Effects of Electrical Stimulation of the Cell: Wound Healing, Cell Proliferation, Apoptosis, and Signal Transduction
Abstract
1. Introduction
2. Electrical Stimulation of Cells
2.1. Cell Proliferation and Apoptosis
2.1.1. Inflammatory Phase in the Healing Process of Wounds
2.1.2. Proliferative Phase in the Wound Healing Process
2.1.3. Remodeling Phase in the Wound Healing Process
2.1.4. Other Aspects of Wound Healing Facilitated by Electrical Stimulation
2.2. Cell Proliferation and Apoptosis
2.2.1. Studies Showing Electrical Stimulation Promotes Apoptosis
2.2.2. Studies Showing Electrical Stimulation Inhibits or Does Not Affect Apoptosis
2.3. Effects of Stimulation on the Proliferation of Cells
2.3.1. Electrical Stimulation Promotes Cell Proliferation
2.3.2. Electrical Stimulation Inhibits Cell Proliferation
3. Molecular Mechanisms in Wound Healing Affected by Electrical Stimulation
3.1. Molecular Mechanisms in Wound Healing
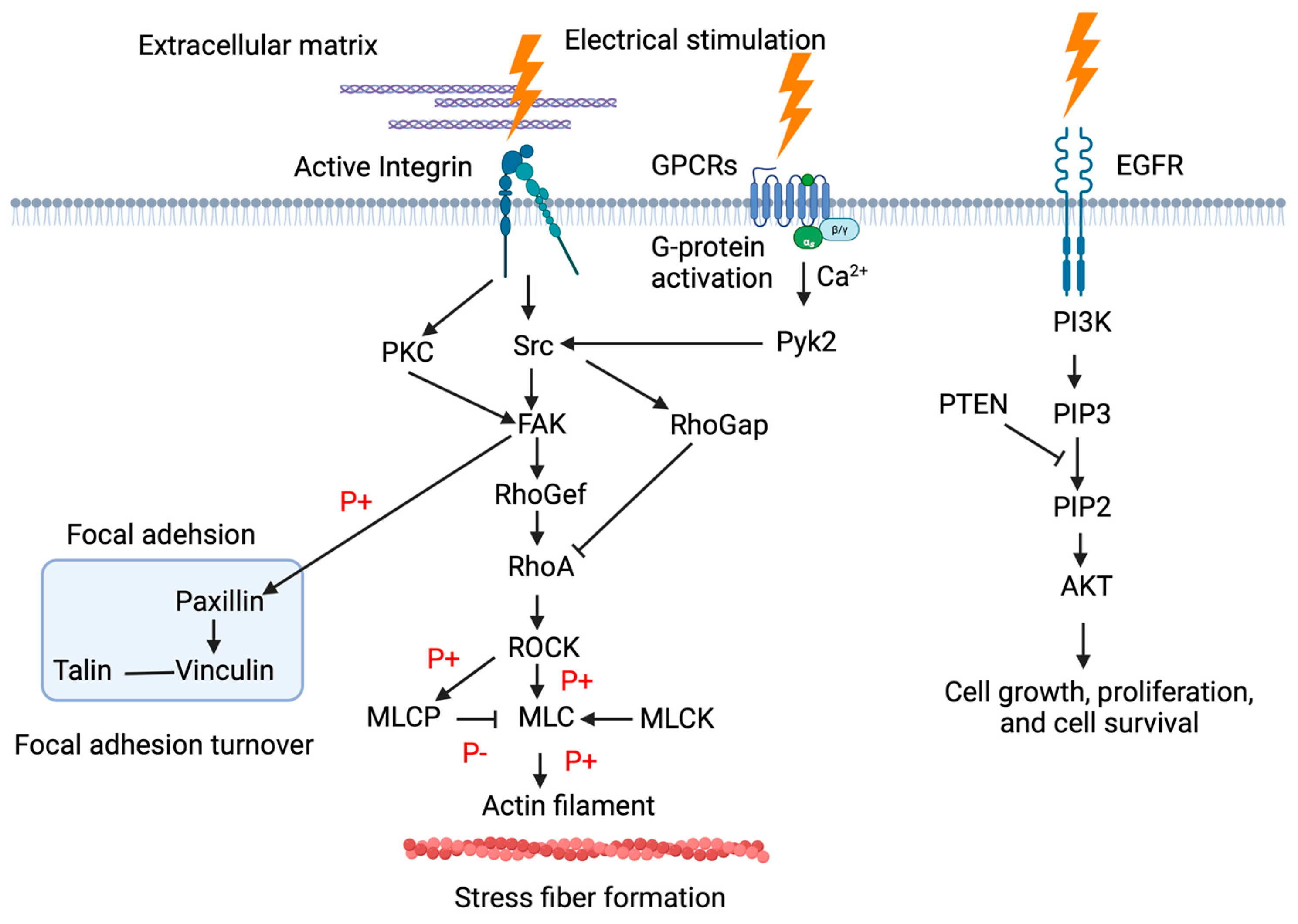
3.2. Signal Transduction Mechanisms in Wound Healing
4. Short Summary
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takayama, S.; Watanabe, M.; Kusuyama, H.; Nagase, S.; Seki, T.; Nakazawa, T.; Yaegashi, N. Evaluation of the effects of acupuncture on blood flow in humans with ultrasound color Doppler imaging. Evid. Based Complement. Alternat. Med. 2012, 2012, 513638. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K. Effects of Electrical Stimulation on the Signal Transduction-Related Proteins, c-Src and Focal Adhesion Kinase, in Fibroblasts. Life 2022, 12, 531. [Google Scholar] [CrossRef]
- Katoh, K. Regulation of Fibroblast Cell Polarity by Src Tyrosine Kinase. Biomedicines 2021, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K. FAK-Dependent Cell Motility and Cell Elongation. Cells 2020, 9, 192. [Google Scholar] [CrossRef]
- Reid, B.; Zhao, M. The Electrical Response to Injury: Molecular Mechanisms and Wound Healing. Adv. Wound Care 2014, 3, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Hoare, J.I.; Rajnicek, A.M.; McCaig, C.D.; Barker, R.N.; Wilson, H.M. Electric fields are novel determinants of human macrophage functions. J. Leukoc. Biol. 2016, 99, 1141–1151. [Google Scholar] [CrossRef]
- Kana, K.; Song, H.; Laschinger, C.; Zandstra, P.W.; Radisic, M. PI3K Phosphorylation Is Linked to Improved Electrical Excitability in an In Vitro Engineered Heart Tissue Disease Model System. Tissue Eng. Part A 2015, 21, 2379–2389. [Google Scholar] [CrossRef]
- Geng, K.; Wang, J.; Liu, P.; Tian, X.; Liu, H.; Wang, X.; Hu, C.; Yan, H. Electrical stimulation facilitates the angiogenesis of human umbilical vein endothelial cells through MAPK/ERK signaling pathway by stimulating FGF2 secretion. Am. J. Physiol. Cell Physiol. 2019, 317, C277–C286. [Google Scholar] [CrossRef]
