Abstract
Prostate cancer (PCa) is the second most common cancer in men. Common treatments include active surveillance, surgery, or radiation. Androgen deprivation therapy and chemotherapy are usually reserved for advanced disease or biochemical recurrence, such as castration-resistant prostate cancer (CRPC), but they are not considered curative because PCa cells eventually develop drug resistance. The latter is achieved through various cellular mechanisms that ultimately circumvent the pharmaceutical’s mode of action. The need for novel therapeutic approaches is necessary under these circumstances. An alternative way to treat PCa is by repurposing of existing drugs that were initially intended for other conditions. By extrapolating the effects of previously approved drugs to the intracellular processes of PCa, treatment options will expand. In addition, drug repurposing is cost-effective and efficient because it utilizes drugs that have already demonstrated safety and efficacy. This review catalogues the drugs that can be repurposed for PCa in preclinical studies as well as clinical trials.
1. Introduction
1.1. Prostate Cancer
In 2022, the United States will have an estimated 268,490 new cases of prostate cancer (PCa) and 34,500 related deaths [1]. Nearly 95% of PCa cases are adenocarcinomas, and 70 to 85% are located in the periphery of the gland [2]. Such tumors tend to extend beyond the prostatic tissue and invade the local perineural space; this histologic finding by itself, though, does not predict positive surgical margins, extraprostatic extension, seminal vesicle invasion, or positive lymph nodes [3]. Lymphovascular invasion, however, was shown to be associated with positive surgical margins, extracapsular extension, seminal vesicle invasion, positive lymph nodes, biochemical recurrence, and decreased overall survival [4]. Metastasis occurs most commonly in regional lymph nodes and bone, followed by lung, then liver [5].
Standard PCa screening consists of measuring the serum prostate-specific antigen (PSA) level and performing a digital rectal exam (DRE); recently, multiparametric magnetic resonance imaging (MRI) has emerged as an additional screening tool [6]. Combining these data with background information, such as family history and race/ethnicity, a shared-decision is usually made between the patient and the urologist on whether or not to pursue prostate biopsy. Prostate biopsy is conducted transperineally or, more commonly, transrectally under ultrasound guidance (TRUS), and it typically consists of 12-core tissue samples that target medial- and lateral- base, mid, and apex regions on both the left and right lobes [7,8].
Clinical staging of PCa combines data from a DRE and needle biopsy results. Though clinical stage correlates closely with pathological stage, pathological stage is the most definitive predictor of biochemical recurrence [9]. Treatment primarily consists of active surveillance (AS), surgery, or radiation, with androgen deprivation therapy (ADT) and chemotherapy used as adjuvants in high-risk disease. AS is a conservative approach considered for patients with Grade group 1 (3 + 3) or 2 (3 + 4) with PSA < 10 ng/mL and involves close monitoring through quarterly PSA tests and an annual prostate biopsy [10]. Patients with Grade group 3 or higher are indicated for definitive therapies: surgery or radiation. High-risk patients, PSA > 20 ng/mL or Grade groups 4 or 5, who choose radiation should receive adjuvant ADT with GnRH agonists or antagonists [11]. High-risk patients with metastasis are managed with ADT and antiandrogens or ADT and docetaxel [12].
Following definitive treatment, 20 to 30% of patients develop biochemical recurrence (BCR) [13,14]. The latter marks the relapse of disease, defined as PSA > 0.2 ng/mL following RP or nadir PSA + 2.0 ng/mL after radiation [12]. Although tumor regression and remission follow ADT, resistance frequently occurs, and in many patients, it is inevitable [15,16]. Resistance to ADT is referred to as castration resistant prostate cancer (CRPC) or hormone-refractory PCa (HRPC). Clinically, patients can progress from non-metastatic CRPC to metastatic CRPC (mCRPC). The role of androgen signaling, as well as other signaling pathways, is important in understanding the mechanisms for CRPC, and using this knowledge facilitates the opportunity for drug targets (Figure 1).
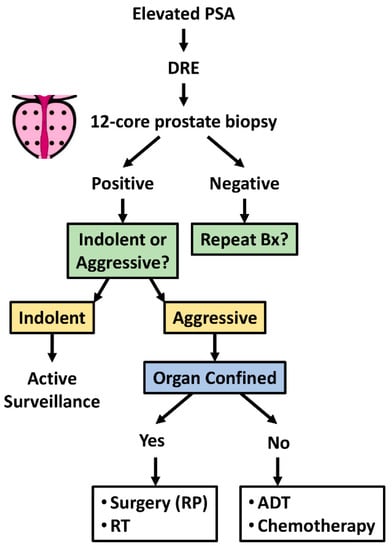
Figure 1.
Clinical diagnosis, patient stratification, and treatment options for prostate cancer. Schematic of the clinical diagnosis and patient stratification for appropriate PCa treatment. Abbreviations: ADT: androgen deprivation therapy; Bx: biopsy; DRE: digital rectal examination; PSA: prostate specific antigen; RP: radical prostatectomy; RT: radiation therapy.
1.2. Therapy Resistance in Prostate Cancer
Physiologically, testosterone passively diffuses into prostate cells where it either directly binds to the androgen receptor (AR) or is converted to dihydrotestosterone, which binds the AR with higher affinity. Bound AR undergoes a conformational change with a dissociation of heat shock proteins, followed by a nuclear translocation, where it dimerizes and serves as a transcription factor that regulates gene expression. In embryology, the prostate does not mature without androgens and becomes atrophic, as seen in individuals with 5 alpha reductase deficiency [17]. The low levels of exposure to androgens are, in fact, protective against development of PCa. The vital role androgens play in PCa was first described by Huggins who showed that prostate tumors regress by removing the primary source of testosterone through orchiectomy [18]. However, deprivation of serum testosterone initially leads to low DHT levels, which is the primary mediator of AR signaling. Withdrawal of these ligands leads to dramatic apoptosis of androgen-dependent tumor cells, leaving a subset of androgen-independent cells in a dormant state [19], some of which have cancer stem cell (CSC) properties. Additionally, ADT has been shown to alter the dynamics of the prostate tumor microenvironment, affecting stromal, endothelial, and immune cells, as well as promoting epithelial-to-mesenchymal transition, which is believed to be a contributor to therapy resistance in PCa [20,21].
There are various methods for PCa cells to achieve resistance, and some are specific to the modality of androgen deprivation. For example, leuprolide, a GnRH agonist that has been a first line treatment for the last 20 years in ADT, inhibits synthesis of androgens by removing the pituitary’s signal to make testosterone. The tumor cells escape by making their own testosterone in an autocrine fashion. By mutations that drive AR gene amplifications or in certain predisposing polymorphisms (such as short CAG sequences found more often in AA men), the AR becomes more sensitive and can respond to minimal intratumoral levels of testosterone [22]. Abiraterone, a relatively novel agent, can block intratumoral testosterone production, but PCa cells have various routes of resistance. For example, gain-in-function mutations in the AR allow it to use other molecules as ligands, such as those downstream of glucocorticoid or IL-6 transmembrane signaling, in order to translocate into the nucleus and cause gene expression [23]. Enzalutamide, one of the strongest anti-androgen agents, can block AR’s promiscuity with other molecules; however, PCa cells are resilient and eventually continue proliferating, even in the absence of AR activity.
1.3. Molecular Landscape in Prostate Cancer
The development of androgen-independent PCa is tightly connected to dysregulations within intracellular signaling cascades. Commonly studied pathways in PCa include PI3K/AKT/mTOR, Ras/Raf/MEK/ERK (MAPK), and WNT/β-catenin. For example, a PTEN gene deletion, found in 16 to 32% of PCa, results in increased mTOR signaling, and increased mTOR signaling is correlated with poor survival in PCa [24]. Likewise, activation of MAPK/ERK signaling, through various mechanisms, has demonstrated resistance to Enzalutamide [25]. WNT signaling dysregulation, either in a β-catenin dependent- or independent-fashion, can also drive aggressive PCa [26].
More important is the cross-communication between different cell signaling pathways in PCa growth and progression. For example, prostate cells with isolated mutations in the Ras/Raf/MEK/ERK signaling are unable to initiate tumorigenesis. However, when combined with alterations to PI3K/AKT/mTOR signaling, such as a PTEN deletion, RAS mutations induce tumorigenesis and facilitate epithelial-to-mesenchymal transition (EMT), making prostate tumors substantially more aggressive [21,27]. Mutations in the Ras pathway can also facilitate resistance to medications targeted towards PI3K/AKT/mTOR pathway [28]. Therefore, the co-administration of drugs targeting multiple signaling pathways can be beneficial. This was demonstrated in a mouse model, where the co-administration of Rapamycin (an inhibitor of mTOR) and Mirdametinib (an inhibitor of MEK) inhibited hormone-refractory PCa cell growth, demonstrating the interdependence of the Ras and AKT signaling cascades in advanced PCa [29].
Another example of dependent cross communication is seen with the gene fusion TMPRSS2-ERG (present in 40 to 80% of PCa) [30,31]. As with isolated Ras/Raf/MEK/ERK mutations, ERG oncogene requires signaling aberrations from other pathways—such as FOXO1 deletions from AKT dysregulation—to have significant tumorigenic and invasive features [32].
Cross communication between aberrant cell signaling pathways is also paramount to the emergence and maintenance of PCa stem cells (PCSCs) in an androgen-resistant tumor state [33]. Studies have shown that interactions between hypoxia induced factors (HIFs) and PI3K, MAPK, or WNT maintain PCa cell “stemness” [34]. In fact, ADT directly drives the release of hypoxia inducible factors [35]. The presence of PCa stem cells might explain the heterogeneous cell populations seen in prostate tumors. PCSCs are attractive drug targets, as more evidence points to these cells as being the origin of CRPC [33]. Therefore, studies such as our own are necessary to explore available United States Food and Drug Administration (FDA)-approved drugs that can inhibit or slow the progression of the disease.
