Antioxidant Defense and Pseudoexfoliation Syndrome: An Updated Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
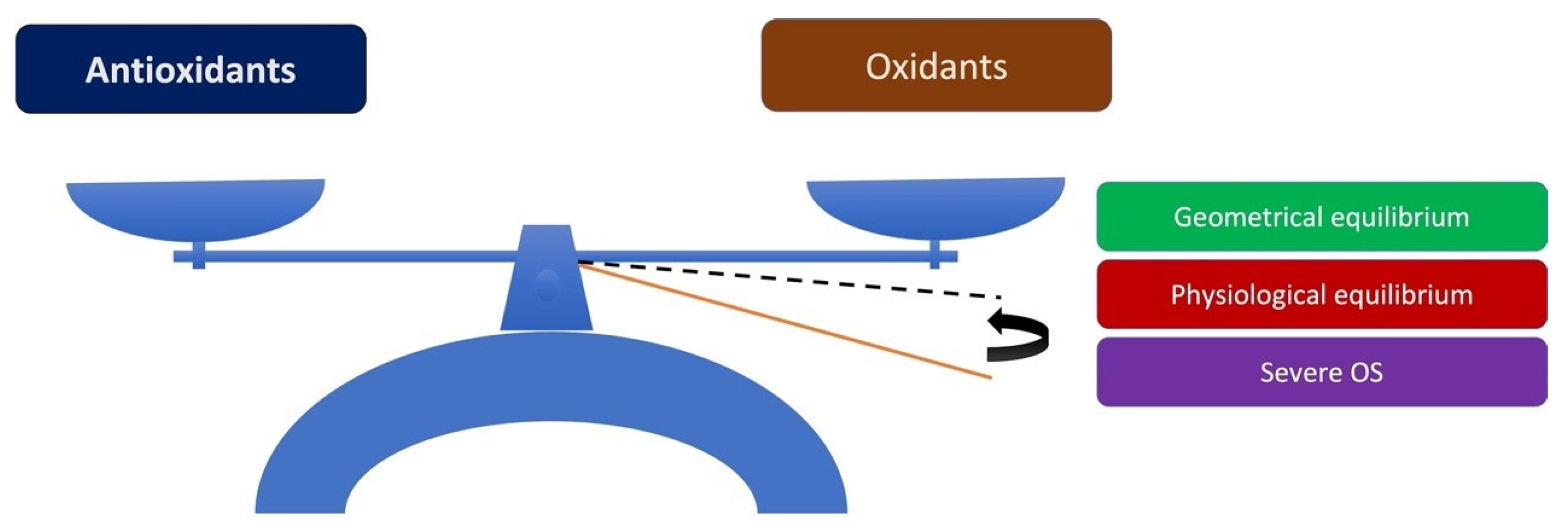
3.1. Disturbance of Antioxidant/Oxidant Equilibrium in the Pathogenesis of PEXS
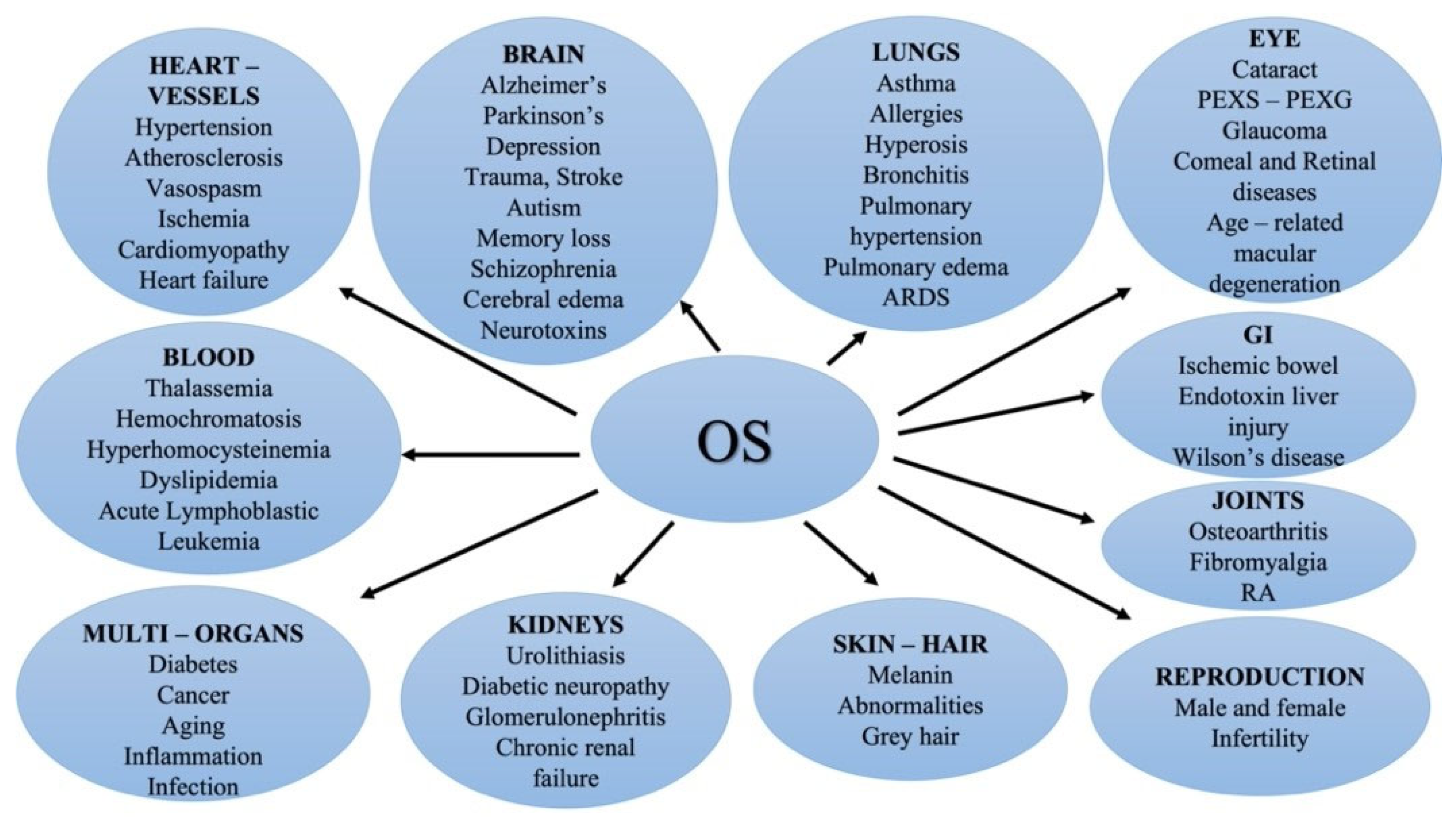
3.2. OS in the Eye
3.2.1. OS in the Lens
3.2.2. OS in the Lens Epithelium
3.2.3. OS in the AH
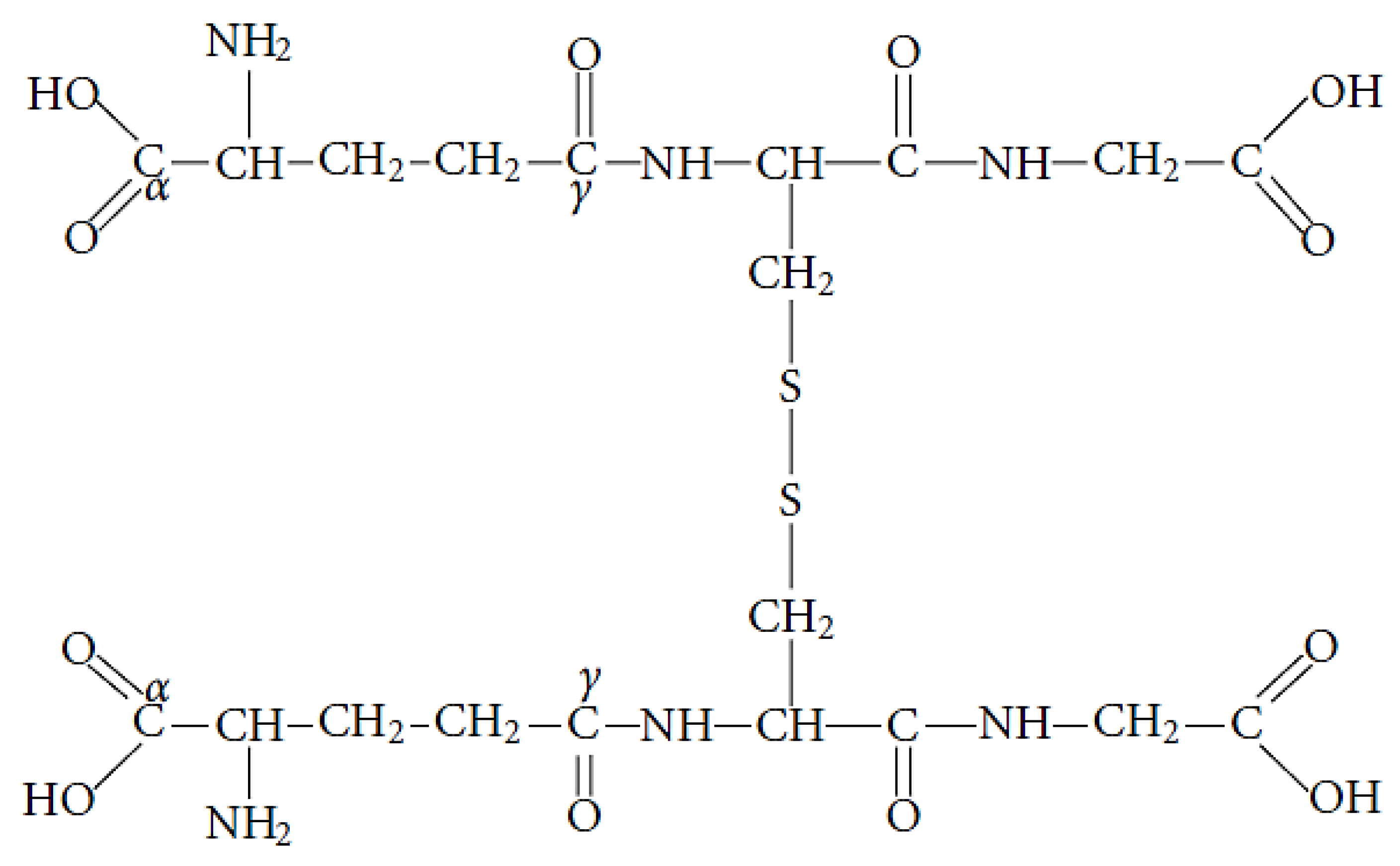
3.3. Antioxidant Defenses in the Eye
- Antioxidants in the crystalline lens: Environmental radiation usually does not harm the human lens due to its protective antioxidant system and chromophore. The lens has various protective and repair systems to deal with OS, mainly ascorbic acid and high levels of reduced GS. Lens also contains antioxidant enzymes, such as SOD, CAT, GPx, other antioxidants like the carotenoids named lutein and zeaxanthin, tocopherols, retinoids, and taurine [128]. In addition, α-crystalline, accounting for over 50% of the total protein mass of the mammalian lens, acts as a molecular chaperone that prevents heat-induced aggregation of many proteins and is also required for the renaturation of chemically denatured proteins. α-crystalline has been demonstrated to inhibit protein aggregation in vitro caused by UVR and OS. Thus, it is believed to protect in vivo lens proteins from photooxidation alterations. Finally, a transsulfuration pathway in the lens has been demonstrated and adjusted under OS conditions, offering different redox potential in cells. This pathway can be characterized as a new defense system against OS [128,129].
- Antioxidants in the crystalline lens epithelium: The lens epithelium has a wide range of antioxidant defense mechanisms, such as the antioxidant enzymes SOD, CAT and the GSH and Trx systems. GSH is also found at high levels in the lens and even higher in the lens epithelium. The total glutathione in a healthy lens epithelium is almost entirely in reduced form (GSH). Still, minimal, virtually undetectable, oxidized levels (GSSG) coexisted. In addition, the lens epithelium contains an active glutathione redox cycle, through which GR, NADPH and the hexose monophosphate shunt (HMPS) pathway efficiently reduce GSSG to GSH. The GSH system includes GPx, NADPH, GR, glutaredoxin (Grx) and GSH levels. The target molecules of the lens epithelial cells protected by the glutathione system are specific cytoskeletal proteins and proteins that maintain average membrane permeability and proteins containing critical sulfhydryl groups necessary for normal epithelial functions (e.g., Na⁺/K⁺-ATPase) [130]. The Trx system has a variety of biochemical processes, such as the detoxification of H2O2, regulation of cell death and activation of transcription factors that regulate cell growth and production of deoxyribonucleotides for DNA synthesis. Both systems can reduce protein disulfide bonds; Trx operates at the micromolar levels and GSH at the millimolar levels. Both systems act selectively on target proteins and metabolic pathways, but the Trx system regulates more proteins and pathways than GSH [131].
- Antioxidants in the AH: The human AH includes non-enzymatic antioxidants such as ascorbic acid (530 μM), L-tyrosine (78 μM), uric acid (43 μM), L-cysteine (14.3 μM) and GSH (5.5 μM). The AH in nocturnal species has a different composition, i.e., the nocturnal rat contains glutathione (125 μM) and L-cysteine (63 μM). The higher concentration of ascorbic acid in the AH of diurnal species compared to that of nocturnal species is a strong indication of the critical protective role of ascorbic acid against UVR. This protection is attributed to direct UVR absorption, fluorescence quenching of biomolecules and control of the fluorescence-mediated biotransformation [132]. L-tyrosine is the second most abundant water-soluble antioxidant in the human AH and is an OH• purifier, 1O2 quencher and weak photosensitiser. Uric acid is a purine derivative found in the lacrimal layer, AH and other extracellular fluids [133]. This water-soluble molecule has high activity against 1O2 and OH•, serving as a possible purifier. It has also been proposed to regulate the glutathione–ascorbic system’s redox status. L-cysteine replenishes its reservoir in the AH and acts as an antioxidant directly through the thiol group. Unlike other ocular tissues, AH contains minimal amounts of protein and antioxidant enzymes. The activity of SOD in human AH is minimal and probably does not contribute significantly to its overall antioxidant defense [132]. Thus, the defense of the AH is mainly based on the extremely high levels of low-molecular-weight antioxidants such as ascorbic acid. Specifically, the concentration of ascorbic acid in the AH is higher than that in the blood plasma [134].
3.3.1. Enzymatic Eye Antioxidants
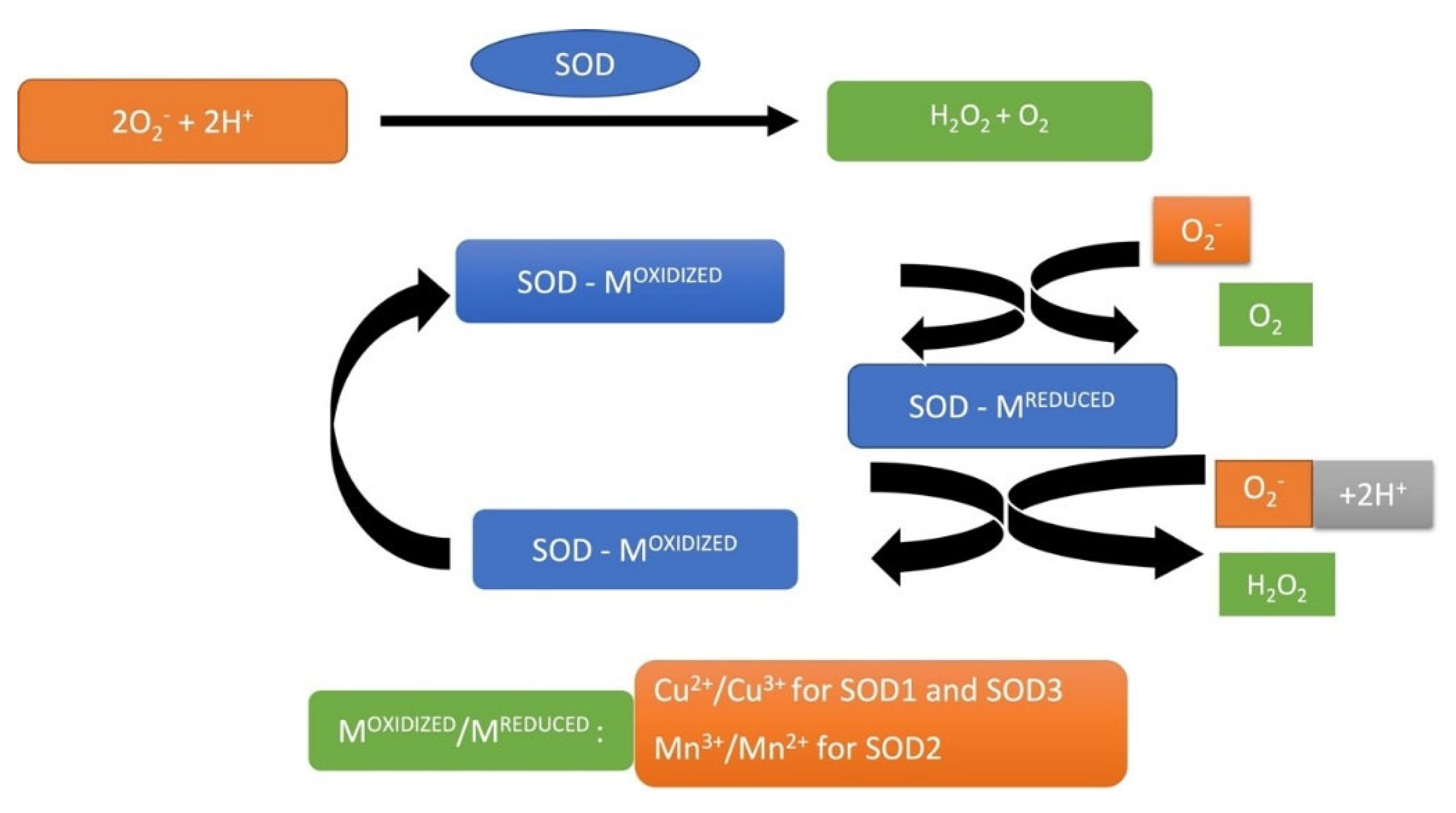
SOD
CAT
GPx
3.3.2. Non-Enzymatic Eye Antioxidants
Vitamin A
Vitamin E
Vitamin C
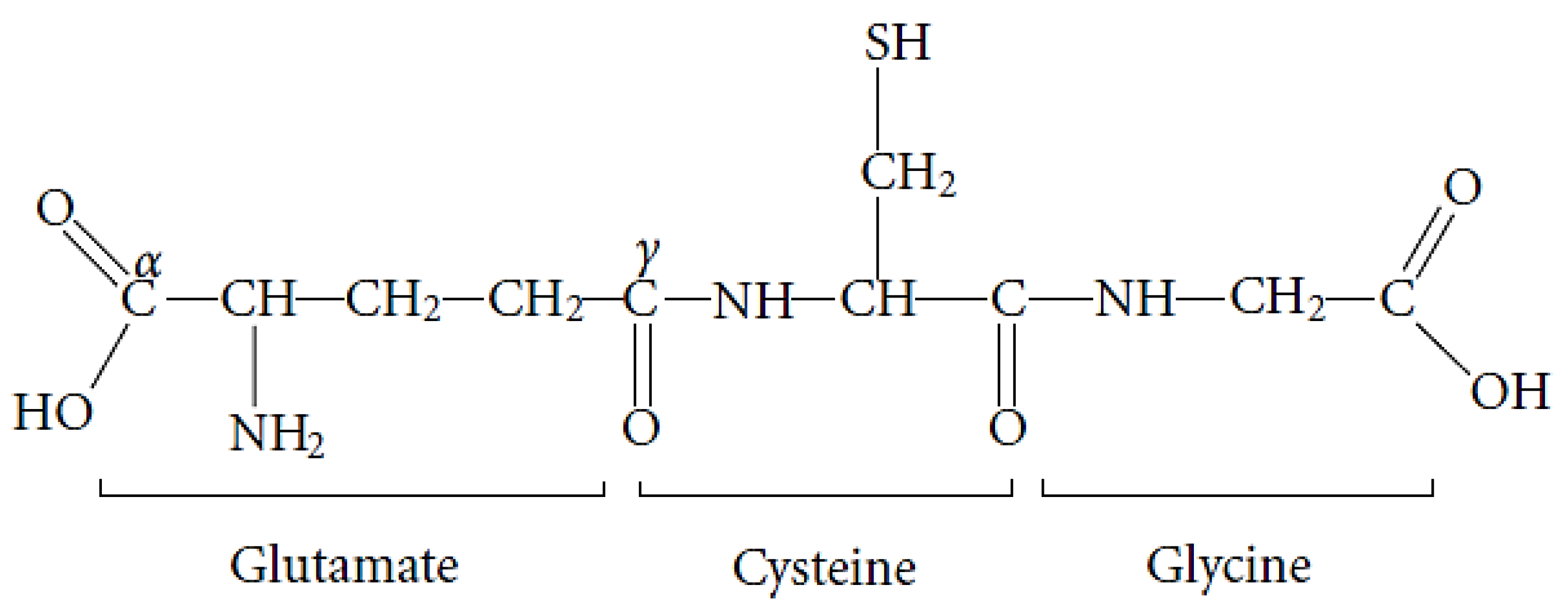
