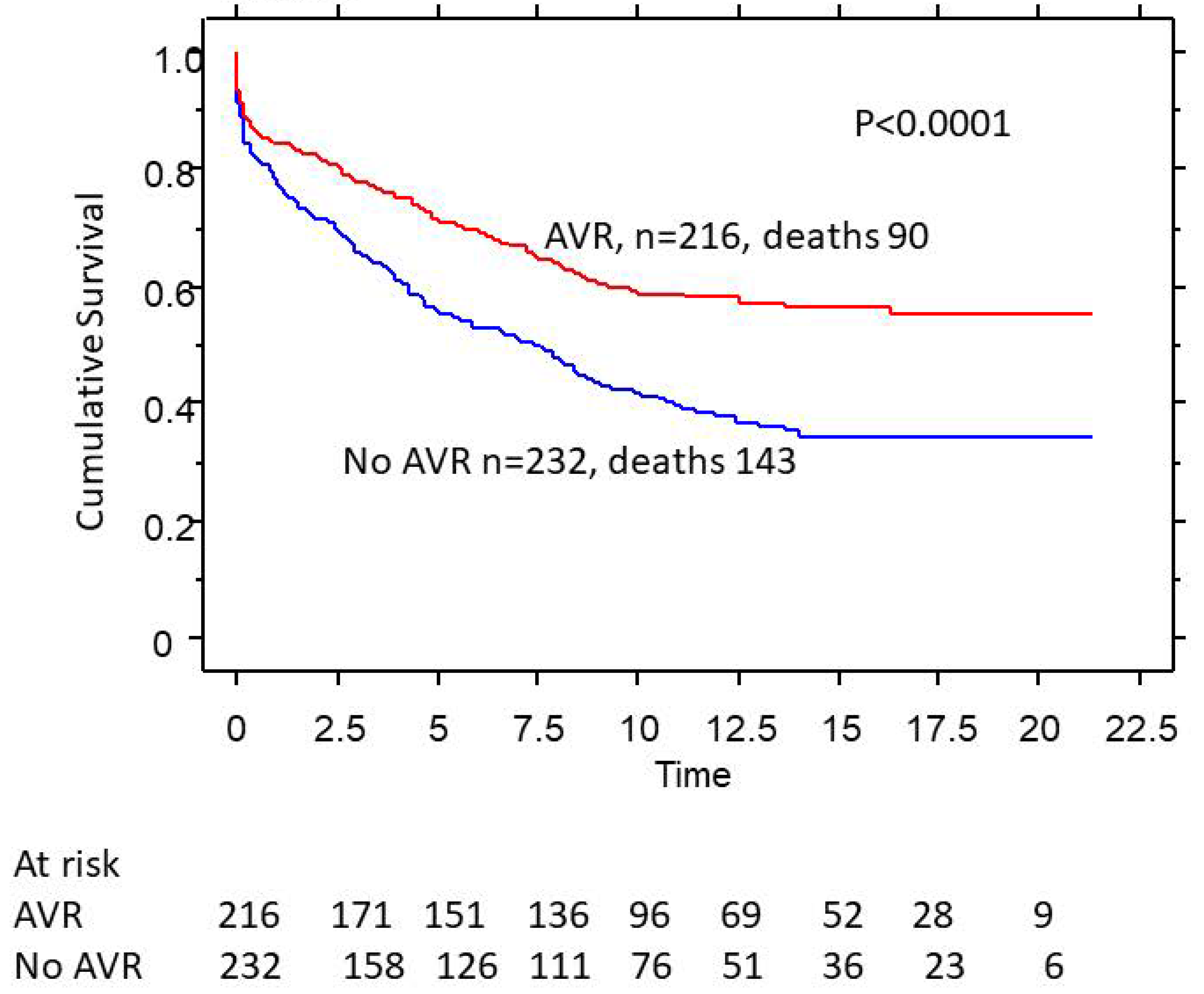
3.4. AVR Rates and Survival as a Function of Gender
3.5. Gender Effects on Survival in Nonsurgical Patients
3.6. Gender Effects on Survival in Surgical Patients
4. Discussion
4.1. Gender and Aortic Valve Disease
4.2. Gender and Volume Overload States
4.3. Aortic Valve Replacement in Females
5. Summary and Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart disease: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Bech-Hanssena, O.; Wallentina, I.; Houltzb, E.; Suurkulaa, M.B.; Larssonc, S.; Caidah, K. Gender differences in patients with severe aortic stenosis: Impact on preoperative left ventricular geometry and function, as well as early postoperative morbidity and mortality. Eur. J. Cardio-Thorac. Surg. 1999, 15, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.O.; Thienelt, C.D.; Katz, S.E.; Bartunek, J.; Tajima, M.; Rohrbach, S.; Douglas, P.S.; Lorell, B.H. Gender differences in molecular remodeling in pressure overload hypertrophy. J. Am. Coll. Cardiol. 1999, 34, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Sampat, U.; Varadarajan, P.; Turk, R.; Kamath, A.; Khandhar, S.; Pai, R.G. Effect of Beta-Blocker Therapy on Survival in Patients With Severe Aortic Regurgitation: Results From a Cohort of 756 Patients. J. Am. Coll. Cardiol. 2009, 54, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.; Varadarajan, P.; Turk, R.; Sampat, U.; Patel, R.; Khandhar, S.; Pai, R.G. Survival in Patients with Severe Aortic Regurgitation and Severe Left Ventricular Dysfunction is Improved by Aortic Valve Replacement: Results from a Cohort of 166 Patients with An Ejection Fraction <35%. Circulation 2009, 120, S134–S138. [Google Scholar] [PubMed]
- Pai, R.G.; Varadarajan, P. Prognostic Implications of Mitral Regurgitation in Patients With Severe Aortic Regurgitation. Circulation 2010, 122, S43–S47. [Google Scholar] [CrossRef] [PubMed]
- Van Royen, N.; Jaffe, C.C.; Krumholz, H.M. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am. J. Cardiol. 1996, 77, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Amico, A.F.; Lictenberg, G.S.; Reisner, S.A.; Stone, C.K.; Schwartz, R.G.; Meltzer, R.S. Superiority of visual versus computerized echocardiographic estimation of radionuclide left ventricular ejection fraction. Am. Heart J. 1989, 118, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Schiller, N.B.; Shah, P.M.; Crawford, M.; DeMaria, A.; Devereux, R.; Feigenbaum, H.; Gutgesell, H.; Reichek, N.; Sahn, D. Schnittger Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J. Am. Soc. Echocardiogr. 1989, 2, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Dent, M.R.; Tappia, P.S.; Dhalla, N.S. Gender Differences in Cardiac Dysfunction and Remodeling due to Volume Overload. J. Card. Fail. 2010, 16, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, C.; Walsh-Wilkinson, E.; Drolet, M.C.; Roussel, E.; Arsenault, M.; Couet, J. Female rats with severe left ventricular volume overload exhibit more cardiac hypertrophy but fewer myocardial transcriptional changes than males. Sci. Rep. 2017, 7, 729. [Google Scholar] [CrossRef] [PubMed]