- Lévêque, M.; Penna, A.; Le Trionnaire, S.; Belleguic, C.; Desrues, B.; Brinchault, G.; Jouneau, S.; Lagadic-Gossmann, D.; Martin-Chouly, C. Phagocytosis depends on TRPV2-mediated calcium influx and requires TRPV2 in lipids rafts: Alteration in macrophages from patients with cystic fibrosis. Sci. Rep. 2018, 8, 4310. [Google Scholar] [CrossRef]
- Rowley, B.A. Electrical current effects on E. coli growth rates. Proc. Soc. Exp. Biol. Med. 1972, 139, 929–934. [Google Scholar] [CrossRef]
- Barranco, S.D.; Spadaro, J.A.; Berger, T.J.; Becker, R.O. In vitro effect of weak direct current on Staphylococcus aureus. Clin. Orthop. Relat. Res. 1974, 100, 250–255. [Google Scholar] [CrossRef]
- Ren, X.; Sun, H.; Liu, J.; Guo, X.; Huang, J.; Jiang, X.; Zhang, Y.; Huang, Y.; Fan, D.; Zhang, J. Keratinocyte electrotaxis induced by physiological pulsed direct current electric fields. Bioelectrochemistry 2019, 127, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Park, H.J.; Abedin-Do, A.; Douville, Y.; Méthot, M.; Zhang, Z. Electrical stimulation promotes the proliferation of human keratinocytes, increases the production of keratin 5 and 14, and increases the phosphorylation of ERK1/2 and p38 MAP kinases. J. Tissue Eng. Regen. Med. 2020, 14, 909–919. [Google Scholar] [CrossRef]
- Zhao, M.; Agius-Fernandez, A.; Forrester, J.V.; McCaig, C.D. Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent. J. Cell Sci. 1996, 109 Pt 6, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gu, Y.; Wang, H.; Liu, Z.; Song, B.; Yin, T. Electric Pulses Can Influence Galvanotaxis of Dictyostelium discoideum. Biomed. Res. Int. 2018, 2018, 2534625. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, R.; Walczysko, P.; Reid, B.; Ou, J.; Leiper, L.J.; Rajnicek, A.M.; McCaig, C.D.; Zhao, M.; Collinson, J.M. The role of electrical signals in murine corneal wound re-epithelialization. J. Cell Physiol. 2011, 226, 1544–1553. [Google Scholar] [CrossRef]
- Casagrande, S.M.; Biondo-Simões, M.d.L.P.; Ioshii, S.; Robes, R.R.; Biondo-Simões, R.; Boeno, B.R.d.O. Histological evaluation of the effect of low-frequency electric stimulation on healing Achilles tendons in rats. Acta Cir. Bras. 2021, 36, e360103. [Google Scholar] [CrossRef]
- Sebastian, A.; Iqbal, S.A.; Colthurst, J.; Volk, S.W.; Bayat, A. Electrical stimulation enhances epidermal proliferation in human cutaneous wounds by modulating p53-SIVA1 interaction. J. Investig. Dermatol. 2015, 135, 1166–1174. [Google Scholar] [CrossRef]
- Wang, Y.; Rouabhia, M.; Zhang, Z. Pulsed electrical stimulation benefits wound healing by activating skin fibroblasts through the TGFβ1/ERK/NF-κB axis. Biochim. Biophys. Acta 2016, 1860, 1551–1559. [Google Scholar] [CrossRef]
- Wang, M.; Luo, Y.; Wang, T.; Wan, C.; Pan, L.; Pan, S.; He, K.; Neo, A.; Chen, X. Artificial skin perception. Adv. Mater. 2021, 33, e2003014. [Google Scholar] [CrossRef]
