Thiotepa, Busulfan, Cyclophosphamide: Effective but Toxic Conditioning Regimen Prior to Autologous Hematopoietic Stem Cell Transplantation in Central Nervous System Lymphoma
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Selection Criteria and Data Collection
2.2. The Conditioning and ASCT Protocols
2.3. Patient Follow-Up and Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Pre-Transplant Treatment
3.3. Engraftment
3.4. Toxicity of Thiotepa, Busulfan, and Cyclophosphamide/ASCT
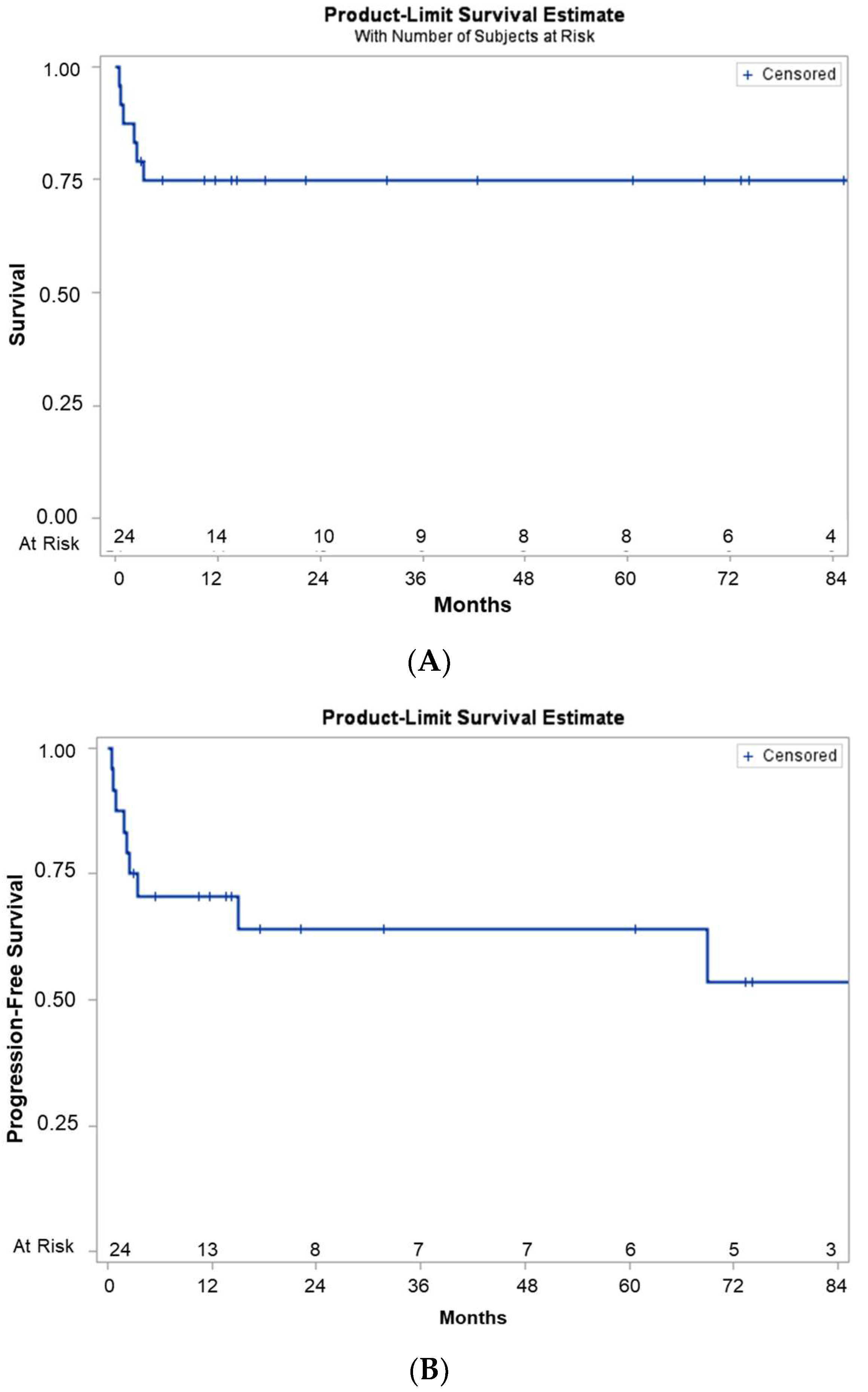
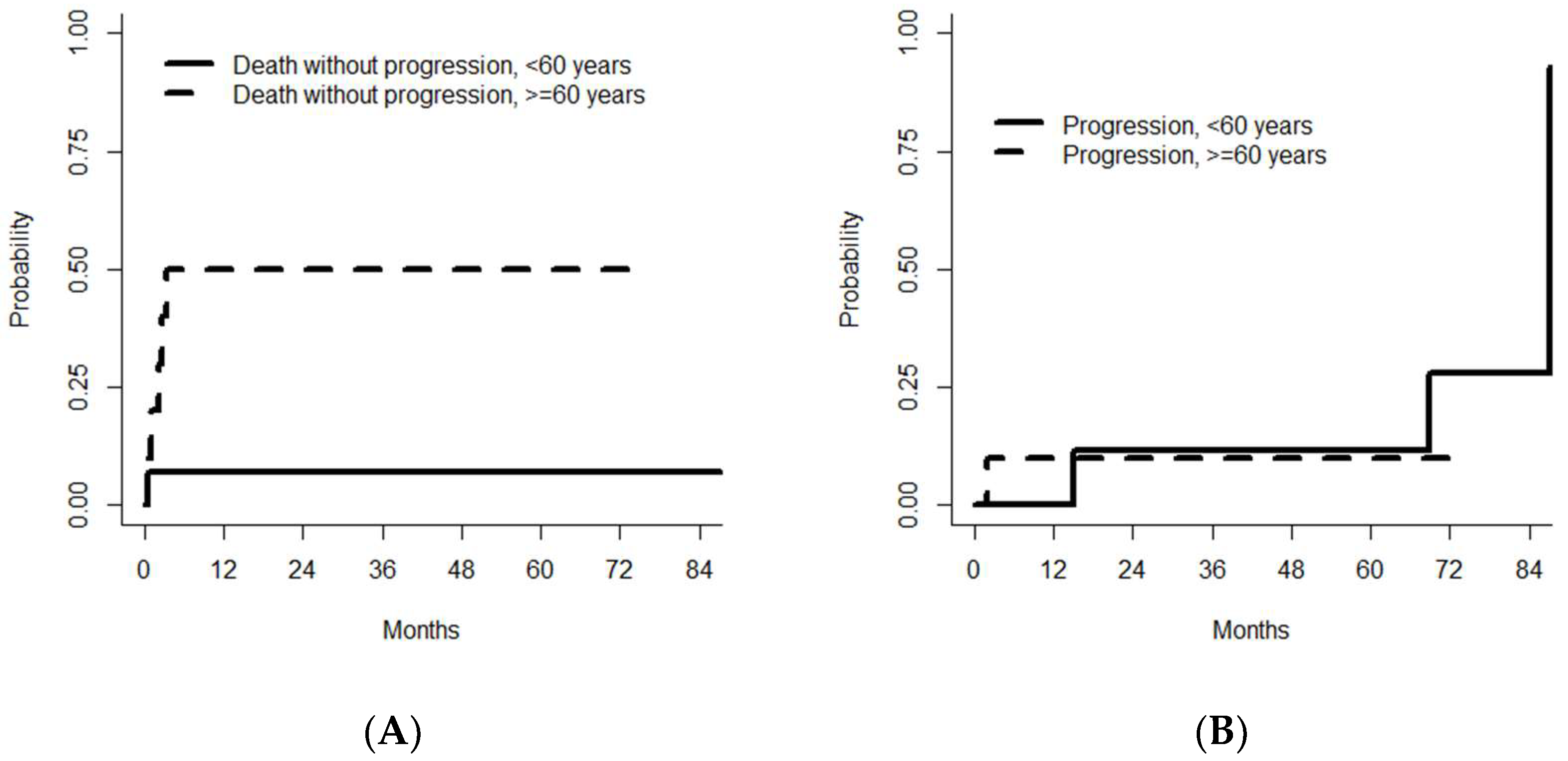
3.5. Outcomes after Thiotepa, Busulfan, and Cyclophosphamide/ASCT
3.6. Prognostic Factors Associated with Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeAngelis, L.M.; Yahalom, J.; Thaler, H.T.; Kher, U. Combined modality therapy for primary CNS lymphoma. J. Clin. Oncol. 1992, 10, 635–643. [Google Scholar] [CrossRef]
- Haas, R.L.M.; Poortmans, P.; de Jong, D.; Aleman, B.M.P.; Dewit, L.G.H.; Verheij, M.; Hart, A.A.M.; van Oers, M.H.J.; van der Hulst, M.; Baars, J.W.; et al. High Response Rates and Lasting Remissions After Low-Dose Involved Field Radiotherapy in Indolent Lymphomas. J. Clin. Oncol. 2003, 21, 2474–2480. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovic, I.T.; Hormigo, A.; Yahalom, J.; DeAngelis, L.M.; Abrey, L.E. Long-Term Follow-Up of High-Dose Methotrexate-Based Therapy with and Without Whole Brain Irradiation for Newly Diagnosed Primary CNS Lymphoma. J. Clin. Oncol. 2006, 24, 4570–4574. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.C.; Roos, D.E.; Pratt, G.; Liew, K.