GSH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, D.P. Redefining Oxidative Stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Modulation of Reactive Oxygen Species in Health and Disease. Antioxidants 2019, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Meduri, A.; Grenga, P.L.; Scorolli, L.; Ceruti, P.; Ferreri, G. Role of Cysteine in Corneal Wound Healing after Photorefractive Keratectomy. Ophthalmic Res. 2009, 41, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Meduri, A.; Scorolli, L.; Scalinci, S.Z.; Grenga, P.L.; Lupo, S.; Rechichi, M.; Meduri, E. Effect of the Combination of Basic Fibroblast Growth Factor and Cysteine on Corneal Epithelial Healing after Photorefractive Keratectomy in Patients Affected by Myopia. Indian J. Ophthalmol. 2014, 62, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Meduri, A.; Scalinci, S.Z.; Morara, M.; Ceruti, P.; Grenga, P.L.; Zigiotti, G.L.; Scorolli, L. Effect of Basic Fibroblast Growth Factor in Transgenic Mice: Corneal Epithelial Healing Process after Excimer Laser Photoablation. Ophthalmologica 2009, 223, 139–144. [Google Scholar] [CrossRef]
- Scorolli, L.; Meduri, A.; Morara, M.; Scalinci, S.Z.; Greco, P.; Meduri, R.A.; Colombati, S. Effect of Cysteine in Transgenic Mice on Healing of Corneal Epithelium after Excimer Laser Photoablation. Ophthalmologica 2008, 222, 380–385. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. Oxidative Stress and Antioxidative Systems: Recipes for Successful Data Collection and Interpretation. Plant Cell Environ. 2016, 39, 1140–1160. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Naumann, G.O.H. Ocular and Systemic Pseudoexfoliation Syndrome. Am. J. Ophthalmol. 2006, 141, 921–937. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U. Oxidative stress and pseudoexfoliation glaucoma. Klin Monbl Augenheilkd 2010, 227, 108–113. [Google Scholar] [CrossRef]
- Zenkel, M.; Pöschl, E.; von der Mark, K.; Hofmann-Rummelt, C.; Naumann, G.O.H.; Kruse, F.E.; Schlötzer-Schrehardt, U. Differential Gene Expression in Pseudoexfoliation Syndrome. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3742–3752. [Google Scholar] [CrossRef]
- Zenkel, M.; Kruse, F.E.; Naumann, G.O.H.; Schlötzer-Schrehardt, U. Impaired Cytoprotective Mechanisms in Eyes with Pseudoexfoliation Syndrome/Glaucoma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5558–5566. [Google Scholar] [CrossRef]
- Mastronikolis, S.; Pagkalou, M.; Plotas, P.; Kagkelaris, K.; Georgakopoulos, C.D. Emerging Roles of Oxidative Stress in the Pathogenesis of Pseudoexfoliation Syndrome (Review). Exp. Ther. Med. 2022, 24, 602. [Google Scholar] [CrossRef]
- Kondkar, A.A.; Azad, T.A.; Sultan, T.; Radhakrishnan, R.; Osman, E.A.; Almobarak, F.A.; Lobo, G.P.; Al-Obeidan, S.A. Polymorphism Rs3742330 in MicroRNA Biogenesis Gene DICER1 Is Associated with Pseudoexfoliation Glaucoma in Saudi Cohort. Genes 2022, 13, 489. [Google Scholar] [CrossRef]
- Krumbiegel, M.; Pasutto, F.; Mardin, C.Y.; Weisschuh, N.; Paoli, D.; Gramer, E.; Zenkel, M.; Weber, B.H.F.; Kruse, F.E.; Schlötzer-Schrehardt, U.; et al. Exploring Functional Candidate Genes for Genetic Association in German Patients with Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2796–2801. [Google Scholar] [CrossRef][Green Version]
- Thorleifsson, G.; Magnusson, K.P.; Sulem, P.; Walters, G.B.; Gudbjartsson, D.F.; Stefansson, H.; Jonsson, T.; Jonasdottir, A.; Jonasdottir, A.; Stefansdottir, G.; et al. Common Sequence Variants in the LOXL1 Gene Confer Susceptibility to Exfoliation Glaucoma. Science 2007, 317, 1397–1400. [Google Scholar] [CrossRef]
- Młynarczyk, M.; Falkowska, M.; Micun, Z.; Obuchowska, I.; Kochanowicz, J.; Socha, K.; Konopińska, J. Diet, Oxidative Stress, and Blood Serum Nutrients in Various Types of Glaucoma: A Systematic Review. Nutrients 2022, 14, 1421. [Google Scholar] [CrossRef]
- Rosell-García, T.; Rivas-Muñoz, S.; Colige, A.; Rodriguez-Pascual, F. Cleavage of LOXL1 by BMP1 and ADAMTS14 Proteases Suggests a Role for Proteolytic Processing in the Regulation of LOXL1 Function. Int. J. Mol. Sci. 2022, 23, 3285. [Google Scholar] [CrossRef]
- Liton, P.B.; Gonzalez, P. Stress Response of the Trabecular Meshwork. J. Glaucoma 2008, 17, 378–385. [Google Scholar] [CrossRef]
- Maggio, M.; Guralnik, J.M.; Longo, D.L.; Ferrucci, L. Interleukin-6 in Aging and Chronic Disease: A Magnificent Pathway. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 575–584. [Google Scholar] [CrossRef]
- Zenkel, M.; Lewczuk, P.; Jünemann, A.; Kruse, F.E.; Naumann, G.O.H.; Schlötzer-Schrehardt, U. Proinflammatory Cytokines Are Involved in the Initiation of the Abnormal Matrix Process in Pseudoexfoliation Syndrome/Glaucoma. Am. J. Pathol. 2010, 176, 2868–2879. [Google Scholar] [CrossRef]
- Gottanka, J.; Flügel-Koch, C.; Martus, P.; Johnson, D.H.; Lütjen-Drecoll, E. Correlation of Pseudoexfoliative Material and Optic Nerve Damage in Pseudoexfoliation Syndrome. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2435–2446. [Google Scholar]
- Wang, H.; Kochevar, I.E. Involvement of UVB-Induced Reactive Oxygen Species in TGF-Beta Biosynthesis and Activation in Keratinocytes. Free Radic. Biol. Med. 2005, 38, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Pasutto, F.; Zenkel, M.; Hoja, U.; Berner, D.; Uebe, S.; Ferrazzi, F.; Schödel, J.; Liravi, P.; Ozaki, M.; Paoli, D.; et al. Pseudoexfoliation Syndrome-Associated Genetic Variants Affect Transcription Factor Binding and Alternative Splicing of LOXL1. Nat. Commun. 2017, 8, 15466. [Google Scholar] [CrossRef] [PubMed]
- Sein, J.; Galor, A.; Sheth, A.; Kruh, J.; Pasquale, L.R.; Karp, C.L. Exfoliation Syndrome: New Genetic and Pathophysiologic Insights. Curr. Opin. Ophthalmol. 2013, 24, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Aydın Yaz, Y.; Yıldırım, N.; Yaz, Y.; Tekin, N.; İnal, M.; Şahin, F.M. Role of Oxidative Stress in Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. Turk. J. Ophthalmol. 2019, 49, 61–67. [Google Scholar] [CrossRef]