- Li, A.; Cho, J.H.; Reid, B.; Tseng, C.C.; He, L.; Tan, P.; Yeh, C.Y.; Wu, P.; Li, Y.; Widelitz, R.B.; et al. Calcium oscillations coordinate feather mesenchymal cell movement by SHH dependent modulation of gap junction networks. Nat. Commun. 2018, 9, 5377. [Google Scholar] [CrossRef]
- Snyder, S.; DeJulius, C.; Willits, R.K. Electrical Stimulation Increases Random Migration of Human Dermal Fibroblasts. Ann. Biomed. Eng. 2017, 45, 2049–2060. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lin, B.J.; Chao, P.H. alpha2beta1 integrin and RhoA mediates electric field-induced ligament fibroblast migration directionality. J. Orthop. Res. 2013, 31, 322–327. [Google Scholar] [CrossRef]
- Bai, H.; Forrester, J.V.; Zhao, M. DC electric stimulation upregulates angiogenic factors in endothelial cells through activation of VEGF receptors. Cytokine 2011, 55, 110–115. [Google Scholar] [CrossRef]
- Cunha, F.; Rajnicek, A.M.; McCaig, C.D. Electrical Stimulation Directs Migration, Enhances and Orients Cell Division and Upregulates the Chemokine Receptors CXCR4 and CXCR2 in Endothelial Cells. J. Vasc. Res. 2019, 56, 39–53. [Google Scholar] [CrossRef]
- Jin, F.; Li, T.; Wei, Z.; Xiong, R.; Qian, L.; Ma, J.; Yuan, T.; Wu, Q.; Lai, C.; Ma, X.; et al. Biofeedback electrostimulation for bionic and long-lasting neural modulation. Nat. Commun. 2022, 13, 5302. [Google Scholar] [CrossRef]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef]
- Sebastian, A.; Syed, F.; Perry, D.; Balamurugan, V.; Colthurst, J.; Chaudhry, I.H.; Bayat, A. Acceleration of cutaneous healing by electrical stimulation: Degenerate electrical waveform down-regulates inflammation, up-regulates angiogenesis and advances remodeling in temporal punch biopsies in a human volunteer study. Wound Repair Regen. 2011, 19, 693–708. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Li, Y.; Yan, H.; Ba, Y.; Wang, X.; Shi, N.; Liu, C. Gastric Electrical Stimulation Increases the Proliferation of Interstitial Cells of Cajal and Alters the Enteric Nervous System in Diabetic Rats. Neuromodulation 2022, 25, 1106–1114. [Google Scholar] [CrossRef]
- Emmerson, E. Efficient Healing Takes Some Nerve: Electrical Stimulation Enhances Innervation in Cutaneous Human Wounds. J. Investig. Dermatol. 2017, 137, 543–545. [Google Scholar] [CrossRef]
- Kajiya, K.; Matsumoto-Okazaki, Y.; Sawane, M.; Fukada, K.; Takasugi, Y.; Akai, T.; Saito, N.; Mori, Y. Electric current-induced lymphatic activation. Exp. Dermatol. 2014, 23, 936–938. [Google Scholar] [CrossRef]
- Luo, R.; Dai, J.; Zhang, J.; Li, Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv. Healthc. Mater. 2021, 10, e2100557. [Google Scholar] [CrossRef]
- Rajendran, S.B.; Challen, K.; Wright, K.L.; Hardy, J.G. Electrical Stimulation to Enhance Wound Healing. J. Funct. Biomater. 2021, 12, 40. [Google Scholar] [CrossRef]
- Love, M.R.; Palee, S.; Chattipakorn, S.C.; Chattipakorn, N. Effects of electrical stimulation on cell proliferation and apoptosis. J. Cell. Physiol. 2018, 233, 1860–1876. [Google Scholar] [CrossRef]
