Hyperbilirubinemia and Hyponatremia as Predictors of Complicated Appendicitis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhangu, A.; Søreide, K.; Saverio, D.S. Acute appendicitis: Modern understanding of pathogenesis, diagnosis, and management. Lancet 2015, 386, 1278–1287. [Google Scholar] [CrossRef]
- Wang, V.; Kriger, D.; Fanous, E. Should all complicated appendicitis be treated the same? The answer is no. Am. Surg. 2019, 85, 1179–1183. [Google Scholar] [PubMed]
- Hajibandeh, S.; Hajibandeh, S.; Hobbs, N. Neutrophil-to-lymphocyte ratio predicts acute appendicitis and distinguishes between complicated and uncomplicated appendicitis: A systematic review and meta-analysis. Am. J. Surg. 2020, 219, 154–163. [Google Scholar] [CrossRef]
- Rawolle, T.; Reismann, M.; Minderjahn, M.I. Sonographic differentiation of complicated from uncomplicated appendicitis. Br. J. Radiol. 2019, 92, 20190102. [Google Scholar] [CrossRef]
- Avanesov, M.; Wiese, N.J.; Karul, M. Diagnostic prediction of complicated appendicitis by combined clinical and radiological appendicitis severity index (APSI). Eur. Radiol. 2018, 28, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, Y.; Itamoto, T.; Takakura, Y. Validity of predictive factors of acute complicated appendicitis. World J. Emerg. Surg. 2016, 11, 48. [Google Scholar] [CrossRef]
- Farooqui, W.; Pommergaard, H.C.; Burcharth, J. The diagnostic value of a panel of serological markers in acute appendicitis. Scand. J. Surg. 2015, 104, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Deiters, A.; Drozd, A.; Parikh, P. Use of the Alvarado score in elderly patients with complicated and uncomplicated appendicitis. Am. Surg. 2019, 85, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Boshnak, N.; Boshnaq, M.; Elgohary, H. Evaluation of platelet indices and red cell distribution width as new biomarkers for the diagnosis of acute appendicitis. J. Investig. Surg. 2018, 31, 121–129. [Google Scholar] [CrossRef]
- Kabir, S.A.; Kabir, S.I.; Sun, R. How to diagnose an acutely inflamed appendix; a systematic review of the latest evidence. Int. J. Surg. 2017, 40, 155–162. [Google Scholar] [CrossRef]
- Giordano, S.; Pääkkönen, M.; Salminen, P. Elevated serum bilirubin in assessing the likelihood of perforation in acute appendicitis: A diagnostic meta-analysis. Int. J. Surg. 2013, 11, 795–800. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, J.H.; Lee, S.S. CT in differentiating complicated from uncomplicated appendicitis: Presence of any of 10 CT features versus radiologists’ gestalt assessment. AJR Am. J. Roentgenol. 2019, 213, W218–W227. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.L.; Jaunoo, S.S. Hyperbilirubinaemia in appendicitis: The diagnostic value for prediction of appendicitis and appendiceal perforation. Eur. J. Trauma Emerg. Surg. 2016, 42, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Kumar, A.; Saxena, N. Hyperbilirubinemia as a predictor of gangrenous/perforated appendicitis: A prospective study. Ann. Gastroenterol. 2013, 26, 325–331. [Google Scholar]