- Chaker, Z.; Badhwar, V.; Alqahtani, F.; Aljohani, S.; Zack, C.J.; Holmes, D.R.; Rihal, C.S.; Alkhouli, M. Sex Differences in the Utilization and Outcomes of Surgical Aortic Valve Replacement for Severe Aortic Stenosis. J. Am. Hear. Assoc. 2017, 6, e006370. [Google Scholar] [CrossRef] [PubMed]
- Hamed, O.; Persson, P.; Engel, A.M.; McDonough, S.; Smith, J.M. Gender differences in outcomes following aortic valve replacement surgery. Int. J. Surg. 2009, 7, 214–217. [Google Scholar] [CrossRef] [PubMed]



| Variables | Males (n = 448) | Females (n = 308) | p Value |
|---|---|---|---|
| Age in years | 59 ± 17 | 64 ± 18 | 0.0002 |
| LVEDD in cm | 6.0 ± 1.0 | 5.2 ± 1.1 | <0.0001 |
| LVESD in cm | 4.2 ± 1.2 | 3.6 ± 1.1 | <0.0001 |
| Septum in cm | 1.3 + 0.2 | 1.2 + 0.3 | 0.0008 |
| Posterior wall in cm | 1.2 + 0.2 | 1.1 + 0.2 | 0.002 |
| Ejection fraction% | 52 ± 18 | 56 ± 17 | 0.003 |
| Hypertension | 62% | 68% | 0.18 |
| Diabetes mellitus | 11% | 18% | 0.006 |
| Coronary artery disease | 35% | 32% | 0.3 |
| Heart failure | 70% | 69% | 0.7 |
| AV replacement | 48% | 24% | <0.0001 |
| RVSP > 59 | 18% | 15% | 0.3 |
| ACE or ARB | 52% | 48% | 0.4 |
| Calcium channel blockers | 28% | 31% | 0.4 |
| Beta Blockers | 50% | 57% | 0.05 |
| Greater than 2+ MR | 40% | 52% | 0.0008 |
| Variables | Exponential Coefficient | 95% CI | p Value |
|---|---|---|---|
| Female gender | 1.0 | 0.77–1.25 | 0.9 |
| Age in years | 1.038 | 1.02–1.04 | <0.0001 |
| LV EDD in mm | 0.88 | 0.76–1.0 | 0.07 |
| EF in % | 0.99 | 0.98–0.99 | 0.009 |
| Diabetes | 1.82 | 1.35–2.37 | <0.0001 |
| AVR | 0.74 | 0.58–1.04 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varadarajan, P.; Pai, R.G. Gender Effects on Left Ventricular Responses and Survival in Patients with Severe Aortic Regurgitation: Results from a Cohort of 756 Patients with up to 22 Years of Follow-Up. Med. Sci. 2023, 11, 36. https://doi.org/10.3390/medsci11020036
Varadarajan P, Pai RG. Gender Effects on Left Ventricular Responses and Survival in Patients with Severe Aortic Regurgitation: Results from a Cohort of 756 Patients with up to 22 Years of Follow-Up. Medical Sciences. 2023; 11(2):36. https://doi.org/10.3390/medsci11020036
Chicago/Turabian StyleVaradarajan, Padmini, and Ramdas G. Pai. 2023. "Gender Effects on Left Ventricular Responses and Survival in Patients with Severe Aortic Regurgitation: Results from a Cohort of 756 Patients with up to 22 Years of Follow-Up" Medical Sciences 11, no. 2: 36. https://doi.org/10.3390/medsci11020036
APA StyleVaradarajan, P., & Pai, R. G. (2023). Gender Effects on Left Ventricular Responses and Survival in Patients with Severe Aortic Regurgitation: Results from a Cohort of 756 Patients with up to 22 Years of Follow-Up. Medical Sciences, 11(2), 36. https://doi.org/10.3390/medsci11020036