-H.; Barton, M.B.; Poulsen, M.G.; Olver, I.N.; Trotter, G.E. Combined-modality therapy for primary central nervous system lymphoma: Long-term data from a Phase II multicenter study (Trans-Tasman Radiation Oncology Group). Int. J. Radiat. Oncol. *Biol. *Phys. 2006, 64, 408–413. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.; Roos, D.; Pratt, G.; Liew, K.; Barton, M.; Poulsen, M.; Olver, I.; Trotter, G. Phase II Multicenter Study of Brief Single-Agent Methotrexate Followed by Irradiation in Primary CNS Lymphoma. J. Clin. Oncol. 2000, 18, 519. [Google Scholar] [CrossRef]
- Kasenda, B.; Schorb, E.; Fritsch, K.; Finke, J.; Illerhaus, G. Prognosis after high-dose chemotherapy followed by autologous stem-cell transplantation as first-line treatment in primary CNS lymphoma—A long-term follow-up study. Ann. Oncol. 2012, 23, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Abrey, L.E.; Moskowitz, C.H.; Mason, W.P.; Crump, M.; Stewart, D.; Forsyth, P.; Paleologos, N.; Correa, D.D.; Anderson, N.D.; Caron, D.; et al. Intensive Methotrexate and Cytarabine Followed by High-Dose Chemotherapy with Autologous Stem-Cell Rescue in Patients with Newly Diagnosed Primary CNS Lymphoma: An Intent-to-Treat Analysis. J. Clin. Oncol. 2003, 21, 4151–4156. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Muldoon, L.L.; Soussain, C.; Jahnke, K.; Johanson, C.; Siegal, T.; Smith, Q.R.; Hall, W.A.; Hynynen, K.; Senter, P.D.; Peereboom, D.M.; et al. Chemotherapy Delivery Issues in Central Nervous System Malignancy: A Reality Check. J. Clin. Oncol. 2007, 25, 2295–2305. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Reni, M.; Foppoli, M.; Martelli, M.; Pangalis, G.A.; Frezzato, M.; Cabras, M.G.; Fabbri, A.; Corazzelli, G.; Ilariucci, F.; et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial. Lancet 2009, 374, 1512–1520. [Google Scholar] [CrossRef]