- Hong, Y.R.; Chung, C.W.; Kim, J.W. Hyperbilirubinemia is a significant indicator for the severity of acute appendicitis. J. Korean Soc. Coloproctol. 2012, 28, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Nassiri, N.; Virgilio, C.D. Association between hyponatremia and complicated appendicitis. JAMA Surg. 2015, 150, 9110912. [Google Scholar] [CrossRef]
- Käser, S.A.; Furler, R.; Evequoz, D.C. Hyponatremia is a specific marker of perforation in sigmoid diverticulitis or appendicitis in patients older than 50 years. Gastroenterol. Res. Pract. 2013, 2013, 462891. [Google Scholar] [CrossRef]
- Pham, X.D.; Sullins, V.F.; Kim, D.Y. Factors predictive of complicated appendicitis in children. J. Surg. Res. 2016, 206, 62–66. [Google Scholar] [CrossRef]
- Giannis, D.; Matenoglou, E.; Moris, D. Hyponatremia as a marker of complicated appendicitis: A systematic review. Surgeon 2020, 18, 295–304. [Google Scholar] [CrossRef]
- Pérez-Soto, R.H.; León-Ballesteros, P.D.; Álvarez Bautista, G.F. Thrombocytosis and hyponatremia as predictors of complicated acute appendicitis: Predictors of appendicitis. J. Surg. Res. 2021, 261, 369–375. [Google Scholar] [CrossRef]
- Eddama, M.; Fragkos, K.C.; Renshaw, S. Logistic regression model to predict acute uncomplicated and complicated appendicitis. Ann. R. Coll. Surg. Engl. 2019, 101, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Al-Qallaf, A.; Shuaib, A.; Al-Sharaf, K. Acute appendicitis as a rare cause of mechanical small bowel obstruction case report. Qatar Med. J. 2017, 2017, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panagiotopoulou, I.G.; Parashar, D.; Lin, R. The diagnostic value of white cell count, C-reactive protein and bilirubin in acute appendicitis and its complications. Ann. R. Coll. Surg. Engl. 2013, 95, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, D.H.; Hulsewé, K.W.; Acker, B.A.V. Evaluation of the diagnostic accuracy of plasma markers for early diagnosis in patients suspected for acute appendicitis. Acad. Emerg. Med. 2013, 20, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, M.; Pazouki, A.; Tamannaie, Z. Comparison of pre-operative bilirubin level in simple appendicitis and perforated appendicitis. Med. J. Islam. Repub. Iran 2013, 27, 109–112. [Google Scholar]