- Hofmann, F.; Ohnimus, H.; Scheller, C.; Strupp, W.; Zimmermann, U.; Jassoy, C. Electric field pulses can induce apoptosis. J. Membr. Biol. 1999, 169, 103–109. [Google Scholar] [CrossRef]
- Guo, B.S.; Cheung, K.K.; Yeung, S.S.; Zhang, B.T.; Yeung, E.W. Electrical stimulation influences satellite cell proliferation and apoptosis in unloading-induced muscle atrophy in mice. PLoS ONE 2012, 7, e30348. [Google Scholar] [CrossRef]
- Liaquat, Z.; Xu, X.; Zilundu, P.L.M.; Fu, R.; Zhou, L. The Current Role of Dexmedetomidine as Neuroprotective Agent: An Updated Review. Brain Sci. 2021, 11, 846. [Google Scholar] [CrossRef]
- Matsuki, N.; Takeda, M.; Ishikawa, T.; Kinjo, A.; Hayasaka, T.; Imai, Y.; Yamaguchi, T. Activation of caspases and apoptosis in response to low-voltage electric pulses. Oncol. Rep. 2010, 23, 1425–1433. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Trillo, M.Á.; Úbeda, A. Molecular mechanisms underlying antiproliferative and differentiating responses of hepatocarcinoma cells to subthermal electric stimulation. PLoS ONE 2014, 9, e84636. [Google Scholar] [CrossRef]
- Baba, T.; Kameda, M.; Yasuhara, T.; Morimoto, T.; Kondo, A.; Shingo, T.; Tajiri, N.; Wang, F.; Miyoshi, Y.; Borlongan, C.V.; et al. Electrical stimulation of the cerebral cortex exerts antiapoptotic, angiogenic, and anti-inflammatory effects in ischemic stroke rats through phosphoinositide 3-kinase/Akt signaling pathway. Stroke 2009, 40, e598–e605. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.; Wang, F.; Guo, A.; Walczysko, P.; Gu, Y.; et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef]
- Zhao, M.; Penninger, J.; Isseroff, R.R. Electrical Activation of Wound-Healing Pathways. Adv. Skin Wound Care 2010, 1, 567–573. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.M.; Qiao, W.L.; Wang, L.; Zhang, J.F. Effects of hypothalamic paraventricular nuclei on apoptosis and proliferation of gastric mucosal cells induced by ischemia/reperfusion in rats. World J. Gastroenterol. 2007, 13, 874. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Z.; He, G.; Liu, J.; Feng, J. Electrical stimulation inhibits neointimal hyperplasia after abdominal aorta balloon injury through the PTEN/p27Kip1 pathway. Acta Biochim. Biophys. Sin. 2010, 42, 807–815. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Madras, G.; Basu, B. Intermittent electrical stimuli for guidance of human mesenchymal stem cell lineage commitment towards neural-like cells on electroconductive substrates. Biomaterials 2014, 35, 6219–6235. [Google Scholar] [CrossRef]
- Huang, J.; Hu, X.; Lu, L.; Ye, Z.; Zhang, Q.; Luo, Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J. Biomed. Mater. Res. A 2010, 93, 164–174. [Google Scholar] [CrossRef]
- Qi, F.; Wang, Y.; Ma, T.; Zhu, S.; Zeng, W.; Hu, X.; Liu, Z.; Huang, J.; Luo, Z. Electrical regulation of olfactory ensheathing cells using conductive polypyrrole/chitosan polymers. Biomaterials 2013, 34, 1799–1809. [Google Scholar] [CrossRef]