Drug repurposing has always been an opportunistic and fortuitous procedure [36,37]. For example, sildenafil citrate, often known as “Viagra”, was first discovered as an antihypertensive medication, then repurposed by Pfizer for the treatment of erectile dysfunction, based on retrospective clinical experience [38,39]. Furthermore, thalidomide, which was formerly used to treat morning sickness in pregnant women, was pulled from the market because of its known association with severe bone birth abnormalities in children [40]. However, serendipity led to the repurposing of thalidomide for the treatment of erythema nodosum leprosum (ENL) [41] and later, in multiple myeloma [42]. Currently, the global SARS-CoV-2 (COVID-19) outbreak has given the medication repositioning strategy a new sense of urgency [43,44,45,46]. Traditional drug research is lengthy, owing to the regulations of creating new medications and treatments. Therefore the quicker repositioning technique has piqued attention to identifying compounds that could counteract the consequences of the viral infection including chloroquine [47], hydroxychloroquine [48], enalapril [49], and remdesivir [47], in addition to others [45].
2. Repurposing Approved Drugs in Cancer
2.1. Introduction to Drug Repurposing
Drug repurposing, also known as drug repositioning, reprofiling, or retasking, is a strategy for finding new uses for approved or investigational pharmaceuticals beyond their original medical indication [41]. Compared to de novo drug development, drug repurposing offers various advantages over developing a new drug for a specific application. Usually, speeding up pharmaceutical research and development is frequently linked to an increase in the development risk. Repositioning candidates have typically undergone research in preclinical models and human clinical trials, as well as safety assessment, optimization, and sometimes, formulation development and, hence, have well-known safety and pharmacokinetic properties. As a result, the time frame for drug development can be greatly reduced, along with the rate of failure in later efficacy trials [38,41]. Relative to the stage of development of the repurposing candidate drug, drug repurposing requires less investment in terms of preclinical and phase I and II expenditures, but the regulatory and phase III costs may be comparable to those for a new drug in the same indication [38,50]. Together, these advantages add up to lower risk and faster return on investment in the development of repurposed pharmaceuticals, as well as lower average associated costs if failures are included.
2.2. A Computational Approach for Drug Repurposing
Computational methods, also known as in silico drug repurposing [51], are critical in predicting novel indications for current medicines, and they largely rely on data from databases such as DrugBank [52], ChemBank [53], Genecards [54], OMIM [55], and PubMed [56]. As a result of the collection and systematic analysis of diverse data, including gene expression, chemical structure, genotype or proteomic data, or electronic health records (EHRs), innovative approaches for drug repositioning can be developed by emphasizing possible candidates for drug-disease connections [57]. Here, we will discuss the most prevalent computational techniques as well as some examples of medication repurposing.
2.2.1. Network-Based Drug Repurposing
Following the advancements in genotyping technology, reduced genotyping costs, and the completion of the Human Genome Project, genome-wide association studies (GWAS) have been performed to find genetic variants that affect common diseases [58]. Using GWAS, new targets that might be shared across the medicines and disease phenotypes examined could be identified, leading to therapeutic repurposing [59,60,61]. In some instances, the genes identified in a GWAS research are not druggable targets. However, a network-based method may reveal genes that are upstream or downstream of the GWAS-associated target and might be used for repurposing [38,62]. The network-based method that integrates information on medicines and their druggable targets constructs drug-disease networks based on various data, or indirectly inferred using computational algorithms, to predict new drug candidates [58,63].
Network-based clustering algorithms were employed to discover subnetworks that allow for prospective candidate drug–target connections [64,65,66]. For instance, vismodegib, a known inhibitor of the Hedgehog signaling pathway, was identified to treat Gorlin syndrome [67], and iloperidone, an antipsychotic for the treatment of schizophrenia, was identified as a novel drug for hypertension [65]. Similarly, network-based propagation techniques are utilized to discover prospective candidate medicines. A random walk propagation algorithm expands a set of disease-associated genes to genes sharing neighbors in a protein-protein network or gene-gene network. Using this strategy, several medicines were identified for novel indications, such as donepezil for Parkinson’s disease, methotrexate for Crohn’s disease, gabapentin for anxiety disorder, risperidone for obsessive-compulsive disorder, in addition to cisplatin for breast cancer [68].
Functional genomics is also widely used to map cancer dependencies and identify therapeutic targets in cancer. This includes genetic screens based on CRISPR/Cas9 technology [69,70,71], reverse genetic screens, and chemogenomic screens [72]. In PCa, this technology has proven to be crucial for clinicians in their decision-making regarding patient treatment, especially in the era of precision medicine. For instance, suppression of cyclin-dependent kinase 12 (CDK12)—which is essential for PCa cell survival—by the covalent inhibitor THZ531 had an anti-PCa effect by downregulating androgen receptor signaling [70]. Another study using chemogenomic screening demonstrated that Hsp70 co-chaperone DNAJA1 is a hub for anticancer drug resistance [72].
2.2.2. Profile-Based Drug Repurposing
Profile-based drug repurposing serves for the comparison of a drug with another drug, disease, or clinical phenotypes using different profiles of the drug to be repurposed [38,73,74]. An example is the expression-based profile, also known as a transcriptome (RNA) signature, which allows for drug-drug similarity and drug-disease similarity [75,76]. The transcriptome signature of a repurposed medication is established by comparing differential gene expression in a cell or tissue before and after therapy, which is then compared to the disease-associated expression profile. For instance, if the drug can reverse the expression pattern of a given set of genes in a particular disease to be closer to that obtained for the healthy state, then that drug might be able to revert the disease phenotype itself. As a result, this computational technique employs the signature reversion principle (SRP), which allows for the comparison of transcriptomic profiles between drugs and diseases and helps in anticipating whether the repurposed drug may be effective on the disease of interest [38,77,78]. Additionally, this concept uncovered novel repurposing prospects in a variety of therapeutic domains [79,80,81,82] in addition to chemo-sensitizers in lymphoid malignancies [83]. Furthermore, the expression-based profile concept requires publicly available gene expression databases. The Broad Institute’s Connectivity Map (cMap) is derived from the outcomes of treating numerous cell types with over 1300 compounds [84]. However, to make cMap more effective, this resource may be coupled with other public sources of gene expression data, including Gene Expression Omnibus and Array Express [38].
The second type of profile-based approach is the structure-based computational strategy. Since drugs of similar chemical structures may share common biological activity, this strategy relies on the drug chemical structures to compare between drugs and identify the new drug-target association [85,86]. Using a similarity ensemble approach (SEA), Keiser and colleagues identified 23 new drug–target associations [73]. Another technique used is molecular docking to identify the ligand-receptor association [87]. Conventional docking, for example, involves evaluating numerous ligands (drugs) against a known receptor in a particular disease. Reverse docking, on the other hand, tests drug libraries against a wide spectrum of receptors to find potential interactions [38,88]. Using a high-throughput computational docking technique, Dakshanamurthy and colleagues have reported that mebendazole, an antiparasitic drug, has the structural capacity to block vascular endothelial growth factor receptor 2 (VEGFR2) [89].
However, this technique is limited due to the lack of 3D structures for some protein targets, lack of macromolecular target databases, a high rate of false positives, some predictability limitations, as well as the questioned docking algorithms utilized due to variances in software packages [38,76]. Nonetheless, recently, a new promising tool, “AlphaFold”, was developed, which counters this issue of structure prediction. It is a neural network-based computational model that can predict protein structures with atomic accuracy [90]. AlphaFold can be used for molecular replacement and for interpreting cryogenic electron microscopy maps. Importantly, developing accurate protein structure prediction algorithms will accelerate the advancement of structural bioinformatics to keep pace with the genomics revolution.
Furthermore, profiling based on side-effect similarities is another resource for computational drug repurposing. Similar side effects between two different drugs are considered to indicate shared physiology, suggesting that they may have a similar mode of action by affecting the same target or pathway [91]. It is also possible that a drug’s impact is comparable to that of a certain illness, indicating common drug-disease physiology [92]. Although this is a good technique for identifying repurposing possibilities, the lack of well-defined adverse effect profiles may restrict its application [76]. On the other hand, artificial intelligence technologies capable of text mining and natural language processing may offer future opportunities to overcome these constraints [93].
2.2.3. Data-Based Drug Repurposing
Some drug repurposing discoveries were made through simple clinical studies or pharmacological analyses, rather than a systematic review of clinical data. Examples of these discoveries are sildenafil for erectile dysfunction [41], aspirin for colorectal cancer [94], raloxifene in breast cancer, and propranolol in osteoporosis [95].
Retrospective clinical data is becoming increasingly popular to discover drug repurposing possibilities [96]. Electronic health records (EHRs) are not only a rich source of laboratory test results and prescription information for patients, but they are also a source of indications for drug repurposing [96,97]. Furthermore, the massive volume of EHR data provides large statistical power [57]. Paik and colleagues have employed this approach to extract clinical data, including laboratory tests and genetic fingerprints, to find over 17,000 known drug-disease correlations and identified terbutaline sulfate as a potential option for the treatment of amyotrophic lateral sclerosis (ALS) [97]. However, obtaining and using EHR data remains challenging due to ethical and legislative hurdles that may limit data access, as well as the difficulty of extracting the unstructured data included in these databases [96].
Text-mining tools, another form of data-based medication repurposing strategy, allows for prioritizing prospective research areas and expedite the disease-drug repurposing process among the extensively accumulating scientific literature [98]. Thus, to discover possible drug-disease connections in the literature, several text mining approaches have been used. In this regard, Li and colleagues have developed disease-specific drug-protein connectivity maps for Alzheimer’s disease through combining gene/protein and drug connection information based on protein interaction networks and literature mining. Their approach revealed diltiazem and quinidine as possible therapeutic candidates for Alzheimer’s disease [99]. A method to construct sentence graph networks was achieved using text mining techniques to find new sarcoidosis disease targets [100]. In addition, Kuusisto and colleagues introduced KinderMiner as a text mining approach for identifying possible indications of old medicines [98], while others published an algorithm to identify potential anti-Alzheimer’s disease drugs, target, and prioritize them, via systematic ‘omics’ mining [101].
2.3. Drug Repurposing Approach in Cancer
Lately, researchers and clinicians are exploring drug repurposing to overcome the shortage of medication for novel cancer treatments [102,103,104]. As such, repurposing drugs with tolerable side effects and known pharmacokinetics and pharmacodynamics profiles offers an alternative to standard anti-cancer chemotherapeutics [51]. Excellent prospects for drug repurposing have been offered for medicines that may have the ability to address more than one target, as detailed in the next section, thanks to advancements in genomic and proteomic tools. As a result, drug repurposing using non-oncology medications has been applied with medicines that are effective for cancer hallmarks but were not designed originally for cancer treatment.