- Soussain, C.; Hoang-Xuan, K.; Taillandier, L.; Fourme, E.; Choquet, S.; Witz, F.; Casasnovas, O.; Dupriez, B.; Souleau, B.; Taksin, A.-L. Intensive Chemotherapy Followed by Hematopoietic Stem-Cell Rescue for Refractory and Recurrent Primary CNS and Intraocular Lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J. Clin. Oncol. 2008, 26, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Omuro, A.; Correa, D.D.; DeAngelis, L.M.; Moskowitz, C.H.; Matasar, M.J.; Kaley, T.J.; Gavrilovic, I.T.; Nolan, C.; Pentsova, E.; Grommes, C.C.; et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015, 125, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Forsyth, P.; Chaudhry, A.; Morris, D.; Glück, S.; Russell, J.A.; Stewart, D.A. High-dose thiotepa, busulfan, cyclophosphamide and ASCT without whole-brain radiotherapy for poor prognosis primary CNS lymphoma. Bone Marrow Transpl. 2003, 31, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Houillier, C.; Taillandier, L.; Dureau, S.; Lamy, T.; Laadhari, M.; Chinot, O.; Moluçon-Chabrot, C.; Soubeyran, P.; Gressin, R.; Choquet, S. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J. Clin. Oncol. 2019, 37, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M.; Grambsch, P.M. The Cox Model. In Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; pp. 39–77. [Google Scholar]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- (2017) Common Terminology Criteria for Adverse Events (CTCAE). 155. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 11 November 2022).