- Willand, M.P.; Rosa, E.; Michalski, B.; Zhang, J.J.; Gordon, T.; Fahnestock, M.; Borschel, G.H. Electrical muscle stimulation elevates intramuscular BDNF and GDNF mRNA following peripheral nerve injury and repair in rats. Neuroscience 2016, 334, 93–104. [Google Scholar] [CrossRef]
- Chang, Y.J.; Hsu, C.M.; Lin, C.H.; Lu, M.S.; Chen, L. Electrical stimulation promotes nerve growth factor-induced neurite outgrowth and signaling. Biochim. Biophys. Acta 2013, 1830, 4130–4136. [Google Scholar] [CrossRef]
- Islamov, R.; Bashirov, F.; Izmailov, A.; Fadeev, F.; Markosyan, V.; Sokolov, M.; Shmarov, M.; Logunov, D.; Naroditsky, B.; Lavrov, I. New Therapy for Spinal Cord Injury: Autologous Genetically-Enriched Leucoconcentrate Integrated with Epidural Electrical Stimulation. Cells 2022, 11, 144. [Google Scholar] [CrossRef]
- Wang, M.; Li, P.; Liu, M.; Song, W.; Wu, Q.; Fan, Y. Potential protective effect of biphasic electrical stimulation against growth factor-deprived apoptosis on olfactory bulb neural progenitor cells through the brain-derived neurotrophic factor-phosphatidylinositol 3′-kinase/Akt pathway. Exp. Biol. Med. 2013, 238, 951–959. [Google Scholar] [CrossRef]
- Huang, L.; Sun, X.; Wang, L.; Pei, G.; Wang, Y.; Zhang, Q.; Liang, Z.; Wang, D.; Fu, C.; He, C.; et al. Enhanced effect of combining bone marrow mesenchymal stem cells (BMMSCs) and pulsed electromagnetic fields (PEMF) to promote recovery after spinal cord injury in mice. MedComm 2022, 3, e160. [Google Scholar] [CrossRef]
- Amori, R.E.; Lau, J.; Pittas, A.G. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. JAMA 2007, 298, 194–206. [Google Scholar] [CrossRef]
- Linkov, G.; Branski, R.C.; Amin, M.; Chernichenko, N.; Chen, C.-H.; Alon, G.; Langmore, S.; Wong, R.J.; Kraus, D.H. Murine model of neuromuscular electrical stimulation on squamous cell carcinoma: Potential implications for dysphagia therapy. Head Neck 2012, 34, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Mamillapalli, R.; Gavrilova, N.; Mihaylova, V.T.; Tsvetkov, L.M.; Wu, H.; Zhang, H.; Sun, H. PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2). Curr. Biol. 2001, 11, 263–267. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Erickson, L.A.; Jin, L.; Kulig, E.; Qian, X.; Cheville, J.C.; Scheithauer, B.W. p27kip1: A multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am. J. Pathol. 1999, 154, 313–323. [Google Scholar] [CrossRef]
- Sebastian, A.; Syed, F.; McGrouther, D.A.; Colthurst, J.; Paus, R.; Bayat, A. A novel in vitro assay for electrophysiological research on human skin fibroblasts: Degenerate electrical waves downregulate collagen I expression in keloid fibroblasts. Exp. Dermatol. 2011, 20, 64–68. [Google Scholar] [CrossRef]