Drug repurposing has many benefits compared to de novo drug studies. These advantages mainly revolve around time and cost, where a study by Kaitin et al. showed that it takes approximately 8.3 years for an anti-cancer medication to get approved, compared with 3 to 4 years for a repurposed drug [105]. Additionally, among the various advantages of drug repurposing is being cost-effective, where a repurposed drug is estimated to cost USD 300 million to reach market, compared with USD 2–3 billion for studying a new chemical entity [104,106].
3. Drug Repurposing in Prostate Cancer
3.1. Clinical Challenges in Prostate Cancer
A major issue with low-risk PCa is that it is frequently overtreated, whereas high-risk PCa is frequently undertreated. Many individuals with high-risk illnesses are only offered palliative androgen deprivation therapy (ADT) rather than intense local therapy. According to the CaPSURE database, ADT is given to 41% of high-risk patients, whereas RP and RT are given to 24% and 28% of high-risk patients, respectively [107,108]. In contrast, local therapy, with either RP or RT, depending on the classification of high-risk illness, results in a 49–80% progression-free probability (PFP) [109,110]. Furthermore, as compared to observation or ADT alone, numerous randomized studies have indicated that active treatment improves survival in individuals with high-risk PCa [111,112].
Another challenge is choosing the appropriate treatment along with the proper timing of administration. When androgen blocking is used as first-line therapy, for example, high response rates are attained; nonetheless, most men proceed to CRPC. Furthermore, systemic chemotherapies have been proven to improve clinical outcomes in individuals with hormone-refractory PCa. However, they are not curative [113].
Furthermore, despite early detection and intervention, advanced PCa might metastasize to lymph nodes and bones, lowering the quality of life and decreasing the median survival rate [114]. As a result, detecting bone metastases is crucial in clinical practice, since the beginning of bone metastasis frequently necessitates the start of chemotherapy and/or bone-targeted treatment [115,116,117]. Another point of contention in PCa care is the timing of hormonal therapy for patients with increasing PSA who have failed initial treatment, as well as if hormones can give an extra advantage to external beam radiotherapy [118].
On the other hand, some challenges in PCa are owed to the limited screening tools and their diagnostic accuracy. Prostate-specific antigen (PSA) is a widely accepted screening tool for PCa. However, elevated PSA levels may result when the normal architecture of the prostate is disrupted, such as in benign prostatic hyperplasia (BPH) and prostatitis. Therefore, PSA is a prostate-specific marker but not PCa-specific [119,120]. Although blood PSA levels associates with clinical stage and tumor phenotype, they are of little use in predicting stage for patients [113,119]. Other tests based on PSA derivatives are required to improve on standard blood PSA, such as PSA density, velocity, and age-specific reference range, and PSA isoforms such as free PSA, pro-PSA, and BPSA (benign PSA) [121,122]. Furthermore, while widespread PSA screening has resulted in early detection and a considerable reduction in PCa staging at diagnosis, it has also resulted in extra, likely unnecessary biopsies, as well as higher over-detection rates [113,121,123].
Moreover, the Gleason score is used to grade biopsies for PCa by histopathological examination of tumor development. Biopsies, on the other hand, may not match the prostatectomy specimen due to sampling issues, as well as the possibility of interobserver variability. In addition, people with morphologically similar PCa may behave differently due to inter- and intra-tumor heterogeneity [124].
Several techniques are available to help determine a patient’s life expectancy; however, their estimation accuracy is limited. Actuarial life tables, which are readily available, offer a fair estimate, but they do not take into consideration specific medical comorbidities [118]. Comorbidity indices, such as the Charlson comorbidity index, predict life expectancy depending on medical conditions; however, it may ignore or exaggerate the relevance of certain morbidities [125]. Nomograms, on the other hand, may offer more precise predictions with a prediction accuracy of 69–84% for life expectancy after therapy for localized PCa [126,127,128].
Also, false-positive and false-negative biopsies reduce the diagnostic accuracy of PCa [129]. Because of the inherent heterogeneity of PCa [130], current systematic biopsy methods done with TRUS guidance often result in underdiagnosis of PCa [131]. To address these limitations, more sensitive and selective serum–tissue biomarkers should be developed, in addition to conjugating new imaging technologies with standard TRUS, to increase biopsy sensitivity [113,132].
3.2. Drugs Repurposed in Prostate Cancer: From Benchside to Bedside
Conventional anti-cancer chemotherapeutic agents have well-known side effects that severely impair cancer patients’ quality of life. Therefore, drug repurposing is an effective alternative method for identifying new anti-cancer candidates from the existing pharmacological pool [51,133]. Here, we will discuss three main categories of drug repurposing studies for PCa based on different discovery and validation methods (Table 1).
The first category is classified based on the knowledge and ability of medical practitioners or researchers to organize scientific observations of random events. For instance, ormeloxifene, a clinically approved selective estrogen receptor modulator, exhibits anti-cancer properties in multiple cancers including ovarian, head and neck, and breast cancers. However, Hafeez et al. reported ormeloxifene–mediated inhibition of oncogenic β-catenin signaling and EMT progression in PCa, mainly by repressing N-cadherin, MMPs (MMP2 and MMP3), β-catenin/TCF-4 transcriptional activity, and inducing pGSK3β expression. In addition, treatment with ormeloxifene inhibited tumorigenic, metastatic, and invasive capacity of PCa cells in vitro and reduced prostate tumors in xenograft mouse models [134]. Naftopidil, a selective adrenoreceptor A1D antagonist, is used for treating lower urinary tract symptoms triggered by benign prostatic hyperplasia [135]. It is also shown to improve the efficiency of radiotherapy (RT) treatment in PC-3 xenograft models as compared with monotherapy with naftopidil or RT [136] (Figure 2).
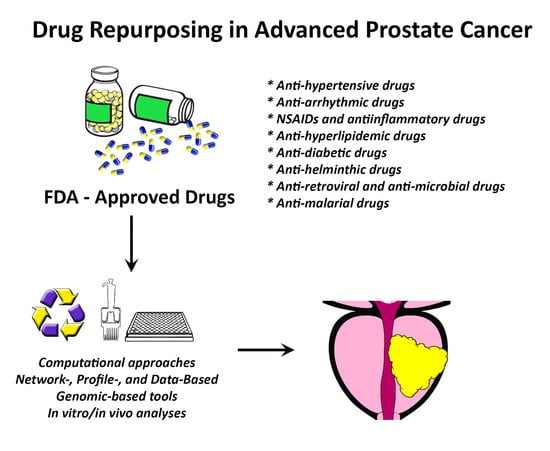
Figure 2.
Drug repurposing in advanced prostate cancer. Using computational approaches and pre-clinical analyses, many Food and Drug Administration (FDA)-approved drugs could be identified and repurposed to treat patients with advanced prostate cancer. Abbreviations: FDA: U.S. Food and Drug Administration; NSAIDs: non-steroidal anti-inflammatory drugs.

Table 1.
Summary table of the drugs that have been repurposed to be used in prostate cancer in pre-clinical models.
Table 1.
Summary table of the drugs that have been repurposed to be used in prostate cancer in pre-clinical models.
| Ref. | Drug | Original Indication | PCa Cell Lines Targeted | In Vivo Studies | Mode(s) of Action | Effect(s) |
|---|---|---|---|---|---|---|
| [137] | Propranolol | Anti-hypertensive | - | - |
|
|
| [138] | Digoxin | Anti-arrhythmic |
| |||
| [138,139] | Ouabain | Anti-arrhythmic | PPC-1 | Male SCID |
| |
| [140] | Aspirin | Anti-inflammatory | LNCaP |
|
| |
| [141] | Celecoxib | Anti-inflammatory | LNCaP & androgen-nonresponsive PC-3 | LNCaP in bovine brain extracts |
|
|
| [142] | dexamethasone | Anti-inflammatory | DU145 PCa cells |
|
| |
| [143,144,145] | Simvastatin | Anti-hyperlipidimic | PC3, 22Rv1, DU145, DU145R80, LNCaP prostate cancer cell lines and EPN normal prostate epithelial cells | DU145R80, 22Rv1 parental and docetaxel resistant cells in xenografts |
|
|
| [146,147,148] | Metformin | Anti-diabetic |
|
| ||
| [149] | Glipizide | Anti-diabetic | PC-3, 22Rv1 and DU145 PC | TRAMP transgenic mouse model |
|
|
| [150] | Mebendazole | Anti-helminthic | LNCaP |
|
| |
| [151] | Niclosamide | Anti-helminthic | LNCaP, VCaP, CWR22Rv1, PC3 and HEK293 | CWR22Rv1 cells in SCID mice |
|
|
| [152,153] | Nelfinavir | Anti-retroviral | DU145 and PC3 cell lines |
|
| |
| [154] | CMT-3 | Anti-microbial | Many lines | xenografts of PC-3 tumors |
|
|
| [155] | Zoledronic acid | Bisphosphonate | LuCaP 23.1, a PSA-producing human CaP xenograft | LuCaP 23.1, a PSA-producing human CaP xenograft |
|
|
| [156] | Valproic acid | Anti-epileptic | AR-positive (LNCaP and C4-2) and AR-negative (DU145 and PC3) | LNCaP, C4-2, and DU145 Xenograft models |
|
|
| [157] | Mifepristone | Anti-progestational steroid |
|
|
Abbreviations: AR: androgen receptor; COX: cyclooxygenase; CRPC: castration-resistant prostate cancer; ECM: extracellular matrix; PCa: prostate cancer; PR: progesterone receptor; SCID: severe combined immunodeficiency.