- Estrada, J.J.; Petrosyan, M.; Barnhart, J. Hyperbilirubinemia in appendicitis: A new predictor of perforation. J. Gastrointest. Surg. 2007, 11, 109–112. [Google Scholar] [CrossRef]
- Akai, M.; Iwakawa, K.; Yasui, Y. Hyperbilirubinemia as a predictor of severity of acute appendicitis. J. Int. Med. Res. 2019, 47, 3663–3669. [Google Scholar] [CrossRef]
- Mcgowan, D.R.; Sims, H.M.; Zia, K. The value of biochemical markers in predicting a perforation in acute appendicitis. ANZ J. Surg. 2013, 83, 79–83. [Google Scholar] [CrossRef]
- Besli, G.E. Predictive value of serum sodium level in determining complicated appendicitis risk in children. Haydarpasa Numune Train. Res. Hosp. Med. J. 2019, 59, 35–40. [Google Scholar] [CrossRef]
- Swart, R.M.; Hoorn, E.J.; Betjes, M.G. Hyponatremia and inflammation: The emerging role of interleukin-6 in osmoregulation. Nephron Physiol. 2011, 118, 45–51. [Google Scholar] [CrossRef]
- Heymowski, A.; Boström, L.; Dahlberg, M. Plasma sodium and age are important markers of risk of perforation in acute appendicitis. J. Gastrointest. Surg. 2021, 25, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Lindestam, U.; Almström, M.; Jacks, J. Low plasma sodium concentration predicts perforated acute appendicitis in children: A prospective diagnostic accuracy study. Eur. J. Pediatr. Surg. 2020, 30, 350–356. [Google Scholar] [PubMed]
- Lin, K.B.; Chan, C.L.; Yang, P.N. Epidemiology of appendicitis and appendectomy for the low-income population in Taiwan. BMC Gastroenterol. 2003, 15, 18. [Google Scholar] [CrossRef]
- Coward, S.; Kareemi, H.; Clement, F. Incidence of appendicitis over time: A comparative analysis of an administrative healthcare database and a pathology-proven appendicitis registry. PLoS ONE 2016, 11, e0165161. [Google Scholar] [CrossRef] [PubMed]
- Nshuti, R.; Kruger, D.; Luvhengo, T.E. Clinical presentation of acute appendicitis in adults at the Chris Hani Baragwanath Academic Hospital. Int. J. Emerg. Med. 2014, 7, 7–12. [Google Scholar] [CrossRef]
- Kirshtein, B.; Bayme, M.; Domchik, S. Complicated appendicitis: Laparoscopic or conventional surgery? World J. Surg. 2007, 31, 744–749. [Google Scholar] [CrossRef]
- Lin, Y.M.; Hsieh, C.H.; Cheng, C.I. Laparoscopic appendectomy for complicated acute appendicitis does not result in increased surgical complications. Asian J. Surg. 2012, 35, 113–116. [Google Scholar] [CrossRef][Green Version]
| Variable | Early Acute (n = 36) | Acute Suppurative (n = 177) | Complicated (n = 32) | Other (n = 2) | p-Value |
|---|---|---|---|---|---|
| Age | 23.9 ± 10.4 | 25.0 ± 12.4 | 23.2 ± 14.9 | 20.0 ± 5.7 | 0.801 |
| (15.0, 31.0) | (14.0, 33.0) | (14.5, 27.0) | (16.0, 24.0) | ||
| Sex (male) | 20 (55.6%) | 116 (65.5%) | 19 (59.4%) | 1 (50.0%) | 0.644 |