- Gartaganis, S.P.; Patsoukis, N.E.; Nikolopoulos, D.K.; Georgiou, C.D. Evidence for Oxidative Stress in Lens Epithelial Cells in Pseudoexfoliation Syndrome. Eye 2007, 21, 1406–1411. [Google Scholar] [CrossRef]
- Tetikoğlu, M.; Sağdik, H.M.; Aktas, S.; Uçar, F.; Özcura, F. Serum Prolidase Activity and Oxidative Stress in Patients with Pseudoexfoliation Syndrome. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1339–1343. [Google Scholar] [CrossRef]
- Fan Gaskin, J.C.; Shah, M.H.; Chan, E.C. Oxidative Stress and the Role of NADPH Oxidase in Glaucoma. Antioxidants 2021, 10, 238. [Google Scholar] [CrossRef]
- Koliakos, G.G.; Konstas, A.G.P.; Schlötzer-Schrehardt, U.; Hollo, G.; Katsimbris, I.E.; Georgiadis, N.; Ritch, R. 8-Isoprostaglandin F2a and Ascorbic Acid Concentration in the Aqueous Humour of Patients with Exfoliation Syndrome. Br. J. Ophthalmol. 2003, 87, 353–356. [Google Scholar] [CrossRef][Green Version]
- Al-Ghananeem, A.M.; Crooks, P.A. Phase I and Phase II Ocular Metabolic Activities and the Role of Metabolism in Ophthalmic Prodrug and Codrug Design and Delivery. Molecules 2007, 12, 373–388. [Google Scholar] [CrossRef]
- CAT-152 0102 Trabeculectomy Study Group; Khaw, P.; Grehn, F.; Holló, G.; Overton, B.; Wilson, R.; Vogel, R.; Smith, Z. A Phase III Study of Subconjunctival Human Anti-Transforming Growth Factor Beta(2) Monoclonal Antibody (CAT-152) to Prevent Scarring after First-Time Trabeculectomy. Ophthalmology 2007, 114, 1822–1830. [Google Scholar] [CrossRef]
- Hyman, M.; Pizzorno, J.; Weil, A. A Rational Approach to Antioxidant Therapy and Vitamin E. Altern. Ther. Health Med. 2005, 11, 14–17. [Google Scholar]
- Botling Taube, A.; Konzer, A.; Alm, A.; Bergquist, J. Proteomic Analysis of the Aqueous Humour in Eyes with Pseudoexfoliation Syndrome. Br. J. Ophthalmol. 2019, 103, 1190–1194. [Google Scholar] [CrossRef]
- Yagci, R.; Ersöz, I.; Erdurmuş, M.; Gürel, A.; Duman, S. Protein Carbonyl Levels in the Aqueous Humour and Serum of Patients with Pseudoexfoliation Syndrome. Eye 2008, 22, 128–131. [Google Scholar] [CrossRef]
- Borazan, M.; Karalezli, A.; Kucukerdonmez, C.; Bayraktar, N.; Kulaksizoglu, S.; Akman, A.; Akova, Y.A. Aqueous Humor and Plasma Levels of Vascular Endothelial Growth Factor and Nitric Oxide in Patients with Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. J. Glaucoma 2010, 19, 207–211. [Google Scholar] [CrossRef]
- Mikropoulos, D.G.; Mallini, P.; Michopoulou, A.; Giannopoulos, T.; Arranz-Marquez, E.; Koliakos, G.G.; Konstas, A.G.P. Asymmetric Dimethyloarginin (ADMA) Concentration in the Aqueous Humor of Patients with Exfoliation Syndrome or Exfoliative Glaucoma. Curr. Eye Res. 2013, 38, 266–270. [Google Scholar] [CrossRef]
- Browne, J.G.; Ho, S.L.; Kane, R.; Oliver, N.; Clark, A.F.; O’Brien, C.J.; Crean, J.K. Connective Tissue Growth Factor Is Increased in Pseudoexfoliation Glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3660–3666. [Google Scholar] [CrossRef]
- Zenkel, M.; Hoja, U.; Gießl, A.; Berner, D.; Hohberger, B.; Weller, J.M.; König, L.; Hübner, L.; Ostermann, T.A.; Gusek-Schneider, G.C.; et al. Dysregulated Retinoic Acid Signaling in the Pathogenesis of Pseudoexfoliation Syndrome. Int. J. Mol. Sci. 2022, 23, 5977. [Google Scholar] [CrossRef]
- Park, D.Y.; Kim, M.; Cha, S.C. Cytokine and Growth Factor Analysis in Exfoliation Syndrome and Glaucoma. Investig. Ophthalmol. Vis. Sci. 2021, 62, 6. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Kondkar, A.A.; Mousa, A.; Osman, E.A.; Al-Obeidan, S.A. Decreased Total Antioxidants Status in the Plasma of Patients with Pseudoexfoliation Glaucoma. Mol Vis 2011, 17, 2769–2775. [Google Scholar]
- Demirdögen, B.C.; Ceylan, O.M.; Işikoğlu, S.; Mumcuoğlu, T.; Erel, O. Evaluation of Oxidative Stress and Paraoxonase Phenotypes in Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma. Clin. Lab. 2014, 60, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ozates, S.; Elgin, K.U.; Yilmaz, N.S.; Demirel, O.O.; Sen, E.; Yilmazbas, P. Evaluation of Oxidative Stress in Pseudo-Exfoliative Glaucoma Patients Treated with and without Topical Coenzyme Q10 and Vitamin E. Eur. J. Ophthalmol. 2019, 29, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Ayaz, L.; Tamer, L. Selenium and Pseudoexfoliation Syndrome. Am. J. Ophthalmol. 2011, 151, 272–276.e1. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Status of Systemic Oxidative Stresses in Patients with Primary Open-Angle Glaucoma and Pseudoexfoliation Syndrome. PLoS ONE 2012, 7, e49680. [Google Scholar] [CrossRef] [PubMed]
- Behndig, A.; Svensson, B.; Marklund, S.L.; Karlsson, K. Superoxide Dismutase Isoenzymes in the Human Eye. Investig. Ophthalmol. Vis. Sci. 1998, 39, 471–475. [Google Scholar]
- Yağci, R.; Gürel, A.; Ersöz, I.; Karadağ, R.; Hepşen, I.F.; Duman, S. The Activities of Paraoxonase, Xanthine Oxidase, Adenosine Deaminase and the Level of Nitrite in Pseudoexfoliation Syndrome. Ophthalmic Res. 2009, 42, 155–159. [Google Scholar] [CrossRef]
- Tetikoğlu, M.; Aktas, S.; Sağdik, H.M.; Özcura, F.; Uçar, F.; Koçak, H.; Neşelioğlu, S.; Erel, Ö. Thiol Disulfide Homeostasis in Pseudoexfoliation Syndrome. Curr. Eye Res. 2017, 42, 876–879. [Google Scholar] [CrossRef]
- Cetinkaya, E.; Duman, R.; Sabaner, M.C.; Erol, M.A.; Duman, R.; Nural, C.; Erel, O. Evaluation of Thiol-Disulfide Homeostasis in Pseudoexfoliation Glaucoma and Primary Open-Angle Glaucoma. Niger. J. Clin. Pract. 2020, 23, 1401–1406. [Google Scholar] [CrossRef]