- Houillier, C.; Dureau, S.; Taillandier, L.; Houot, R.; Chinot, O.; Moluçon-Chabrot, C.; Schmitt, A.; Gressin, R.; Choquet, S.; Damaj, G. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients Age 60 Years and Younger: Long-Term Results of the Randomized Phase II PRECIS Study. J. Clin. Oncol. 2022, 40, 3692–3698. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.; Christofides, A.; Visani, G. Novel regimens prior to autologous stem cell transplantation for the management of adults with relapsed/refractory non-Hodgkin lymphoma and Hodgkin lymphoma: Alternatives to BEAM conditioning. Leuk. Lymphoma 2016, 57, 2499–2509. [Google Scholar] [CrossRef]
- Soussain, C.; Suzan, F.; Hoang-Xuan, K.; Cassoux, N.; Levy, V.; Azar, N.; Belanger, C.; Achour, E.; Ribrag, V.; Gerber, S. Results of Intensive Chemotherapy Followed by Hematopoietic Stem-Cell Rescue in 22 Patients with Refractory or Recurrent Primary CNS Lymphoma or Intraocular Lymphoma. J. Clin. Oncol. 2001, 19, 742–749. [Google Scholar] [CrossRef]
- Soussain, C.; Choquet, S.; Fourme, E.; Delgadillo, D.; Bouabdallah, K.; Ghesquières, H.; Damaj, G.; Dupriez, B.; Vargaftig, J.; Gonzalez, A.; et al. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: A retrospective study of 79 cases. Haematologica 2012, 97, 1751–1756. [Google Scholar] [CrossRef]
- Welch, M.R.; Sauter, C.S.; Matasar, M.J.; Faivre, G.; Weaver, S.A.; Moskowitz, C.H.; Omuro, A.M. Autologous stem cell transplant in recurrent or refractory primary or secondary central nervous system lymphoma using thiotepa, busulfan and cyclophosphamide. Leuk. Lymphoma 2015, 56, 361–367. [Google Scholar] [CrossRef]
- Jantunen, E.; Salonen, J.; Juvonen, E.; Koivunen, E.; Siitonen, T.; Lehtinen, T.; Kuittinen, O.; Leppä, S.; Anttila, V.-J.; Itälä, M.; et al. Invasive fungal infections in autologous stem cell transplant recipients: A nation-wide study of 1188 transplanted patients: Fungal infections in autologous SCT recipients. Eur. J. Haematol. 2004, 73, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.A.; Girmenia, C.; Brüggemann, R.J.; Duarte, R.F.; Kibbler, C.C.; Ljungman, P.; Racil, Z.; Ribaud, P.; Slavin, M.A.; Cornely, O.A.; et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: Summary of the updated recommendations from the European Conference on Infections in Leukaemia. J. Antimicrob. Chemother. 2018, 73, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Christopeit, M.; Schmidt-Hieber, M.; Sprute, R.; Buchheidt, D.; Hentrich, M.; Karthaus, M.; Penack, O.; Ruhnke, M.; Weissinger, F.; Cornely, O.A.; et al. Prophylaxis, diagnosis and therapy of infections in patients undergoing high-dose chemotherapy and autologous haematopoietic stem cell transplantation. 2020 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2020, 100, 321–336. [Google Scholar] [PubMed]