Nitroxoline, a widely used antibiotic for treating urinary tract infections, inhibits endothelial cell proliferation for different solid cancer types [158,159]. It also mediates AMPK-dependent inhibition of the mTOR signaling pathway and cyclin D1-Rb-Cdc25A axis suggesting its potential role for therapeutic development against PCa [160]. Nelfinavir, a human immunodeficiency virus (HIV) protease inhibitor authorized by the FDA, is typically used in therapies for HIV patients [161]. Studies have identified certain nitroxoline-mediated anti-cancer mechanisms, such as inhibition of (PI3K)/Akt signaling pathway, the proteasome, and HIF-1α which limit angiogenesis, as well as activation of endoplasmic reticulum (ER) stress, autophagy, and apoptosis [162]. Interestingly, nelfinavir shows promising therapeutic potential for the treatment of CRPC through blocking site-2 protease cleavage [153].
Second are the drugs that have been evaluated in assays and categorized according to their activity. Itraconazole, the antifungal drug, which inhibits angiogenesis and the Hedgehog signaling pathway, has been tested in phase II clinical trials and found effective in men with metastatic CRPC. In addition, digoxin was repurposed for PCa treatment as it inhibits HIF-1α synthesis and tumor growth and was clinically trialed in patients with recurrent PCa [163,164]. Clofoctol, an antibiotic used to treat upper respiratory tract infections, is a promising inhibitor of PCa. Wang and colleagues reported that clofoctol reduced the development of PCa cells at clinically feasible doses. It also induced ER stress and activated all three Unfolded Protein Response (UPR) pathways, resulting in indirect suppression of protein translation and subsequent reduction in the amounts of G1/S cyclins, resulting in G1 cell cycle arrest. Furthermore, clofoctol was found to be effective in a PCa xenograft model in animals, making it a promising anti-PCa medication candidate suitable for human testing [165]. Several fatty acid synthase (FASN) inhibitors, including antifungal agent cerulenin, its synthetic derivative C75, and triclosan have been shown to inhibit cancer cell growth by inducing cell death. However, their evaluation in clinical trials was challenged due to pharmacological limitations [166,167]. Triclosan (TSC) is an approved bactericide in personal hygiene products that possesses a good safety profile, oral bioavailability, and stability in plasma [168]. Triclosan was found to be a superior alternative to C75 and orlistat in triggering cell death in PCa cells via the inhibition of FASN. In addition, it induced G0/G1 cell cycle arrest and dose-dependent reduction in total lipid content of PCa cells [167]. As a result, TCS-mediated suppression of the metabolic oncogene FASN has the potential to be developed as a treatment for advanced PCa.
Other drugs are classified using the in-silico drug repurposing approach. For example, risperidone, an antipsychotic agent, is used for the treatment of central nervous system disorders owing to its binding affinity for dopamine D2 and serotonin 5-HT2 receptors [169]. On the other hand, 17-β-hydroxysteroid dehydrogenase 10 (17HSD10) is responsible for Alzheimer’s disease pathogenesis and PCa cell survival upon ADT. Since risperidone targets 17HSD10, it is a possible treatment choice for both Alzheimer’s disease and PCa [170].
Furthermore, Zenarestat, an aldose reductase inhibitor, may be an effective cancer chemotherapeutic drug since aldose reductases promote tumor development by activating the transcription of NF-kB and AP-1 [171]. Based on thorough gene expression profiling and disease-gene-drug association data, zenarestat was repurposed to serve as a potential medication for treatment in PCa [172].
3.2.1. Anti-Hypertensives and Anti-Arrhythmic Drugs
Beta-blockers act by blockage of beta-adrenergic receptors in the body and have been traditionally utilized for their anti-hypertensive and anti-arrhythmic properties [173]. One such beta blocker is the non-selective propranolol, which has been shown to exhibit anticancer properties, encompassing PCa [137]. Specifically, it has been shown to halt PCa proliferation and induce apoptosis, in synergism with a glucose analog, 2-deoxy-d-glucose (2DG). Propranolol also acts via inhibition of phosphatidic acid phosphatase (PAP), which has been shown to be overexpressed in certain cancers [174]. After induction by 2DG, propranolol’s blockade of PAPs leads to accumulation of LC3-II and p62, markers of autophagy, thus promoting cancer cell death [139].
Cardiac glycosides, such as digoxin and ouabain, exert their anti-arrhythmic properties via Na+/K+ ATPase pump inhibition, which increases intracellular Ca2+ levels [139]. Simspon et al. showed that ouabain sensitized resistant prostate adenocarcinoma cells (PPC-1) to aniokis, the process by which normal cells undergo apoptosis by detaching from the extracellular matrix (ECM) via a caspase-dependent mechanism [138].
Prazosin is a selective inhibitor of the alpha-1 adrenergic receptor and has been authorized for the treatment of hypertension [175]. Additionally, it is used to treat a variety of clinical conditions, including benign prostatic hyperplasia, Raynaud’s illness, and congestive heart failure [175,176,177]. In patient-derived glioblastoma-initiating cells (GICs), prazosin mediated growth inhibition through inhibiting the PKCδ- dependent AKT signaling pathway compared to neural stem cells that lack PKCδ [178]. Interestingly, prazosin has been shown to display antiproliferative activity, superior to that of other α1-blockers, by inducing G2 checkpoint arrest and subsequent apoptosis in PCa cell lines [179].
3.2.2. NSAIDS, Anti-Inflammatory Drugs and Aspirin
Non-steroidal anti-inflammatory drugs (NSAIDs) act via inhibition of the cyclooxygenase (COX) pathway, thus limiting prostaglandin (PG) synthesis, and are one of the most widely used drugs for their anti-inflammatory, antipyretic, and analgesic effects [180]. NSAIDs have been shown to affect the proliferative, apoptotic, resistance, and metastatic potential of many PCa cell lines via both COX-dependent and independent mechanisms. The aforementioned results highlight its potential for use in therapy-resistant PCa [181]. An example of the COX-independent mechanism is demonstrated in the antitumorigenic effects of statin and aspirin combination on LNCaP cells, which are androgen-sensitive human PCa cells, via reduction in cyclin D1 levels [140]. Celecoxib, a selective COX-2 inhibitor, blocks Akt phosphorylation and activation, which, in turn, leads to apoptosis in PCa cells [141]. Another anti-inflammatory agent, the glucocorticoid dexamethasone, inhibited ERG activity, an oncogenic transcription factor, of prostate tumor cells in an in-silico model [142].
According to clinical studies, patients undergoing long-term NSAID treatment have a decreased chance of acquiring cancer [182,183]. Indomethacin, an NSAID used for rheumatic disease treatment [184,185], is a notable antineoplastic agent. Mechanistically, it inhibits Cox-1/2-dependent angiogenesis [186,187] as well as MAPK pathways [188]. It also inhibits cancer cell growth via impairing PKC-p38-DRP1 axis-dependent mitochondrial dynamics or downregulating Wnt/β-catenin signaling [189]. A clinical trial is currently ongoing (ClinicalTrials.gov; NCT02935205) to study the combined effect of hormone therapy using enzalutamide and indomethacin on PCa by lowering the amount of androgen the body makes and/or blocking the use of androgen by the tumor cells.
3.2.3. Anti-Hyperlipidemic Drugs
Statins act as lipid-lowering agents by inhibiting the HMG-CoA reductase enzyme and thus inhibiting the rate-limiting step cholesterol biosynthesis [190]. However, preclinical and clinical evidence show that statins exhibit anti-neoplastic activity in various cancers [191,192]. Ianelli et al. demonstrated the synergistic effects of simvastatin and valproic acid in sensitizing PCa to docletaxel and decreasing resistance rates. This combination method targets the CSCs by inhibiting the action of the Yes-associated protein (YAP) oncogene, a transcriptional regulator implicated in many cancers, including PCa [143,144]. Zhuang et al. demonstrated that simvastatin acts to promote apoptosis via cholesterol depletion. Specifically, simvastatin acts via depletion of cholesterol-containing lipid rafts of PCa cells, which, in turn, inhibits protein kinase B (also known as AKT) signaling pathway [193]. Statins also have a role in sensitizing PCa cells to ADT. Kong et al. have shown that simvastatin treatment helps to re-sensitize PCa cell lines that have developed resistance to enzalutamide, an FDA approved anti-androgenic agent for the treatment of CRPC [145].
In a retrospective cohort study of men who underwent prostate biopsy, statin decreased risk of PCa and resulted in a better prognosis and reduction in PCa volume and PSA levels [194]. Other studies have indicated that statins reduce PCa-related mortality and risk [195,196], while others have found no impact [194]. Inconsistent clinical results make a definite link between statin usage and PCa difficult to establish [197].
3.2.4. Anti-Diabetic Drugs
Metformin, a member of the biguanide family, acts as an anti-diabetic agent by increasing sensitivity to insulin, inhibiting hepatic production of glucose, and restricting hepatic gluconeogenesis [198]. Aside from its anti-diabetic properties, metformin has shown the potential to be used for its anti-cancer properties, particularly in PCa. Metformin inhibits the mitochondrial complex I of the electron transport chain, which leads to an increase in adenosine monophosphate-activated protein kinase (AMPK), in turn leading to the inhibition of the mammalian target of rapamycin (mTOR) and activation of tuberous sclerosis complex-2, a tumor suppressor gene [146]. By decreasing the levels of insulin in circulation, metformin downregulates phosphoinositide-3-kinase (PI3K) axis, a main contributor to cell growth, proliferation, and differentiation [147]. Furthermore, metformin has been shown to decrease androgen receptor expression, which could establish its role as an adjunct to ADT [148]. Glipizide, another antidiabetic drug that stimulates beta-cell insulin secretion, has shown tumor suppressive effects of PCa cells in a TRAMP transgenic mouse model. It does so mainly via inhibition of angiogenesis, particularly by targeting HMGIY/ANGPT1 signaling pathway [149].
Metformin also has significant anti-cancer activity [199,200,201] in breast, prostate, lung, cervix, ovarian, and CNS cancers, and it is being used in phase 1–4 clinical trials for cancer therapy [202]. Metformin reduced the risk of PCa diagnosis in comparison to other oral hypoglycemics [203]. In xenograft models, the combination of metformin and chemotherapy inhibits tumor development and prevents recurrence of PCa cells via inhibiting inflammatory pathways [204]. In addition, metformin and valproic acid (VPA) synergistically suppressed proliferation in both LNCaP (androgen dependent) and PC-3 (androgen independent) cell lines and enhanced apoptosis in patient-derived prostate tumor explants [205].