- Paulson, C.; Thomas, S.C.; Gonzalez, O.; Taylor, S.; Swiston, C.; Herrick, J.S.; McCoy, L.; Curtin, K.; Chaya, C.J.; Stagg, B.C.; et al. Exfoliation Syndrome in Baja Verapaz Guatemala: A Cross-Sectional Study and Review of the Literature. J. Clin. Med. 2022, 11, 1795. [Google Scholar] [CrossRef]
- Hicks, P.M.; Au, E.; Self, W.; Haaland, B.; Feehan, M.; Owen, L.A.; Siedlecki, A.; Nuttall, E.; Harrison, D.; Reynolds, A.L.; et al. Pseudoexfoliation and Cataract Syndrome Associated with Genetic and Epidemiological Factors in a Mayan Cohort of Guatemala. Int. J. Environ. Res. Public Health 2021, 18, 7231. [Google Scholar] [CrossRef]
- Patil, A.; Swiston, C.; Wallace, R.T.; Paulson, C.; Conley, M.E.; McCoy, L.; Chaya, C.; Wirostko, B. Exfoliation Syndrome and Exfoliation Glaucoma in the Navajo Nation. Vision 2022, 6, 61. [Google Scholar] [CrossRef]
- Türkcü, F.M.; Köz, O.G.; Yarangümeli, A.; Oner, V.; Kural, G. Plasma Homocysteine, Folic Acid, and Vitamin B₁₂ Levels in Patients with Pseudoexfoliation Syndrome, Pseudoexfoliation Glaucoma, and Normotensive Glaucoma. Medicina 2013, 49, 214–218. [Google Scholar] [CrossRef]
- Arnarsson, A.; Damji, K.F.; Sasaki, H.; Sverrisson, T.; Jonasson, F. Pseudoexfoliation in the Reykjavik Eye Study: Five-Year Incidence and Changes in Related Ophthalmologic Variables. Am. J. Ophthalmol. 2009, 148, 291–297. [Google Scholar] [CrossRef]
- Romeo Villadóniga, S.; Rodríguez García, E.; Sagastagoia Epelde, O.; Álvarez Díaz, M.D.; Domingo Pedrol, J.C. Effects of Oral Supplementation with Docosahexaenoic Acid (DHA) plus Antioxidants in Pseudoexfoliative Glaucoma: A 6-Month Open-Label Randomized Trial. J. Ophthalmol. 2018, 2018, 8259371. [Google Scholar] [CrossRef]
- Dursun, F.; Vural Ozec, A.; Aydin, H.; Topalkara, A.; Dursun, A.; Toker, M.I.; Erdogan, H.; Arici, M.K. Total Oxidative Stress, Paraoxonase and Arylesterase Levels at Patients with Pseudoexfoliation Syndrome and Pseudoexfoliative Glaucoma. Int. J. Ophthalmol. 2015, 8, 985–990. [Google Scholar] [CrossRef]
- Kang, J.H.; Loomis, S.J.; Wiggs, J.L.; Willett, W.C.; Pasquale, L.R. A Prospective Study of Folate, Vitamin B₆, and Vitamin B₁₂ Intake in Relation to Exfoliation Glaucoma or Suspected Exfoliation Glaucoma. JAMA Ophthalmol. 2014, 132, 549–559. [Google Scholar] [CrossRef]
- Puska, P.M. Unilateral Exfoliation Syndrome: Conversion to Bilateral Exfoliation and to Glaucoma: A Prospective 10-Year Follow-up Study. J. Glaucoma 2002, 11, 517–524. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.M.; Koca, M.R.; Naumann, G.O.; Volkholz, H. Pseudoexfoliation Syndrome. Ocular Manifestation of a Systemic Disorder? Arch. Ophthalmol. 1992, 110, 1752–1756. [Google Scholar] [CrossRef]
- Hammer, T.; Schlötzer-Schrehardt, U.; Naumann, G.O. Unilateral or Asymmetric Pseudoexfoliation Syndrome? An Ultrastructural Study. Arch. Ophthalmol. 2001, 119, 1023–1031. [Google Scholar] [CrossRef]
- Plateroti, P.; Plateroti, A.M.; Abdolrahimzadeh, S.; Scuderi, G. Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma: A Review of the Literature with Updates on Surgical Management. J. Ophthalmol. 2015, 2015, 370371. [Google Scholar] [CrossRef]
- Cabrera, M.P.; Chihuailaf, R.H. Antioxidants and the Integrity of Ocular Tissues. Vet. Med. Int. 2011, 2011, 905153. [Google Scholar] [CrossRef] [PubMed]
- Imaz Aristimuño, N.; Rodriguez Agirretxe, I.; San Vicente Blanco, R.; Rotaeche Del Campo, R.; Mendicute Del Barrio, J. Comparison of Cardiovascular Risk and Events among Spanish Patients with and without Ocular Pseudoexfoliation. J. Clin. Med. 2022, 11, 2153. [Google Scholar] [CrossRef] [PubMed]
- Berner, D.; Hoja, U.; Zenkel, M.; Ross, J.J.; Uebe, S.; Paoli, D.; Frezzotti, P.; Rautenbach, R.M.; Ziskind, A.; Williams, S.E.; et al. The Protective Variant Rs7173049 at LOXL1 Locus Impacts on Retinoic Acid Signaling Pathway in Pseudoexfoliation Syndrome. Hum. Mol. Genet. 2019, 28, 2531–2548. [Google Scholar] [CrossRef] [PubMed]
- Benoist d’Azy, C.; Pereira, B.; Chiambaretta, F.; Dutheil, F. Oxidative and Anti-Oxidative Stress Markers in Chronic Glaucoma: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166915. [Google Scholar] [CrossRef] [PubMed]
- Faschinger, C.; Schmut, O.; Wachswender, C.; Mossböck, G. Glaucoma and oxidative stress. Determination of malondialdehyde—A product of lipid peroxidation. Ophthalmologe 2006, 103, 953–959. [Google Scholar] [CrossRef]
- Shirakami, T.; Yamanaka, M.; Fujihara, J.; Matsuoka, Y.; Gohto, Y.; Obana, A.; Tanito, M. Advanced Glycation End Product Accumulation in Subjects with Open-Angle Glaucoma with and without Exfoliation. Antioxidants 2020, 9, 755. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, J.W. Effect of Advanced Glycation End Products on Oxidative Stress and Senescence of Trabecular Meshwork Cells. Korean J. Ophthalmol. 2012, 26, 123–131. [Google Scholar] [CrossRef]
- Dembski, M.; Nowińska, A.; Ulfik-Dembska, K.; Wylęgała, E. Swept Source Optical Coherence Tomography Analysis of a Selected Eye’s Anterior Segment Parameters in Patients with Pseudoexfoliation Syndrome. J. Clin. Med. 2022, 11, 268. [Google Scholar] [CrossRef]
- Chakraborty, M.; Sahay, P.; Rao, A. Primary Human Trabecular Meshwork Model for Pseudoexfoliation. Cells 2021, 10, 3448. [Google Scholar] [CrossRef]
- Voloshenyuk, T.G.; Hart, A.D.; Khoutorova, E.; Gardner, J.D. TNF-α Increases Cardiac Fibroblast Lysyl Oxidase Expression through TGF-β and PI3Kinase Signaling Pathways. Biochem. Biophys. Res. Commun. 2011, 413, 370–375. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X.; Cai, J. Proteomic Identification of Oxidatively Modified Retinal Proteins in a Chronic Pressure-Induced Rat Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3177–3187. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Rezania, F.; Trounce, I.A.; Crowston, J.G. Oxidative Stress and Mitochondrial Dysfunction in Glaucoma. Curr. Opin. Pharmacol. 2013, 13, 12–15. [Google Scholar] [CrossRef]