- Alimohamed, N.; Daly, A.; Owen, C.; Duggan, P.; Stewart, D.A. Upfront thiotepa, busulfan, cyclophosphamide, and autologous stem cell transplantation for primary CNS lymphoma: A single centre experience. Leuk. Lymphoma 2012, 53, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, V.J.; Smith, B.R.; DeGregorio, M.W.; Rappeport, J.M. Pharmacology of agents used in bone marrow transplant conditioning regimens. Crit. Rev. Oncol./Hematol. 1992, 13, 241–270. [Google Scholar] [CrossRef] [PubMed]
- Illerhaus, G. Effects on Survival of Non-Myeloablative Chemoimmunotherapy Compared to High-Dosse Chemotherapy Followed by Autologous Stem Cell Transplantation (HDC-ASCT) As Consolidation Therapy in Patients with Primary CNS Lymphoma—Results of an International Randomized Phase III Trial (MATRix/IELSG43). Blood 2022, 140, LBA-3. [Google Scholar]
- Hoang-Xuan, K.; Deckert, M.; Ferreri, A.J.M.; Furtner, J.; Perez-Larraya, J.G.; Henriksson, R.; Hottinger, A.F.; Kasenda, B.; Lefranc, F.; Lossos, A.; et al. European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro-Oncology 2023, 25, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Samhouri, Y.; Mustafa Ali, M.K.; Law, J.; Khan, C.; Wegner, R.; Lee, S.T.; Lister, J. Consolidative Autologous Stem Cell Transplantation Versus Whole Brain Radiation in PCNSL.; A Nationwide Analysis. Clin. Lymphoma Myeloma Leuk. 2022, 22, 735–743. [Google Scholar] [CrossRef]

| Primary CNSL (n = 15) | Secondary CNSL (n = 9) | Whole Study Population (n = 24) | |
|---|---|---|---|
| Age at diagnosis, years, median (range) | 55 | 57 | 56.5 |
| Sex (%) | |||
| Male | 10 (66) | 5 (56) | 15 (63) |
| Female | 5 (33) | 4 (44) | 9 (38) |
| PS at inclusion (%) | |||
| 0 | 3 (20) | 0 (0) | 3 (13) |
| 1–2 | 7 (47) | 4 (44) | 11 (46) |
| 3–4 | 1 (7) | 1 (12) | 2 (8) |
| Missing data | 4 (26) | 4 (44) | 8 (33) |
| Ocular involvement (%) | 4 (26) | 1 (11) | 5 (21) |
| CSF positive (%) | 1 (6) | 3 (33) | 4 (17) |
| Histology (%) | |||
| Diffuse large B-cell lymphoma | 14 (93) | 8 (89) | 22 (92) |
| Follicular lymphoma | 1 (6) | 1 (11) | 2 (8) |
| Number of courses of chemotherapy (%) | |||
| 1 | 11 (73) | 3 (33) | 14 (58) |
| 2 | 4 (26) | 5 (56) | 9 (38) |
| >2 | 0 (0) | 1 (11) | 1 (5) |
| Intrathecal treatment (%) | 0 (0) | 4 (44) | 4 (17) |
| Radiotherapy (%) | 2 (13) | 1 (11) | 3 (5) |
| Indication for ASCT (%) | |||
| First-line treatment | 10 (66) | 3 (33) | 13 (54) |
| Relapsed disease | 4 (26) | 5 (56) | 9 (38) |
| Refractory disease | 1 (6) | 1 (11) | 2 (8) |
| Time interval between diagnosis and ASCT, months, median (range) | 6 (3–100) | 20 (5–81) | 6 (3–100) |
| Age at the time of ASCT (%) | |||
| ≤60 60–65 | 9 (60) 3 (20) | 5 (55) 3 (34) | 14 (58) 6 (25) |