Long-term metformin therapy has also been found to significantly reduce the incidence of breast cancer in women with type 2 diabetes [206]. By reducing elevated insulin levels, metformin inhibits the growth of tumors expressing insulin receptors [207,208]. Metformin has also been shown to activate AMPK, a critical energy sensor in cellular metabolism, and to limit cancer cell proliferation via the negative regulation of mTOR needed for tumor survival [209].
3.2.5. Anti-Helminthic Drugs
Mebendazole is a microtubule inhibiting drug that fights parasitic infections. Docetaxel is a treatment option for metastatic PCa; however, it had a modest effect on increasing the median survival time [210]. Rushworth et al. showed that mebendazole and docetaxel act synergistically to inhibit both in vitro and in vivo tumor growth of murine-derived PCC. This synergism is proposed to be through the inhibition of microtubule assembly at distinct areas, increasing the mitotic blockade of G2/M and thus, promoting cell death [150]. Niclosamide, another anthelminthic drug, has been shown to potently decrease expression of the androgen receptor variant AR-V7, which drives castration resistant PCa (CRPC). Consequently, it has potential to sensitize treatment to enzalutamide, an anti-androgen of the second generation, which is approved for use in treatment of patients with CRPC that are no longer responsive to docetaxel [151].
Niclosamide, an FDA-approved antihelminthic drug, exerts an anticancer effect in multiple types of cancer [211,212]. For example, niclosamide inhibits colorectal cancer cells’ liver metastases by downregulating S100A4 and blocking the Wnt/β-catenin signaling pathway [213,214]. It decreases lung cancer invasion by inhibiting the S100A4/NF-B/MMP9 axis: 350 reduces both the breast cancer cell pulmonary metastases and metastatic potential of lapatinib-resistant breast cancer cells by inhibiting the STAT3-FAK-Src axis [215] and epithelial-mesenchymal transition (EMT) [216], respectively. Furthermore, it inhibits the IL6-STAT3-AR signaling pathways in enzalutamide-resistant advanced PCa cells, reversing drug resistance and cellular invasion [217].
3.2.6. Anti-Retroviral Drugs
Nelfinavir, a protease inhibitor used in HIV combination therapy, has shown potential to be repurposed in cancer therapy [152]. Guan et al. demonstrated that nelfinavir and its analogues help combat CRPC by directly inhibiting Site-2 Protease (S2P) cleavage and Regulated Intramembrane Processing (RIP) [153].
3.2.7. Anti-Microbial Drugs
Minocycline, a semi-synthetic tetracycline derivative, was initially given FDA-approval for acne and some STDs, but it was later shown to have other non-antibiotic beneficial properties, including usage in inflammatory diseases [218]. Lokeshwar showed that chemically modified tetracyclines, particularly CMT-3, was able to promote apoptosis in PCa cell lines via activation of caspase-3 and caspase-9 [154].
Antifungal itraconazole, which has a high safety profile, has been noted for its anti-angiogenesis properties [219,220]. It inhibits mTOR, which reduces angiogenesis through the cholesterol trafficking route [221]. Itraconazole binds to the sterol-sensing domain of NPC1 and targets the mitochondrial protein VDAC1 to regulate AMPK and MTOR, resulting in the suppression of cell proliferation and angiogenesis [222]. In addition, it downregulates the PDGF/PI3K/Akt/mTOR pathway in infantile hemangioma [223]. A non-comparative, randomized, phase II study was conducted to evaluate the antitumor efficacy of two doses of oral itraconazole in men with metastatic PCa. Results showed that high-dose itraconazole (600 mg/day) has a modest antitumor activity in men with metastatic CRPC [219].
3.2.8. Anti-Malarial Drugs
Artemisinin (ARS) is a well-known antimalarial medication that is used to treat about 600 million cases of malaria every year [224]. According to recent research, ARS and its derivatives exhibit anticancer activity as they promote non-apoptotic programmed cell death [225,226,227]. For example, artesunate (ART)-based drugs increased ROS production and favored lysosomal over autophagic ferritin degradation in cancer cells [228].
Similarly, chloroquine (CQ) and its derivative, hydroxychloroquine (HCQ), are antimalarial drugs that also treat several diseases, including rheumatoid arthritis, discoid lupus erythematosus, and systemic lupus erythematosus [229,230]. CQ or HCQ, the only FDA-approved autophagy flux inhibitors, have been used to treat pancreatic and other cancers [231,232].
Quinacrine, an antimalarial drug discovered in the 1920s, aids in the treatment of a variety of malignancies, owing to its ability to activate the p53 gene [233]. Mainly, quinacrine induces p53 expression by facilitating the chromatin transcription (FACT) protein complex, which is trapped onto the chromatin, thereby inhibiting CK2-mediated phosphorylation of p53 [234,235].
3.2.9. Others
Zoledronic acid (ZA), a member of the bisphosphonates class of drugs, inhibits bone resorption via inhibition of osteoclast activity, hence its role in treatment of osteoporosis [236]. This repurposed drug has been approved for clinical use in treating PCa. Corey et al. demonstrated the anti-PCa effects of ZA, both in vivo by decreasing metastatic potential and in vitro by inducing G1 arrest, proliferation decline, and apoptosis [155]. In regard to PCa, ZA inhibits osteoblastic and osteolytic metastasis [155], while it clinically reduces skeletal complications in PCa patients with bone metastases [237]. Although the treatment with celecoxib, a COX-2 inhibitor, in combination with ZA therapy, is considered complementary for the treatment of advanced or metastatic PCa, it did not improve the survival rate [238]. In contrast, the combination of ZA and docetaxel enhanced PCa patients’ survival [210]. Based on early clinical trials, ZA is typically given every three weeks; however, current evidence suggests that giving ZA every 12 weeks results in similar outcomes in men with CRPC bone metastases [239].
Valproic acid (VPA) is used in the treatment of epileptic, bipolar, and schizophrenic disorders by inhibiting class I histone deacetylases (HDAC) [240]. Xia et al. demonstrated that chronic VPA administration results in reduced PCa cellular proliferation and upregulated caspase-2 and caspase-3 activity [156].
Mifepristone, an anti-progesterone with potent progesterone receptor affinity, is used for pregnancy termination. Etreby et al. showed mifepristone’s anti-cancer effects in both androgen sensitive and insensitive PCa, via induction of TFG-β-1, a pro-apoptotic transcription factor [157]. In fact, mifepristone’s anticancer activity is known for its ability to inhibit tumor growth via blocking the overexpressed cell surface receptors, such as progesterone, estrogen, and glucocorticoid receptors, in CRPC cells [241]. With combined estrogen treatment, anti-progesterone delivery to PCa cells suppressed the development of androgen-insensitive PCa in vivo [242]. The anti-tumor activity of mifepristone was reported against multiple cancer types, including androgen-sensitive and androgen-insensitive PCa [243]. In addition, a phase II study, performed by Taplin et al., suggested that the combination therapy of mifepristone with corticosteroids, ketoconazole, or 5-α reductase inhibitors might prevent the compensatory surge in adrenal androgens in patients with CRPC [244].
Disulfiram was first employed in the rubber vulcanization process, but it has been utilized as an alcohol-aversion medication to treat alcoholism [245,246]. Disulfiram’s mechanisms of action are closely linked to the acetaldehyde dehydrogenase (ALDH)-related cellular metabolic processes. For example, disulfiram-mediated acetaldehyde metabolism is a viable therapeutic target for BRCA1/2-deficient cancer cells [247]. ALDH positive atypical teratoid/rhabdoid tumor cells also had their metabolism decreased by disulfiram. Furthermore, because of its ability to block ALDH, disulfiram inhibits formaldehyde oxidation in cancer cells, resulting in cell death [248,249]. In addition, ALDH maintains the stemness of cancer cells, which explains disulfiram’s anticancer action in PCSCs [250,251]. Disulfiram serves as a DNA methyltransferase (DNMT1) inhibitor that restores tumor suppressor genes and DNA demethylation in PCa cells. Mainly, it reduces 5-methyl cytosine (5meC) content and methylation in APC and RARB gene promoters [252]. Hence, in a pilot trial for recurrent PCa, Schweizer and colleagues tested for disulfiram in epigenetic therapy via evaluating 5meC content in peripheral blood mononuclear cells (PBMC) [253]. Additionally, enhanced reduction in tumor growth was obtained upon the combination of disulfiram with Cu2+ in CRPC xenografts [254]. In addition, diocarb (disulfiram metabolite) and copper complex may potentially target NPL4 protein to regulate protein turnover of tumorigenesis, promoting stress-response pathways [255].
Rapamycin has been repurposed for cancer therapy after being authorized as an immunosuppressant for kidney transplantation [256] and an anti-restenosis drug [257,258]. Rapamycin inhibits mTORC1 by binding to the FRB domain of mTOR and hence its use in cancer treatment [259]. Treatment with rapamycin reduced leukemic progenitor cells in patients with acute myeloid leukemia [260,261]. It also demonstrated efficient antitumor activity in patients with drug-resistant chronic myelogenous leukemia, with very minor adverse effects in the majority of cases [262,263]. A study by our group demonstrated that Rapamycin is effective in the in vitro treatment of glioblastoma and neuroblastoma by targeting their CSC population [264].
Thalidomide, a glutamic acid derivative, was used as a sedative in 1957 and to treat morning sickness in pregnant women [265]. Nowadays, thalidomide is widely recognized as an antiangiogenic agent that inhibits VEGF, bFGF, tumor necrosis factor-alpha (TNF-α), and various other pro-angiogenic factors [266].
Interestingly, a study by our group assessed the in vitro and in vivo antitumor properties and mechanisms of action of ST1926 synthetic retinoids in targeting the cancer stem-like cells population of human PCa. Our results revealed that ST1926 substantially reduced proliferation of PCa cells and induced cell cycle arrest, p53-independent apoptosis, and early DNA damage. It also significantly reduced prostate spheres’ formation ability in vitro, denoting sufficient eradication of the self-renewal ability of the highly androgen-resistant CSCs [267].
4. Clinical Trials of Drugs Repurposed in PCa
Typically used cancer therapies are known to carry severe side effects. Findings of medications that are not typically used for this disease is encouraging for several reasons: they are already discovered, most have been on the market for a long time, proving their safety, they can help with diagnosing or treating, many are cost-effective when compared to chemotherapy or radiation, and they may improve quality of life, with minimal side effects. This applies to PCa.