- Izzotti, A.; Bagnis, A.; Saccà, S.C. The Role of Oxidative Stress in Glaucoma. Mutat. Res. 2006, 612, 105–114. [Google Scholar] [CrossRef]
- Sarenac Vulovic, T.S.; Pavlovic, S.M.; Jakovljevic, V.L.; Janicijevic, K.B.; Zdravkovic, N.S. Nitric Oxide and Tumour Necrosis Factor Alpha in the Process of Pseudoexfoliation Glaucoma. Int. J. Ophthalmol. 2016, 9, 1138–1142. [Google Scholar] [CrossRef]
- Sekeroglu, M.A.; Irkec, M.; Mocan, M.C.; Ileri, E.; Dikmenoglu, N.; Seringec, N.; Karaosmanoglu, D.; Orhan, M. The Association of Ocular Blood Flow with Haemorheological Parameters in Primary Open-Angle and Exfoliative Glaucoma. Acta Ophthalmol. 2011, 89, 429–434. [Google Scholar] [CrossRef]
- Sorkhabi, R.; Ghorbanihaghjo, A.; Ahoor, M.; Nahaei, M.; Rashtchizadeh, N. High-Sensitivity C-Reactive Protein and Tumor Necrosis Factor Alpha in Pseudoexfoliation Syndrome. Oman Med. J. 2013, 28, 16–19. [Google Scholar] [CrossRef]
- Paroni, R.; Fermo, I.; Fiorina, P.; Cighetti, G. Determination of Asymmetric and Symmetric Dimethylarginines in Plasma of Hyperhomocysteinemic Subjects. Amino Acids 2005, 28, 389–394. [Google Scholar] [CrossRef]
- Koukoula, S.C.; Katsanos, A.; Tentes, I.K.; Labiris, G.; Kozobolis, V.P. Retrobulbar Hemodynamics and Aqueous Humor Levels of Endothelin-1 in Exfoliation Syndrome and Exfoliation Glaucoma. Clin. Ophthalmol. 2018, 12, 1199–1204. [Google Scholar] [CrossRef]
- Haefliger, I.O.; Dettmann, E.; Liu, R.; Meyer, P.; Prünte, C.; Messerli, J.; Flammer, J. Potential Role of Nitric Oxide and Endothelin in the Pathogenesis of Glaucoma. Surv. Ophthalmol. 1999, 43 (Suppl. 1), S51–S58. [Google Scholar] [CrossRef]
- Sen, C.K. Cellular Thiols and Redox-Regulated Signal Transduction. Curr. Top. Cell Regul. 2000, 36, 1–30. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The Thiol Pool in Human Plasma: The Central Contribution of Albumin to Redox Processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Rossi, R.; Milzani, A.; Colombo, R.; Dalle-Donne, I. S-Glutathionylation: From Redox Regulation of Protein Functions to Human Diseases. J. Cell Mol. Med. 2004, 8, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Gartaganis, S.P.; Georgakopoulos, C.D.; Patsoukis, N.E.; Gotsis, S.S.; Gartaganis, V.S.; Georgiou, C.D. Glutathione and Lipid Peroxide Changes in Pseudoexfoliation Syndrome. Curr. Eye Res. 2005, 30, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Strzalka-Mrozik, B.; Prudlo, L.; Kimsa, M.W.; Kimsa, M.C.; Kapral, M.; Nita, M.; Mazurek, U. Quantitative Analysis of SOD2, ALDH1A1 and MGST1 Messenger Ribonucleic Acid in Anterior Lens Epithelium of Patients with Pseudoexfoliation Syndrome. Mol. Vis. 2013, 19, 1341–1349. [Google Scholar] [PubMed]
- Johansson, K.; Järvliden, J.; Gogvadze, V.; Morgenstern, R. Multiple Roles of Microsomal Glutathione Transferase 1 in Cellular Protection: A Mechanistic Study. Free Radic. Biol. Med. 2010, 49, 1638–1645. [Google Scholar] [CrossRef]
- Gasińska, K.; Czop, M.; Kosior-Jarecka, E.; Wróbel-Dudzińska, D.; Kocki, J.; Żarnowski, T. Small Nucleolar RNAs in Pseudoexfoliation Glaucoma. Cells 2022, 11, 2738. [Google Scholar] [CrossRef]
- Tomczyk-Socha, M.; Kręcicka, J.; Misiuk-Hojło, M.; Turno-Kręcicka, A. MicroRNA Expression in Pseudoexfoliation Syndrome with the Use of Next-Generation Sequencing. Genes 2022, 13, 582. [Google Scholar] [CrossRef]
- Stafiej, J.; Hałas-Wiśniewska, M.; Izdebska, M.; Gagat, M.; Grzanka, D.; Grzanka, A.; Malukiewicz, G. Immunohistochemical Analysis of Microsomal Glutathione S-Transferase 1 and Clusterin Expression in Lens Epithelial Cells of Patients with Pseudoexfoliation Syndrome. Exp. Ther. Med. 2017, 13, 1057–1063. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Koppaka, V.; Thompson, D.C.; Vasiliou, V. Focus on Molecules: ALDH1A1: From Lens and Corneal Crystallin to Stem Cell Marker. Exp. Eye Res. 2012, 102, 105–106. [Google Scholar] [CrossRef]
- Choudhary, S.; Xiao, T.; Vergara, L.A.; Srivastava, S.; Nees, D.; Piatigorsky, J.; Ansari, N.H. Role of Aldehyde Dehydrogenase Isozymes in the Defense of Rat Lens and Human Lens Epithelial Cells against Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2005, 46, 259–267. [Google Scholar] [CrossRef]
- Koliakos, G.G.; Befani, C.D.; Mikropoulos, D.; Ziakas, N.G.; Konstas, A.G.P. Prooxidant-Antioxidant Balance, Peroxide and Catalase Activity in the Aqueous Humour and Serum of Patients with Exfoliation Syndrome or Exfoliative Glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1477–1483. [Google Scholar] [CrossRef]
- Can Demirdöğen, B.; Koçan Akçin, C.; Göksoy, E.; Yakar, G.; Öztepe, T.; Demirkaya-Budak, S.; Oflaz, S. Paraoxonase 1 (PON1) Promoter (-107T/C) and Coding Region (192Q/R and 55L/M) Genetic Variations in Pseudoexfoliation Syndrome and Pseudoexfoliative Glaucoma Risk. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2257–2270. [Google Scholar] [CrossRef]
- Simavli, H.; Tosun, M.; Bucak, Y.Y.; Erdurmus, M.; Ocak, Z.; Onder, H.I.; Acar, M. Serum and Aqueous Xanthine Oxidase Levels, and MRNA Expression in Anterior Lens Epithelial Cells in Pseudoexfoliation. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1161–1167. [Google Scholar] [CrossRef]