| >65 | 3 (20) | 1 (11) | 4 (17) |
| Response status at the time of ASCT (%) | |||
| CR | 9 (60) | 6 (67) | 15 (63) |
| PR | 6 (40) | 3 (33) | 9 (38) |
| PS at the time of ASCT * (%) | |||
| 0 | 9 (60) | 5 (56) | 14 (58) |
| 1 | 6 (40) | 3 (33) | 9 (38) |
| Grade 1/2 | Grade 3 | Grade 4 | All | |
|---|---|---|---|---|
| Infections | 13 | 2 | 9 | 24 |
| Neurological events | 4 | 1 | 4 | 9 |
| Mucositis * | 5 | 7 | 3 | 15 |
| Cutaneous events | 5 | 2 | 0 | 7 |
| Other complications | ||||
| Colitis | 18 out of 24 (75%) had a CTCAE grade 3 event | |||
| Renal dysfunction | 2 out of 24 | |||
| Hemorrhagic cystitis | 2 out of 24 | |||
| Fine and Gray Model | Cause Specific Hazard Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Reference | 3-yr CIF | Hazard Ratio | LCL | UCL | p Value | Hazard Ratio | p Value |
| Age | 8.003 | 0.89 | 72.9 | 0.06 | 8.72 | 0.048 | ||
| <60 | reference | 0.07 | 0.06 | 0.08 | ||||
| >=60 | 0.5 | 0.44 | 0.56 | |||||
| cd34 | 0.12 | 0.014 | 1.12 | 0.06 | 0.13 | 0.05 | ||
| <4.6 | reference | 0.5 | 0.44 | 0.56 | ||||
| >=4.6 | 0.07 | 0.06 | 0.08 | |||||
| status before ASCT | 4.57 | 0.96 | 21.8 | 0.05 | 4.41 | 0.08 | ||
| CR | reference | 0.13 | 0.11 | 0.15 | ||||
| PR | 0.44 | 0.38 | 0.5 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delphine, L.; Pierre-Edouard, D.; Bruno, R.; Bérengère, G.; Magalie, J.; Patrick, V.; Jean-Pierre, M.; Pierre, M. Thiotepa, Busulfan, Cyclophosphamide: Effective but Toxic Conditioning Regimen Prior to Autologous Hematopoietic Stem Cell Transplantation in Central Nervous System Lymphoma. Med. Sci. 2023, 11, 14. https://doi.org/10.3390/medsci11010014
Delphine L, Pierre-Edouard D, Bruno R, Bérengère G, Magalie J, Patrick V, Jean-Pierre M, Pierre M. Thiotepa, Busulfan, Cyclophosphamide: Effective but Toxic Conditioning Regimen Prior to Autologous Hematopoietic Stem Cell Transplantation in Central Nervous System Lymphoma. Medical Sciences. 2023; 11(1):14. https://doi.org/10.3390/medsci11010014
Chicago/Turabian StyleDelphine, Lebon, Debureaux Pierre-Edouard, Royer Bruno, Gruson Bérengère, Joris Magalie, Votte Patrick, Marolleau Jean-Pierre, and Morel Pierre. 2023. "Thiotepa, Busulfan, Cyclophosphamide: Effective but Toxic Conditioning Regimen Prior to Autologous Hematopoietic Stem Cell Transplantation in Central Nervous System Lymphoma" Medical Sciences 11, no. 1: 14. https://doi.org/10.3390/medsci11010014
APA StyleDelphine, L., Pierre-Edouard, D., Bruno, R., Bérengère, G., Magalie, J., Patrick, V., Jean-Pierre, M., & Pierre, M. (2023). Thiotepa, Busulfan, Cyclophosphamide: Effective but Toxic Conditioning Regimen Prior to Autologous Hematopoietic Stem Cell Transplantation in Central Nervous System Lymphoma. Medical Sciences, 11(1), 14. https://doi.org/10.3390/medsci11010014