We have shown that many drugs have been tested in preclinical studies to assess their anti-tumor activity in PCa. However, many of those drugs have already made it into clinical trials. Categories of those medications include anti-inflammatory (both steroidal and non-steroidal), anti-arrhythmic, anti-diabetic, anti-helminthic, anti-fungal, antibiotics, lipid-lowering agents, anticoagulants, bisphosphonates, anti-parasitics, immunomodulators, growth factor inhibitors, farnesyltransferase inhibitors, ribonucleotide reductase inhibitors, polysaccharide, retinoid derivatives, hormones, sugars, cyclic peptides, alpha-keto acids, fatty acids, vitamins, and chemical elements (Table 2).

Table 2.
Summary table of the drugs that have been repurposed to be used in prostate cancer in clinical trials.
5. Conclusions and Future Directions
Prostate cancer cells exploit various cell signaling channels to become castration resistant. Blockade of these channels can terminate or slow the growth of resistant tumors. A plethora of drugs designed for other diseases target the channels used by CRPC. Thus, repurposing these drugs to treat PCa will increase the treatment repertoire. Repurposing offers the advantage of bypassing Phase I and II clinical trials and, thus, speeds up drug approval. Many clinical trials have been designed that repurpose drugs to treat PCa, and more will follow. As electronic health records become more integrated, and artificial intelligence and precision medicine advance, more drugs will be strategically repurposed to treat PCa. Drugs that target the nuances of patient-specific tumors will be identified and hopefully improve patient life expectancy, cost of treatment, and quality of life. Functional genomics is also widely used to map cancer dependencies and identify therapeutic targets in cancer. This includes genetic screens based on CRISPR/Cas9 technology [69,70,71], reverse genetic screens, and chemogenomic screens [72]. In the era of personalized medicine, drug repurposing presents an opportunity and should be implicated, using computational genomic and proteomic technologies, to guide clinicians in their decision-making regarding patient treatment. Studies should continue reviewing the current trends of repurposing, as this is an efficient and valuable practice for drug discovery.
Author Contributions
Conceptualization, H.F.B.; methodology, H.F.B., T.D., D.D., M.M.M., J.C.A.M., F.P. and A.M.; validation, H.F.B., T.D., D.D., M.M.M., J.C.A.M., F.P., O.L., A.M., W.A.-K., A.M.N., R.P. and Y.O.; investigation, H.F.B. and T.D.; resources, H.F.B., T.D., D.D., M.M.M., J.C.A.M., O.L., F.P., A.M., W.A.-K., A.M.N., R.P. and Y.O.; data curation, H.F.B., T.D., D.D., M.M.M., J.C.A.M., F.P. and A.M.; writing—original draft preparation, H.F.B., T.D., D.D., M.M.M., J.C.A.M., F.P. and A.M.; writing—review and editing, H.F.B., T.D., D.D., M.M.M., J.C.A.M., F.P., O.L., A.M., W.A.-K., A.M.N., R.P. and Y.O.; visualization, H.F.B., W.A.-K., A.M.N., R.P. and Y.O.; supervision, H.F.B., W.A.-K., A.M.N., R.P. and Y.O.; project administration, H.F.B., R.P. and Y.O.; funding acquisition, H.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank all members of the Arkadi M. Rywlin M.D. Department of Pathology and Laboratory Medicine, Mount Sinai Medical Center (Miami Beach, FL, USA) and the Herbert Wertheim College of Medicine, Florida International University (Miami, FL, USA), and the Abou-Kheir’s group for their help with this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Stamey, T.A.; Donaldson, A.N.; Yemoto, C.E.; McNeal, J.E.; Sözen, S.; Gill, H. Histological and clinical findings in 896 consecutive prostates treated only with radical retropubic prostatectomy: Epidemiologic significance of annual changes. J. Urol. 1998, 160, 2412–2417. [Google Scholar] [CrossRef]
- Al-Hussain, T.; Carter, H.B.; Epstein, J.I. Significance of prostate adenocarcinoma perineural invasion on biopsy in patients who are otherwise candidates for active surveillance. J. Urol. 2011, 186, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Rakic, N.; Jamil, M.; Keeley, J.; Sood, A.; Vetterlein, M.; Dalela, D.; Arora, S.; Modonutti, D.; Bronkema, C.; Novara, G.; et al. Evaluation of lymphovascular invasion as a prognostic predictor of overall survival after radical prostatectomy. Urol. Oncol. 2021, 39, 495.e1–495.e6. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Iida, M.; Yamaguchi, M.; Shida, K. Analysis of bone metastasis of prostatic adenocarcinoma in 137 autopsy cases. Adv. Exp. Med. Biol. 1992, 324, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Karakiewicz, P.I.; Benayoun, S.; Kattan, M.W.; Perrotte, P.; Valiquette, L.; Scardino, P.T.; Cagiannos, I.; Heinzer, H.; Tanguay, S.; Aprikian, A.G.; et al. Development and validation of a nomogram predicting the outcome of prostate biopsy based on patient age, digital rectal examination and serum prostate specific antigen. J. Urol. 2005, 173, 1930–1934. [Google Scholar] [CrossRef]
- Bjurlin, M.A.; Taneja, S.S. Standards for prostate biopsy. Curr. Opin. Urol. 2014, 24, 155–161. [Google Scholar] [CrossRef]
- Pound, C.R.; Partin, A.W.; Epstein, J.I.; Walsh, P.C. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol. Clin. N. Am. 1997, 24, 395–406. [Google Scholar] [CrossRef]
- Chen, R.C.; Rumble, R.B.; Loblaw, D.A.; Finelli, A.; Ehdaie, B.; Cooperberg, M.R.; Morgan, S.C.; Tyldesley, S.; Haluschak, J.J.; Tan, W.; et al. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J. Clin. Oncol. 2016, 34, 2182–2190. [Google Scholar] [CrossRef]
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J. Urol. 2018, 199, 683–690. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J. Urol. 2021, 205, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, M. Management of biochemically recurrent prostate cancer following local therapy. Am. J. Manag. Care 2014, 20, S273–S281. [Google Scholar] [PubMed]
- Han, M.; Partin, A.W.; Zahurak, M.; Piantadosi, S.; Epstein, J.I.; Walsh, P.C. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J. Urol. 2003, 169, 517–523. [Google Scholar] [CrossRef]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, A.E.; Davis, D.L.; Milatovich, A.; Mendonca, B.B.; Imperato-McGinley, J.; Griffin, J.E.; Francke, U.; Wilson, J.D.; Russell, D.W. Molecular genetics of steroid 5 alpha-reductase 2 deficiency. J. Clin. Investig. 1992, 90, 799–809. [Google Scholar] [CrossRef] [PubMed]
- HUGGINS, C.; JOHNSON, M.A. Cancer of the bladder and prostate. J. Am. Med. Assoc. 1947, 135, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Paller, C.; Kyprianou, N. Mechanisms of Therapeutic Resistance in Prostate Cancer. Curr. Oncol. Rep. 2017, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Chen, W.Y.; Abou-Kheir, W.; Zeng, T.; Yin, J.J.; Bahmad, H.; Lee, Y.C.; Liu, Y.N. Androgen deprivation therapy-induced epithelial-mesenchymal transition of prostate cancer through downregulating SPDEF and activating CCL2. Biochim. Biophys. Acta Mol. Basis. Dis. 2018, 1864, 1717–1727. [Google Scholar] [CrossRef]
- Cheaito, K.A.; Bahmad, H.F.; Hadadeh, O.; Saleh, E.; Dagher, C.; Hammoud, M.S.; Shahait, M.; Mrad, Z.A.; Nassif, S.; Tawil, A.; et al. EMT Markers in Locally-Advanced Prostate Cancer: Predicting Recurrence? Front. Oncol. 2019, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Li, S.; Huang, J.Y.; He, Z.Q.; Meng, X.Y.; Cao, Y.; Fang, C.; Zeng, X.T. Androgen receptor gene polymorphisms and risk of prostate cancer: A meta-analysis. Sci. Rep. 2017, 7, 40554. [Google Scholar] [CrossRef] [PubMed]
- Maitland, N.J. Resistance to Antiandrogens in Prostate Cancer: Is It Inevitable, Intrinsic or Induced? Cancers 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.B.; Li, J.; Meniel, V.S.; Fennell, C.M.; Waring, P.; Montgomery, K.G.; Rebello, R.J.; Macpherson, A.A.; Koushyar, S.; Furic, L.; et al. Identification of Pik3ca Mutation as a Genetic Driver of Prostate Cancer That Cooperates with Pten Loss to Accelerate Progression and Castration-Resistant Growth. Cancer Discov. 2018, 8, 764–779. [Google Scholar] [CrossRef]
- Li, S.; Fong, K.W.; Gritsina, G.; Zhang, A.; Zhao, J.C.; Kim, J.; Sharp, A.; Yuan, W.; Aversa, C.; Yang, X.J.; et al. Activation of MAPK Signaling by CXCR7 Leads to Enzalutamide Resistance in Prostate Cancer. Cancer Res. 2019, 79, 2580–2592. [Google Scholar] [CrossRef]
- Yamamoto, H.; Oue, N.; Sato, A.; Hasegawa, Y.; Matsubara, A.; Yasui, W.; Kikuchi, A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene 2010, 29, 2036–2046. [Google Scholar] [CrossRef]
- Mulholland, D.J.; Kobayashi, N.; Ruscetti, M.; Zhi, A.; Tran, L.M.; Huang, J.; Gleave, M.; Wu, H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012, 72, 1878–1889. [Google Scholar] [CrossRef]
- Carracedo, A.; Ma, L.; Teruya-Feldstein, J.; Rojo, F.; Salmena, L.; Alimonti, A.; Egia, A.; Sasaki, A.T.; Thomas, G.; Kozma, S.C.; et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Investig. 2008, 118, 3065–3074. [Google Scholar] [CrossRef]
- Kinkade, C.W.; Castillo-Martin, M.; Puzio-Kuter, A.; Yan, J.; Foster, T.H.; Gao, H.; Sun, Y.; Ouyang, X.; Gerald, W.L.; Cordon-Cardo, C.; et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J. Clin. Investig. 2008, 118, 3051–3064. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef]
- Kim, J.; Wu, L.; Zhao, J.C.; Jin, H.J.; Yu, J. TMPRSS2-ERG gene fusions induce prostate tumorigenesis by modulating microRNA miR-200c. Oncogene 2014, 33, 5183–5192. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Blee, A.M.; Wang, D.; An, J.; Pan, Y.; Yan, Y.; Ma, T.; He, Y.; Dugdale, J.; Hou, X.; et al. Loss of FOXO1 Cooperates with TMPRSS2-ERG Overexpression to Promote Prostate Tumorigenesis and Cell Invasion. Cancer Res. 2017, 77, 6524–6537. [Google Scholar] [CrossRef] [PubMed]
- Rybak, A.P.; Bristow, R.G.; Kapoor, A. Prostate cancer stem cells: Deciphering the origins and pathways involved in prostate tumorigenesis and aggression. Oncotarget 2015, 6, 1900–1919. [Google Scholar] [CrossRef]
- Vadde, R.; Vemula, S.; Jinka, R.; Merchant, N.; Bramhachari, P.V.; Nagaraju, G.P. Role of hypoxia-inducible factors (HIF) in the maintenance of stemness and malignancy of colorectal cancer. Crit. Rev. Oncol. Hematol. 2017, 113, 22–27. [Google Scholar] [CrossRef]
- O’Reilly, D.; Johnson, P.; Buchanan, P.J. Hypoxia induced cancer stem cell enrichment promotes resistance to androgen deprivation therapy in prostate cancer. Steroids 2019, 152, 108497. [Google Scholar] [CrossRef]
- Swinney, D.C. Phenotypic vs. target-based drug discovery for first-in-class medicines. Clin. Pharmacol. Ther. 2013, 93, 299–301. [Google Scholar] [CrossRef]
- Eder, J.; Sedrani, R.; Wiesmann, C. The discovery of first-in-class drugs: Origins and evolution. Nat. Rev. Drug Discov. 2014, 13, 577–587. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Phillips, D.J. Pfizer’s Expiring Viagra Patent Adversely Affects Other Drugmakers Too. Available online: https://www.forbes.com/sites/investor/2013/12/20/pfizers-expiring-viagra-patent-adversely-affects-other-drugmakers-too/?sh=5349531468d4 (accessed on 11 January 2021).