- Turan, G.; Turan, M. The Evaluation of TUNEL, PCNA and SOX2 Expressions in Lens Epithelial Cells of Cataract Patients with Pseudoexfoliation Syndrome. Curr. Eye Res. 2020, 45, 12–16. [Google Scholar] [CrossRef]
- Dmuchowska, D.A.; Pietrowska, K.; Krasnicki, P.; Kowalczyk, T.; Misiura, M.; Grochowski, E.T.; Mariak, Z.; Kretowski, A.; Ciborowski, M. Metabolomics Reveals Differences in Aqueous Humor Composition in Patients with and without Pseudoexfoliation Syndrome. Front. Mol. Biosci. 2021, 8, 682600. [Google Scholar] [CrossRef]
- Shoham, A.; Hadziahmetovic, M.; Dunaief, J.L.; Mydlarski, M.B.; Schipper, H.M. Oxidative Stress in Diseases of the Human Cornea. Free Radic. Biol. Med. 2008, 45, 1047–1055. [Google Scholar] [CrossRef]
- Cejka, C.; Cejkova, J. Oxidative Stress to the Cornea, Changes in Corneal Optical Properties, and Advances in Treatment of Corneal Oxidative Injuries. Oxid. Med. Cell Longev. 2015, 2015, 591530. [Google Scholar] [CrossRef]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant Defenses in the Ocular Surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef]
- Cai, C.X.; Birk, D.E.; Linsenmayer, T.F. Nuclear Ferritin Protects DNA From UV Damage in Corneal Epithelial Cells. Mol. Biol. Cell 1998, 9, 1037–1051. [Google Scholar] [CrossRef]
- Sacca, S.C.; Bolognesi, C.; Battistella, A.; Bagnis, A.; Izzotti, A. Gene-Environment Interactions in Ocular Diseases. Mutat. Res. 2009, 667, 98–117. [Google Scholar] [CrossRef]
- Hicks, P.M.; Siedlecki, A.; Haaland, B.; Owen, L.A.; Au, E.; Feehan, M.; Murtaugh, M.A.; Sieminski, S.; Reynolds, A.; Lillvis, J.; et al. A Global Genetic Epidemiological Review of Pseudoexfoliation Syndrome. Explor. Med. 2021, 2, 527–543. [Google Scholar] [CrossRef]
- Aström, S.; Lindén, C. Incidence and Prevalence of Pseudoexfoliation and Open-Angle Glaucoma in Northern Sweden: I. Baseline Report. Acta Ophthalmol. Scand. 2007, 85, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Ritch, R.; Schlötzer-Schrehardt, U. Exfoliation (Pseudoexfoliation) Syndrome: Toward a New Understanding: Proceedings of the First International Think Tank. Acta Ophthalmol. Scand. 2001, 79, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, N.; Yasar, E.; Gursoy, H.; Colak, E. Prevalence of Pseudoexfoliation Syndrome and Its Association with Ocular and Systemic Diseases in Eskisehir, Turkey. Int. J. Ophthalmol. 2017, 10, 128–134. [Google Scholar] [CrossRef]
- Beebe, D.C.; Holekamp, N.M.; Shui, Y.-B. Oxidative Damage and the Prevention of Age-Related Cataracts. Ophthalmic Res. 2010, 44, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, V.M.; Beyer, E.C. Oxidative Stress, Lens Gap Junctions, and Cataracts. Antioxid. Redox Signal 2009, 11, 339–353. [Google Scholar] [CrossRef]
- Bodaness, R.S.; Leclair, M.; Zigler, J.S. An Analysis of the H2O2-Mediated Crosslinking of Lens Crystallins Catalyzed by the Heme-Undecapeptide from Cytochrome c. Arch. Biochem. Biophys. 1984, 231, 461–469. [Google Scholar] [CrossRef]
- Zigler, J.S.; Huang, Q.L.; Du, X.Y. Oxidative Modification of Lens Crystallins by H2O2 and Chelated Iron. Free Radic. Biol. Med. 1989, 7, 499–505. [Google Scholar] [CrossRef]
- McNamara, M.; Augusteyn, R.C. The Effects of Hydrogen Peroxide on Lens Proteins: A Possible Model for Nuclear Cataract. Exp. Eye Res. 1984, 38, 45–56. [Google Scholar] [CrossRef]
- Rose, R.C.; Richer, S.P.; Bode, A.M. Ocular Oxidants and Antioxidant Protection. Proc. Soc. Exp. Biol. Med. 1998, 217, 397–407. [Google Scholar] [CrossRef]
- Saxena, P.; Saxena, A.K.; Cui, X.L.; Obrenovich, M.; Gudipaty, K.; Monnier, V.M. Transition Metal-Catalyzed Oxidation of Ascorbate in Human Cataract Extracts: Possible Role of Advanced Glycation End Products. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1473–1481. [Google Scholar]
- Ozaki, Y.; Mizuno, A.; Itoh, K.; Iriyama, K. Inter- and Intramolecular Disulfide Bond Formation and Related Structural Changes in the Lens Proteins. A Raman Spectroscopic Study in Vivo of Lens Aging. J. Biol. Chem. 1987, 262, 15545–15551. [Google Scholar] [CrossRef]
- Dillon, J.; Zheng, L.; Merriam, J.C.; Gaillard, E.R. The Optical Properties of the Anterior Segment of the Eye: Implications for Cortical Cataract. Exp. Eye Res. 1999, 68, 785–795. [Google Scholar] [CrossRef]
- Dairou, J.; Malecaze, F.; Dupret, J.-M.; Rodrigues-Lima, F. The Xenobiotic-Metabolizing Enzymes Arylamine N-Acetyltransferases in Human Lens Epithelial Cells: Inactivation by Cellular Oxidants and UVB-Induced Oxidative Stress. Mol. Pharmacol. 2005, 67, 1299–1306. [Google Scholar] [CrossRef]
- Reddan, J.R.; Steiger, C.A.; Dziedzic, D.C.; Gordon, S.R. Regional Differences in the Distribution of Catalase in the Epithelium of the Ocular Lens. Cell. Mol. Biol. 1996, 42, 209–219. [Google Scholar]
- Hosler, M.R.; Wang-Su, S.-T.; Wagner, B.J. Targeted Disruption of Specific Steps of the Ubiquitin-Proteasome Pathway by Oxidation in Lens Epithelial Cells. Int. J. Biochem. Cell Biol. 2003, 35, 685–697. [Google Scholar] [CrossRef]
- Giblin, F.J.; McCready, J.P.; Schrimscher, L.; Reddy, V.N. Peroxide-Induced Effects on Lens Cation Transport Following Inhibition of Glutathione Reductase Activity in Vitro. Exp. Eye Res. 1987, 45, 77–91. [Google Scholar] [CrossRef]
- Rogers, C.S.; Chan, L.-M.; Sims, Y.S.; Byrd, K.D.; Hinton, D.L.; Twining, S.S. The Effects of Sub-Solar Levels of UV-A and UV-B on Rabbit Corneal and Lens Epithelial Cells. Exp. Eye Res. 2004, 78, 1007–1014. [Google Scholar] [CrossRef]