- Tuan, T.L.; Wu, H.; Huang, E.Y.; Chong, S.S.; Laug, W.; Messadi, D.; Kelly, P.; Le, A. Increased plasminogen activator inhibitor-1 in keloid fibroblasts may account for their elevated collagen accumulation in fibrin gel cultures. Am. J. Pathol. 2003, 162, 1579–1589. [Google Scholar] [CrossRef]
- Li, A.; Zhou, J.; Widelitz, R.B.; Chow, R.H.; Chuong, C.M. Integrating Bioelectrical Currents and Ca(2+) Signaling with Biochemical Signaling in Development and Pathogenesis. Bioelectricity 2020, 2, 210–220. [Google Scholar] [CrossRef]
- Hurt, K.J.; Musicki, B.; Palese, M.A.; Crone, J.K.; Becker, R.E.; Moriarity, J.L.; Snyder, S.H.; Burnett, A.L. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc. Natl. Acad. Sci. USA 2002, 99, 4061–4066. [Google Scholar] [CrossRef]
- Wolf-Goldberg, T.; Barbul, A.; Ben-Dov, N.; Korenstein, R. Low electric fields induce ligand-independent activation of EGF receptor and ERK via electrochemical elevation of H(+) and ROS concentrations. Biochim. Biophys. Acta 2013, 1833, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, S.; Kim, Y.S. Insight into Pain Modulation: Nociceptors Sensitization and Therapeutic Targets. Curr. Drug Targets 2019, 20, 775–788. [Google Scholar] [CrossRef]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef] [PubMed]

| Factors | Protein Names |
|---|---|
| Ca2+ Influx | Ca2+ Channels [1,2] |
| Signal transduction proteins to the nucleus | EGFR [3] ERK [4,5,6,7] PI3K [4,5,8,9] TRV2 [10] Atk [11] ERK1/2 [12] p38 MAP kinase [12,13] MAPK [14] JNK [13,15] |
| Cell adhesion and cytoskeletal proteins | Integrin α2β1 [16] FAK [17,18] Src [17,18] α-SMA [19] TUBB3 [20] cytokeratin-10 [21] |
| Neurotransmitter proteins | substance P [22] PGP (Protein Gene Product) 9.5 [22] |
| Apoptosis associated proteins | P53 [23] Bcl-2 [23] PCNA [24] HDM2 [24] SIVA1 [24] |
| Cytokines, Chemokines | IL6 [12] IL 8 [12] CXCR2 [25,26] CXCR4 [25,26] |
| Neurotrophic Factors | BDNF [29,30] GDNF [29] NGF [30,31] N-CAM [32] NOGO-A (neurite growth inhibitor) [33] PTEN (inhibit PI3kinase) [34] p27kip1 (cell cycle inhibitor) [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katoh, K. Effects of Electrical Stimulation of the Cell: Wound Healing, Cell Proliferation, Apoptosis, and Signal Transduction. Med. Sci. 2023, 11, 11. https://doi.org/10.3390/medsci11010011
Katoh K. Effects of Electrical Stimulation of the Cell: Wound Healing, Cell Proliferation, Apoptosis, and Signal Transduction. Medical Sciences. 2023; 11(1):11. https://doi.org/10.3390/medsci11010011
Chicago/Turabian StyleKatoh, Kazuo. 2023. "Effects of Electrical Stimulation of the Cell: Wound Healing, Cell Proliferation, Apoptosis, and Signal Transduction" Medical Sciences 11, no. 1: 11. https://doi.org/10.3390/medsci11010011
APA StyleKatoh, K. (2023). Effects of Electrical Stimulation of the Cell: Wound Healing, Cell Proliferation, Apoptosis, and Signal Transduction. Medical Sciences, 11(1), 11. https://doi.org/10.3390/medsci11010011