- Kim, J.H.; Scialli, A.R. Thalidomide: The tragedy of birth defects and the effective treatment of disease. Toxicol. Sci. 2011, 122, 1–6. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Singhal, S.; Mehta, J.; Desikan, R.; Ayers, D.; Roberson, P.; Eddlemon, P.; Munshi, N.; Anaissie, E.; Wilson, C.; Dhodapkar, M.; et al. Antitumor activity of thalidomide in refractory multiple myeloma. N. Engl. J. Med. 1999, 341, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Ye, D.; Liu, Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents 2020, 55, 105948. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.K.; DiPaola, R.S.; Romanelli, F.; Dutch, R.E. Rapid repurposing of drugs for COVID-19. Science 2020, 368, 829–830. [Google Scholar] [CrossRef] [PubMed]
- Serafin, M.B.; Bottega, A.; Foletto, V.S.; da Rosa, T.F.; Horner, A.; Horner, R. Drug repositioning is an alternative for the treatment of coronavirus COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105969. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Abou-Kheir, W. Crosstalk between COVID-19 and prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 561–563. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef]
- Sun, M.L.; Yang, J.M.; Sun, Y.P.; Su, G.H. Inhibitors of RAS Might Be a Good Choice for the Therapy of COVID-19 Pneumonia. Chin. J. Tuberc. Respir. Dis. 2020, 43, 219–222. [Google Scholar] [CrossRef]
- Breckenridge, A.; Jacob, R. Overcoming the legal and regulatory barriers to drug repurposing. Nat. Rev. Drug Discov. 2019, 18, 1–2. [Google Scholar] [CrossRef]
- Shim, J.S.; Liu, J.O. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int. J. Biol. Sci. 2014, 10, 654–663. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Seiler, K.P.; George, G.A.; Happ, M.P.; Bodycombe, N.E.; Carrinski, H.A.; Norton, S.; Brudz, S.; Sullivan, J.P.; Muhlich, J.; Serrano, M.; et al. ChemBank: A small-molecule screening and cheminformatics resource database. Nucleic Acids Res. 2008, 36, D351–D359. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.org: Online Mendelian Inheritance in Man (OMIM(R)), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Hurle, M.R.; Yang, L.; Xie, Q.; Rajpal, D.K.; Sanseau, P.; Agarwal, P. Computational drug repositioning: From data to therapeutics. Clin. Pharmacol. Ther. 2013, 93, 335–341. [Google Scholar] [CrossRef]
- Sanseau, P.; Agarwal, P.; Barnes, M.R.; Pastinen, T.; Richards, J.B.; Cardon, L.R.; Mooser, V. Use of genome-wide association studies for drug repositioning. Nat. Biotechnol. 2012, 30, 317–320. [Google Scholar] [CrossRef]
- Willyard, C. New human gene tally reignites debate. Nature 2018, 558, 354–355. [Google Scholar] [CrossRef]
- Grover, M.P.; Ballouz, S.; Mohanasundaram, K.A.; George, R.A.; Goscinski, A.; Crowley, T.M.; Sherman, C.D.; Wouters, M.A. Novel therapeutics for coronary artery disease from genome-wide association study data. BMC Med. Genom. 2015, 8 (Suppl. S2), S1. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, H.Y. Rational drug repositioning by medical genetics. Nat. Biotechnol. 2013, 31, 1080–1082. [Google Scholar] [CrossRef]
- Greene, C.S.; Voight, B.F. Pathway and network-based strategies to translate genetic discoveries into effective therapies. Hum. Mol. Genet. 2016, 25, R94–R98. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; McGovern, D.P.; Barrett, J.C.; Wang, K.; Radford-Smith, G.L.; Ahmad, T.; Lees, C.W.; Balschun, T.; Lee, J.; Roberts, R.; et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 2010, 42, 1118–1125. [Google Scholar] [CrossRef]
- Wu, H.; Gao, L.; Dong, J.; Yang, X. Detecting overlapping protein complexes by rough-fuzzy clustering in protein-protein interaction networks. PLoS ONE 2014, 9, e91856. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Huang, J.; Ma, Z.; Zhang, J.; Zou, Y.; Gao, L. Inferring drug-disease associations based on known protein complexes. BMC Med. Genom. 2015, 8 (Suppl. S2), S2. [Google Scholar] [CrossRef]
- Lu, J.; Chen, L.; Yin, J.; Huang, T.; Bi, Y.; Kong, X.; Zheng, M.; Cai, Y.D. Identification of new candidate drugs for lung cancer using chemical-chemical interactions, chemical-protein interactions and a K-means clustering algorithm. J. Biomol. Struct. Dyn. 2016, 34, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Subelj, L.; Bajec, M. Unfolding communities in large complex networks: Combining defensive and offensive label propagation for core extraction. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2011, 83, 036103. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Navarro, C.; Cano, C.; Fajardo, W.; Blanco, A. DrugNet: Network-based drug-disease prioritization by integrating heterogeneous data. Artif. Intell. Med. 2015, 63, 41–49. [Google Scholar] [CrossRef]
- Fei, T.; Chen, Y.; Xiao, T.; Li, W.; Cato, L.; Zhang, P.; Cotter, M.B.; Bowden, M.; Lis, R.T.; Zhao, S.G.; et al. Genome-wide CRISPR screen identifies HNRNPL as a prostate cancer dependency regulating RNA splicing. Proc. Natl. Acad. Sci. USA 2017, 114, E5207–E5215. [Google Scholar] [CrossRef]
- Lei, H.; Wang, Z.; Jiang, D.; Liu, F.; Liu, M.; Lei, X.; Yang, Y.; He, B.; Yan, M.; Huang, H.; et al. CRISPR screening identifies CDK12 as a conservative vulnerability of prostate cancer. Cell Death Dis. 2021, 12, 740. [Google Scholar] [CrossRef]
- Tsujino, T.; Komura, K.; Inamoto, T.; Azuma, H. CRISPR Screen Contributes to Novel Target Discovery in Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 12777. [Google Scholar] [CrossRef]
- Blackman, J.S.; Knighton, L.E.; Takakuwa, J.E.; Calderwood, S.K.; Truman, A.W. Chemogenomic screening identifies the Hsp70 co-chaperone DNAJA1 as a hub for anticancer drug resistance. Sci. Rep. 2020, 10, 13831. [Google Scholar] [CrossRef]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting new molecular targets for known drugs. Nature 2009, 462, 175–181. [Google Scholar] [CrossRef]
- Hieronymus, H.; Lamb, J.; Ross, K.N.; Peng, X.P.; Clement, C.; Rodina, A.; Nieto, M.; Du, J.; Stegmaier, K.; Raj, S.M.; et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell 2006, 10, 321–330. [Google Scholar] [CrossRef]
- Iorio, F.; Rittman, T.; Ge, H.; Menden, M.; Saez-Rodriguez, J. Transcriptional data: A new gateway to drug repositioning? Drug Discov. Today 2013, 18, 350–357. [Google Scholar] [CrossRef]
- Dudley, J.T.; Deshpande, T.; Butte, A.J. Exploiting drug-disease relationships for computational drug repositioning. Brief. Bioinform. 2011, 12, 303–311. [Google Scholar] [CrossRef]
- Sirota, M.; Dudley, J.T.; Kim, J.; Chiang, A.P.; Morgan, A.A.; Sweet-Cordero, A.; Sage, J.; Butte, A.J. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci. Transl. Med. 2011, 3, 96ra77. [Google Scholar] [CrossRef]
- Dudley, J.T.; Sirota, M.; Shenoy, M.; Pai, R.K.; Roedder, S.; Chiang, A.P.; Morgan, A.A.; Sarwal, M.M.; Pasricha, P.J.; Butte, A.J. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci. Transl. Med. 2011, 3, 96ra76. [Google Scholar] [CrossRef]
- Mirza, N.; Sills, G.J.; Pirmohamed, M.; Marson, A.G. Identifying new antiepileptic drugs through genomics-based drug repurposing. Hum. Mol. Genet. 2017, 26, 527–537. [Google Scholar] [CrossRef]
- Shin, E.; Lee, Y.C.; Kim, S.R.; Kim, S.H.; Park, J. Drug Signature-based Finding of Additional Clinical Use of LC28-0126 for Neutrophilic Bronchial Asthma. Sci. Rep. 2015, 5, 17784. [Google Scholar] [CrossRef]
- Malcomson, B.; Wilson, H.; Veglia, E.; Thillaiyampalam, G.; Barsden, R.; Donegan, S.; El Banna, A.; Elborn, J.S.; Ennis, M.; Kelly, C.; et al. Connectivity mapping (ssCMap) to predict A20-inducing drugs and their antiinflammatory action in cystic fibrosis. Proc. Natl. Acad. Sci. USA 2016, 113, E3725–E3734. [Google Scholar] [CrossRef]