- Long, A.C.; Colitz, C.M.H.; Bomser, J.A. Apoptotic and Necrotic Mechanisms of Stress-Induced Human Lens Epithelial Cell Death. Exp. Biol. Med. 2004, 229, 1072–1080. [Google Scholar] [CrossRef]
- Cejková, J.; Stípek, S.; Crkovská, J.; Ardan, T.; Pláteník, J.; Cejka, C.; Midelfart, A. UV Rays, the Prooxidant/Antioxidant Imbalance in the Cornea and Oxidative Eye Damage. Physiol. Res. 2004, 53, 1–10. [Google Scholar] [CrossRef]
- Barros, P.S.M.; Padovani, C.F.; Silva, V.V.; Queiroz, L.; Barros, S.B.M. Antioxidant Status of Dog Aqueous Humor after Extracapsular Lens Extraction. Braz. J. Med. Biol. Res. 2003, 36, 1491–1494. [Google Scholar] [CrossRef][Green Version]
- Ringvold, A.; Anderssen, E.; Jellum, E.; Bjerkås, E.; Sonerud, G.A.; Haaland, P.J.; Devor, T.P.; Kjønniksen, I. UV-Absorbing Compounds in the Aqueous Humor from Aquatic Mammals and Various Non-Mammalian Vertebrates. Ophthalmic Res. 2003, 35, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Wielgus, A.R.; Sarna, T. Ascorbate Enhances Photogeneration of Hydrogen Peroxide Mediated by the Iris Melanin. Photochem. Photobiol. 2008, 84, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Megaw, J.M. Gluthathione and Ocular Photobiology. Curr. Eye Res. 1984, 3, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, Ş.; Gülçin, İ. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Sies, H. Total Antioxidant Capacity: Appraisal of a Concept. J. Nutr. 2007, 137, 1493–1495. [Google Scholar] [CrossRef]
- Shin, A.H.; Oh, C.J.; Park, J.-W. Glycation-Induced Inactivation of Antioxidant Enzymes and Modulation of Cellular Redox Status in Lens Cells. Arch. Pharm. Res. 2006, 29, 577–581. [Google Scholar] [CrossRef]
- Babizhayev, M.A. Structural and Functional Properties, Chaperone Activity and Posttranslational Modifications of Alpha-Crystallin and Its Related Subunits in the Crystalline Lens: N-Acetylcarnosine, Carnosine and Carcinine Act as Alpha- Crystallin/Small Heat Shock Protein Enhancers in Prevention and Dissolution of Cataract in Ocular Drug Delivery Formulations of Novel Therapeutic Agents. Recent Pat. Drug Deliv. Formul. 2012, 6, 107–148. [Google Scholar] [CrossRef]
- Giblin, F.J. Glutathione: A Vital Lens Antioxidant. J. Ocul. Pharmacol. Ther. 2000, 16, 121–135. [Google Scholar] [CrossRef]
- Go, Y.-M.; Roede, J.R.; Walker, D.I.; Duong, D.M.; Seyfried, N.T.; Orr, M.; Liang, Y.; Pennell, K.D.; Jones, D.P. Selective Targeting of the Cysteine Proteome by Thioredoxin and Glutathione Redox Systems. Mol. Cell Proteom. 2013, 12, 3285–3296. [Google Scholar] [CrossRef]
- Ringvold, A. The Significance of Ascorbate in the Aqueous Humour Protection against UV-A and UV-B. Exp. Eye Res. 1996, 62, 261–264. [Google Scholar] [CrossRef]
- Horwath-Winter, J.; Kirchengast, S.; Meinitzer, A.; Wachswender, C.; Faschinger, C.; Schmut, O. Determination of Uric Acid Concentrations in Human Tear Fluid, Aqueous Humour and Serum. Acta Ophthalmol. 2009, 87, 188–192. [Google Scholar] [CrossRef]
- Badhu, B.; Baral, N.; Lamsal, M.; Das, H.; Dhital Badhu, A. Plasma and Aqueous Humur Ascorbic Acid Levels in People with Cataract from Diverse Geographical Regions of Nepal. Southeast Asian J. Trop. Med. Public Health 2007, 38, 582–585. [Google Scholar]
- Crapo, J.D.; Oury, T.; Rabouille, C.; Slot, J.W.; Chang, L.Y. Copper, Zinc Superoxide Dismutase Is Primarily a Cytosolic Protein in Human Cells. Proc. Natl. Acad. Sci. USA 1992, 89, 10405–10409. [Google Scholar] [CrossRef]
- Sturtz, L.A.; Diekert, K.; Jensen, L.T.; Lill, R.; Culotta, V.C. A Fraction of Yeast Cu,Zn-Superoxide Dismutase and Its Metallochaperone, CCS, Localize to the Intermembrane Space of Mitochondria. A Physiological Role for SOD1 in Guarding against Mitochondrial Oxidative Damage. J. Biol. Chem. 2001, 276, 38084–38089. [Google Scholar] [CrossRef]
- Chang, L.Y.; Slot, J.W.; Geuze, H.J.; Crapo, J.D. Molecular Immunocytochemistry of the CuZn Superoxide Dismutase in Rat Hepatocytes. J. Cell Biol. 1988, 107, 2169–2179. [Google Scholar] [CrossRef]
- Marklund, S.L. Extracellular Superoxide Dismutase and Other Superoxide Dismutase Isoenzymes in Tissues from Nine Mammalian Species. Biochem. J. 1984, 222, 649–655. [Google Scholar] [CrossRef]
- Switala, J.; Loewen, P.C. Diversity of Properties among Catalases. Arch. Biochem. Biophys. 2002, 401, 145–154. [Google Scholar] [CrossRef]
- Deisseroth, A.; Dounce, A.L. Catalase: Physical and Chemical Properties, Mechanism of Catalysis, and Physiological Role. Physiol. Rev. 1970, 50, 319–375. [Google Scholar] [CrossRef]
- Atalla, L.; Fernandez, M.A.; Rao, N.A. Immunohistochemical Localization of Catalase in Ocular Tissue. Curr. Eye Res. 1987, 6, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Gudkov, S.V.; Lankin, V.Z.; Novoselov, V.I. Role of Glutathione Peroxidases and Peroxiredoxins in Free Radical-Induced Pathologies. Biochemistry 2021, 86, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M.; Brigelius-Flohé, R.; Aumann, K.D.; Roveri, A.; Schomburg, D.; Flohé, L. Diversity of Glutathione Peroxidases. Methods Enzymol. 1995, 252, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Satici, A.; Guzey, M.; Gurler, B.; Vural, H.; Gurkan, T. Malondialdehyde and Antioxidant Enzyme Levels in the Aqueous Humor of Rabbits in Endotoxin-Induced Uveitis. Eur. J. Ophthalmol. 2003, 13, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Bekhor, I. Levels of Expression of the Genes for Glutathione Reductase, Glutathione Peroxidase, Catalase and CuZn-Superoxide Dismutase in Rat Lens and Liver. Exp. Eye Res. 1994, 59, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Chaudière, J.; Ferrari-Iliou, R. Intracellular Antioxidants: From Chemical to Biochemical Mechanisms. Food Chem. Toxicol. 1999, 37, 949–962. [Google Scholar] [CrossRef]