| TBIL (µmol/L) | 14.2 ± 5.1 | 17.0 ± 8.0 | 23.3 ± 6.3 | 6.8 ± 1.9 | <0.001 |
| (11.0, 16.9) | (11.6, 20.9) | (17.7, 27.5) | (6.0, 8.7) | ||
| Hyperbilirubinemia | 5 (13.9%) | 48 (27.1%) | 15 (46.8%) | 0 (0.0%) | <0.001 |
| Serum sodium levels | 135.9 ± 1.79 | 134.7 ± 2.7 | 132.6 ± 2.6 | 137.0 ± 2.8 | <0.001 |
| (mEq/L) | (135.0, 137.0) | (130.0, 134.0) | (130.0, 134.0) | (135.0, 139.0) | |
| Hyponatremia | 13 (36.1%) | 79 (44.6%) | 22 (68.8%) | 0 (0.0%) | <0.001 |
| Hyperbilirubinemia + hyponatremia | 1 (2.7%) | 29 (16.4%) | 18 (56.3%) | 0 (0.0%) | <0.001 |
| Appendectomy | 0.743 | ||||
| Laparoscopic | 34 (94.4%) | 163 (92.1%) | 31 (96.9%) | 2 (100.0%) | |
| Open | 2 (5.6%) | 14 (7.9%) | 1 (3.1%) | 0 (0.0%) |
| Variables | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | Lower | Upper | p-Value | OR | Lower | Upper | p-Value | |
| Sex (male) | 1.202 | 0.563 | 2.565 | 0.636 | 1.544 | 0.670 | 3.557 | 0.308 |
| TBIL level (>20 µmol/L) | 1.098 | 1.052 | 1.147 | <0.001 | 1.083 | 1.033 | 1.135 | <0.001 |
| Serum sodium level (<135 mEg/L) | 0.743 | 0.646 | 0.855 | <0.001 | 0.789 | 0.681 | 0.914 | 0.002 |
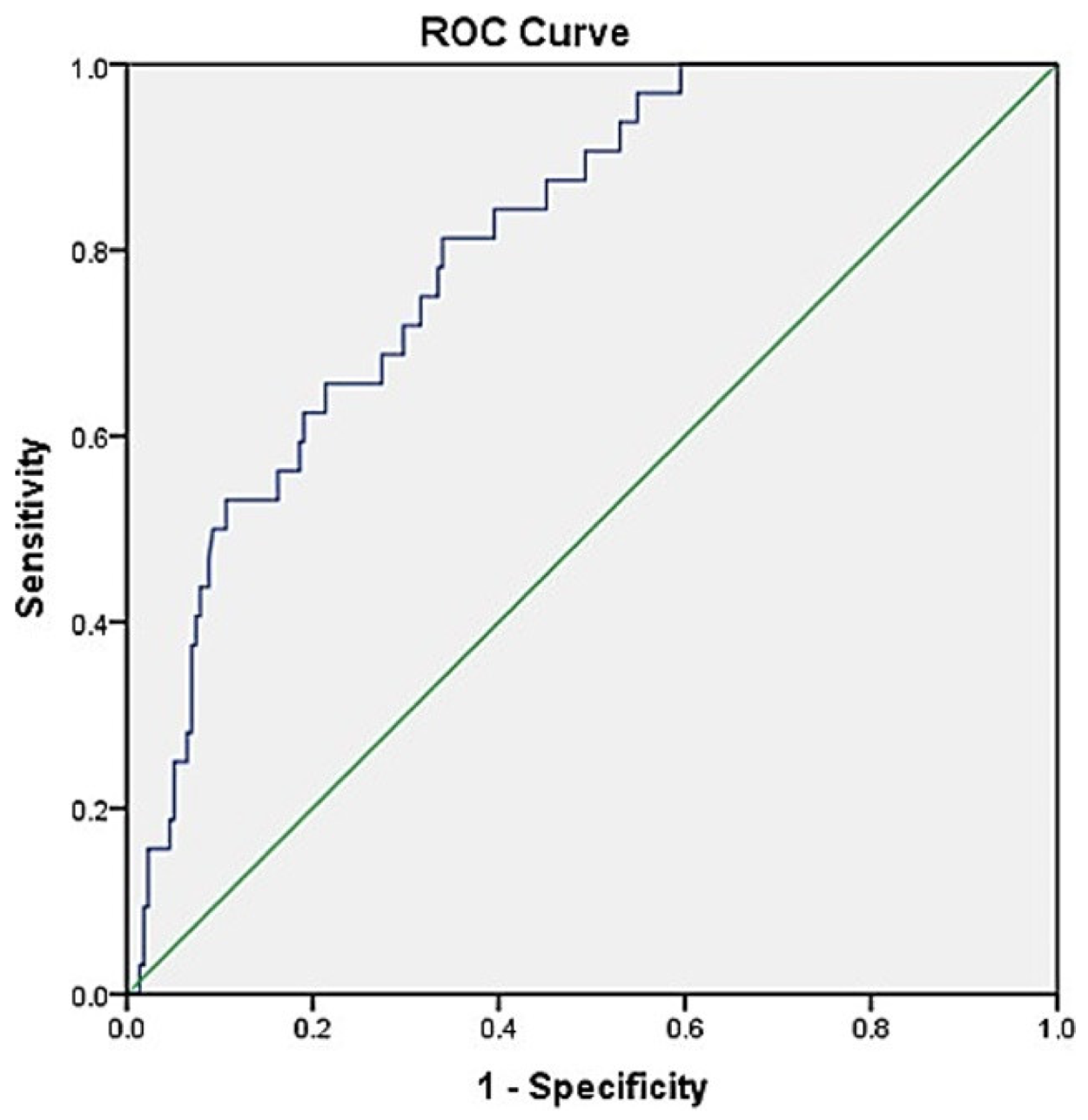
| Variable | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|
| (%, 95% CI) | (%, 95% CI) | (%, 95% CI) | (%, 95% CI) | (%, 95% CI) | |
| Hyperbilirubinemia | 65.6 | 75.4 | 28.4 | 93.6 | 0.79 |
| (46.8–81.4) | (69.0–81.0) | (22.0–35.8) | (90.1–96.0) | (0.737–0.842) | |
| Hyponatremia | 84.4 | 45.6 | 18.8 | 95.1 | 0.73 |
| (67.2–94.7) | (38.8–52.5) | (16.0–21.9) | (89.6–97.8) | (0.671–0.785) | |
| Hyperbilirubinemia + hyponatremia | 81.3 | 64.65 | 25.5 | 95.9 | 0.80 |
| (63.6–92.8) | (57.9–71.0) | (21.1–30.4) | (91.8–98.0) | (0.748–0.851) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuaib, A.; Alhamdan, N.; Arian, H.; Sallam, M.A.; Shuaib, A. Hyperbilirubinemia and Hyponatremia as Predictors of Complicated Appendicitis. Med. Sci. 2022, 10, 36. https://doi.org/10.3390/medsci10030036
Shuaib A, Alhamdan N, Arian H, Sallam MA, Shuaib A. Hyperbilirubinemia and Hyponatremia as Predictors of Complicated Appendicitis. Medical Sciences. 2022; 10(3):36. https://doi.org/10.3390/medsci10030036
Chicago/Turabian StyleShuaib, Abdullah, Nour Alhamdan, Husain Arian, Mohamed Alaa Sallam, and Ali Shuaib. 2022. "Hyperbilirubinemia and Hyponatremia as Predictors of Complicated Appendicitis" Medical Sciences 10, no. 3: 36. https://doi.org/10.3390/medsci10030036
APA StyleShuaib, A., Alhamdan, N., Arian, H., Sallam, M. A., & Shuaib, A. (2022). Hyperbilirubinemia and Hyponatremia as Predictors of Complicated Appendicitis. Medical Sciences, 10(3), 36. https://doi.org/10.3390/medsci10030036