- Chug-Ahuja, J.K.; Holden, J.M.; Forman, M.R.; Mangels, A.R.; Beecher, G.R.; Lanza, E. The Development and Application of a Carotenoid Database for Fruits, Vegetables, and Selected Multicomponent Foods. J. Am. Diet Assoc. 1993, 93, 318–323. [Google Scholar] [CrossRef]
- Khachik, F.; Spangler, C.J.; Smith, J.C.; Canfield, L.M.; Steck, A.; Pfander, H. Identification, Quantification, and Relative Concentrations of Carotenoids and Their Metabolites in Human Milk and Serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar] [CrossRef]
- Das, N.P. Effects of Vitamin A and Its Analogs on Nonenzymatic Lipid Peroxidation in Rat Brain Mitochondria. J. Neurochem. 1989, 52, 585–588. [Google Scholar] [CrossRef]
- Livrea, M.A.; Tesoriere, L.; Bongiorno, A.; Pintaudi, A.M.; Ciaccio, M.; Riccio, A. Contribution of Vitamin A to the Oxidation Resistance of Human Low Density Lipoproteins. Free Radic. Biol. Med. 1995, 18, 401–409. [Google Scholar] [CrossRef]
- Navigatore-Fonzo, L.S.; Delgado, S.M.; Gimenez, M.S.; Anzulovich, A.C. Daily Rhythms of Catalase and Glutathione Peroxidase Expression and Activity Are Endogenously Driven in the Hippocampus and Are Modified by a Vitamin A-Free Diet. Nutr. Neurosci. 2014, 17, 21–30. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Beta-Carotene: An Unusual Type of Lipid Antioxidant. Science 1984, 224, 569–573. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid Radical Chemistry and Antioxidant/pro-Oxidant Properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Khachik, F.; Carvalho, L.S.; Muir, G.J.; Zhao, D.Y.; Katz, N.B. Identification and Quantitation of Carotenoids and Their Metabolites in the Tissues of the Human Eye. Exp. Eye Res. 2001, 72, 215–223. [Google Scholar] [CrossRef]
- Wolf, G. The Discovery of the Antioxidant Function of Vitamin E: The Contribution of Henry A. Mattill. J. Nutr. 2005, 135, 363–366. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.A. The Chemistry and Antioxidant Properties of Tocopherols and Tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Yeum, K.J.; Taylor, A.; Tang, G.; Russell, R.M. Measurement of Carotenoids, Retinoids, and Tocopherols in Human Lenses. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2756–2761. [Google Scholar]
- Goyal, A.; Srivastava, A.; Sihota, R.; Kaur, J. Evaluation of Oxidative Stress Markers in Aqueous Humor of Primary Open Angle Glaucoma and Primary Angle Closure Glaucoma Patients. Curr. Eye Res. 2014, 39, 823–829. [Google Scholar] [CrossRef]
- Buettner, G.R. The Pecking Order of Free Radicals and Antioxidants: Lipid Peroxidation, Alpha-Tocopherol, and Ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
- Thomas, C.E.; McLean, L.R.; Parker, R.A.; Ohlweiler, D.F. Ascorbate and Phenolic Antioxidant Interactions in Prevention of Liposomal Oxidation. Lipids 1992, 27, 543–550. [Google Scholar] [CrossRef]
- Brubaker, R.F.; Bourne, W.M.; Bachman, L.A.; McLaren, J.W. Ascorbic Acid Content of Human Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1681–1683. [Google Scholar]
- Nemet, I.; Monnier, V.M. Vitamin C Degradation Products and Pathways in the Human Lens. J. Biol. Chem. 2011, 286, 37128–37136. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Biosynthesis and Functions of Glutathione, an Essential Biofactor. J. Nutr. Sci. Vitaminol. 1992, 38, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Wendel, A. The Physiological Consequences of Glutathione Variations. Life Sci. 1992, 51, 1083–1094. [Google Scholar] [CrossRef]
- Dominko, K.; Đikić, D. Glutathionylation: A Regulatory Role of Glutathione in Physiological Processes. Arh. Hig. Rada Toksikol. 2018, 69, 1–24. [Google Scholar] [CrossRef]
- Sagone, A.L.; Husney, R.M.; O’Dorisio, M.S.; Metz, E.N. Mechanisms for the Oxidation of Reduced Gluthathione by Stimulated Granulocytes. Blood 1984, 63, 96–104. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Tissue-Specific Functions of Individual Glutathione Peroxidases. Free Radic. Biol. Med. 1999, 27, 951–965. [Google Scholar] [CrossRef]
- Ganea, E.; Harding, J.J. Glutathione-Related Enzymes and the Eye. Curr. Eye Res. 2006, 31, 1–11. [Google Scholar] [CrossRef]

| Eye Antioxidants | |
|---|---|
| Enzymatic | Non-Enzymatic |
| SOD | Vitamin A Vitamin E |
| CAT | Vitamin C GSH Cysteine |
| GPx | Carotenoids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastronikolis, S.; Kagkelaris, K.; Pagkalou, M.; Tsiambas, E.; Plotas, P.; Georgakopoulos, C.D. Antioxidant Defense and Pseudoexfoliation Syndrome: An Updated Review. Med. Sci. 2022, 10, 68. https://doi.org/10.3390/medsci10040068
Mastronikolis S, Kagkelaris K, Pagkalou M, Tsiambas E, Plotas P, Georgakopoulos CD. Antioxidant Defense and Pseudoexfoliation Syndrome: An Updated Review. Medical Sciences. 2022; 10(4):68. https://doi.org/10.3390/medsci10040068
Chicago/Turabian StyleMastronikolis, Stylianos, Konstantinos Kagkelaris, Marina Pagkalou, Evangelos Tsiambas, Panagiotis Plotas, and Constantinos D. Georgakopoulos. 2022. "Antioxidant Defense and Pseudoexfoliation Syndrome: An Updated Review" Medical Sciences 10, no. 4: 68. https://doi.org/10.3390/medsci10040068
APA StyleMastronikolis, S., Kagkelaris, K., Pagkalou, M., Tsiambas, E., Plotas, P., & Georgakopoulos, C. D. (2022). Antioxidant Defense and Pseudoexfoliation Syndrome: An Updated Review. Medical Sciences, 10(4), 68. https://doi.org/10.3390/medsci10040068