- Wagner, A.; Cohen, N.; Kelder, T.; Amit, U.; Liebman, E.; Steinberg, D.M.; Radonjic, M.; Ruppin, E. Drugs that reverse disease transcriptomic signatures are more effective in a mouse model of dyslipidemia. Mol. Syst. Biol. 2015, 11, 791. [Google Scholar] [CrossRef]
- Wei, G.; Twomey, D.; Lamb, J.; Schlis, K.; Agarwal, J.; Stam, R.W.; Opferman, J.T.; Sallan, S.E.; den Boer, M.L.; Pieters, R.; et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell 2006, 10, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I.; Tropsha, A.; Faulon, J.L.; Rintoul, M.D. Systems chemical biology. Nat. Chem. Biol. 2007, 3, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Iorio, F.; Isacchi, A.; di Bernardo, D.; Brunetti-Pierri, N. Identification of small molecules enhancing autophagic function from drug network analysis. Autophagy 2010, 6, 1204–1205. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Lee, A.; Lee, K.; Kim, D. Using reverse docking for target identification and its applications for drug discovery. Expert Opin. Drug Discov. 2016, 11, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Dakshanamurthy, S.; Issa, N.T.; Assefnia, S.; Seshasayee, A.; Peters, O.J.; Madhavan, S.; Uren, A.; Brown, M.L.; Byers, S.W. Predicting new indications for approved drugs using a proteochemometric method. J. Med. Chem. 2012, 55, 6832–6848. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Campillos, M.; Kuhn, M.; Gavin, A.C.; Jensen, L.J.; Bork, P. Drug target identification using side-effect similarity. Science 2008, 321, 263–266. [Google Scholar] [CrossRef]
- Yang, L.; Agarwal, P. Systematic drug repositioning based on clinical side-effects. PLoS ONE 2011, 6, e28025. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Lee, T.; Hwang, S.; Park, C.; Ahn, J.; Seo, S.; Hwang, Y.; Yoon, Y. PISTON: Predicting drug indications and side effects using topic modeling and natural language processing. J. Biomed. Inform. 2018, 87, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Bibbins-Domingo, K.; Force, U.S.P.S.T. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 836–845. [Google Scholar] [CrossRef]
- Cavalla, D.; Singal, C. Retrospective clinical analysis for drug rescue: For new indications or stratified patient groups. Drug Discov. Today 2012, 17, 104–109. [Google Scholar] [CrossRef]
- Jensen, P.B.; Jensen, L.J.; Brunak, S. Mining electronic health records: Towards better research applications and clinical care. Nat. Rev. Genet. 2012, 13, 395–405. [Google Scholar] [CrossRef]
- Paik, H.; Chung, A.Y.; Park, H.C.; Park, R.W.; Suk, K.; Kim, J.; Kim, H.; Lee, K.; Butte, A.J. Repurpose terbutaline sulfate for amyotrophic lateral sclerosis using electronic medical records. Sci. Rep. 2015, 5, 8580. [Google Scholar] [CrossRef]
- Kuusisto, F.; Steill, J.; Kuang, Z.; Thomson, J.; Page, D.; Stewart, R. A Simple Text Mining Approach for Ranking Pairwise Associations in Biomedical Applications. AMIA Summits Transl. Sci. Proc. 2017, 2017, 166–174. [Google Scholar]
- Li, J.; Zhu, X.; Chen, J.Y. Building disease-specific drug-protein connectivity maps from molecular interaction networks and PubMed abstracts. PLoS Comput. Biol. 2009, 5, e1000450. [Google Scholar] [CrossRef]
- Gramatica, R.; Di Matteo, T.; Giorgetti, S.; Barbiani, M.; Bevec, D.; Aste, T. Graph theory enables drug repurposing--how a mathematical model can drive the discovery of hidden mechanisms of action. PLoS ONE 2014, 9, e84912. [Google Scholar] [CrossRef]
- Zhang, M.; Schmitt-Ulms, G.; Sato, C.; Xi, Z.; Zhang, Y.; Zhou, Y.; St George-Hyslop, P.; Rogaeva, E. Drug Repositioning for Alzheimer’s Disease Based on Systematic ’omics’ Data Mining. PLoS ONE 2016, 11, e0168812. [Google Scholar] [CrossRef]
- Hammoud, H.; Saker, Z.; Harati, H.; Fares, Y.; Bahmad, H.F.; Nabha, S. Drug Repurposing in Medulloblastoma: Challenges and Recommendations. Curr. Treat. Options Oncol. 2020, 22, 6. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Elajami, M.K.; El Zarif, T.; Bou-Gharios, J.; Abou-Antoun, T.; Abou-Kheir, W. Drug repurposing towards targeting cancer stem cells in pediatric brain tumors. Cancer Metastasis Rev. 2020, 39, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Bahmad, H.F.; Daher, D.; Aljamal, A.A.; Elajami, M.K.; Oh, K.S.; Alvarez Moreno, J.C.; Delgado, R.; Suarez, R.; Zaldivar, A.; Azimi, R.; et al. Repurposing of Anticancer Stem Cell Drugs in Brain Tumors. J. Histochem. Cytochem. 2021, 69, 749–773. [Google Scholar] [CrossRef] [PubMed]
- Kaitin, K.I.; DiMasi, J.A. Pharmaceutical innovation in the 21st century: New drug approvals in the first decade, 2000–2009. Clin. Pharmacol. Ther. 2011, 89, 183–188. [Google Scholar] [CrossRef]
- Nosengo, N. Can you teach old drugs new tricks? Nature 2016, 534, 314–316. [Google Scholar] [CrossRef]
- Hamilton, A.S.; Albertsen, P.C.; Johnson, T.K.; Hoffman, R.; Morrell, D.; Deapen, D.; Penson, D.F. Trends in the treatment of localized prostate cancer using supplemented cancer registry data. BJU Int. 2011, 107, 576–584. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Zhang, Y.; Schroeck, F.R.; Skolarus, T.A.; Wei, J.T.; Montie, J.E.; Gilbert, S.M.; Strope, S.A.; Dunn, R.L.; Miller, D.C.; et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA 2013, 309, 2587–2595. [Google Scholar] [CrossRef]
- Walz, J.; Joniau, S.; Chun, F.K.; Isbarn, H.; Jeldres, C.; Yossepowitch, O.; Chao-Yu, H.; Klein, E.A.; Scardino, P.T.; Reuther, A.; et al. Pathological results and rates of treatment failure in high-risk prostate cancer patients after radical prostatectomy. BJU Int. 2011, 107, 765–770. [Google Scholar] [CrossRef]
- Yossepowitch, O.; Eggener, S.E.; Bianco, F.J., Jr.; Carver, B.S.; Serio, A.; Scardino, P.T.; Eastham, J.A. Radical prostatectomy for clinically localized, high risk prostate cancer: Critical analysis of risk assessment methods. J. Urol. 2007, 178, 493–499. [Google Scholar] [CrossRef]
- Bill-Axelson, A.; Holmberg, L.; Ruutu, M.; Garmo, H.; Stark, J.R.; Busch, C.; Nordling, S.; Haggman, M.; Andersson, S.O.; Bratell, S.; et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N. Engl. J. Med. 2011, 364, 1708–1717. [Google Scholar] [CrossRef]
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M.; et al. Radical prostatectomy versus observation for localized prostate cancer. N. Engl. J. Med. 2012, 367, 203–213. [Google Scholar] [CrossRef]
- Kelloff, G.J.; Choyke, P.; Coffey, D.S.; Prostate Cancer Imaging Working, G. Challenges in clinical prostate cancer: Role of imaging. AJR Am. J. Roentgenol. 2009, 192, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.C.; Choueiri, M.; Tu, S.M.; Lin, S.H. Biology and clinical management of prostate cancer bone metastasis. Front. Biosci. 2007, 12, 3273–3286. [Google Scholar] [CrossRef] [PubMed]
- Mollica, V.; Rizzo, A.; Rosellini, M.; Marchetti, A.; Ricci, A.D.; Cimadamore, A.; Scarpelli, M.; Bonucci, C.; Andrini, E.; Errani, C.; et al. Bone Targeting Agents in Patients with Metastatic Prostate Cancer: State of the Art. Cancers 2021, 13, 546. [Google Scholar] [CrossRef] [PubMed]
- Suzman, D.L.; Boikos, S.A.; Carducci, M.A. Bone-targeting agents in prostate cancer. Cancer Metastasis Rev. 2014, 33, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.C.; Carducci, M.A. Early use of chemotherapy in metastatic prostate cancer. Cancer Treat. Rev. 2017, 55, 218–224. [Google Scholar] [CrossRef]
- Silberstein, J.L.; Pal, S.K.; Lewis, B.; Sartor, O. Current clinical challenges in prostate cancer. Transl. Androl. Urol. 2013, 2, 122–136. [Google Scholar] [CrossRef]
- Bostwick, D.G. Prostate-specific antigen. Current role in diagnostic pathology of prostate cancer. Am. J. Clin. Pathol. 1994, 102, S31–S37. [Google Scholar]
- Nogueira, L.; Corradi, R.; Eastham, J.A. Prostatic specific antigen for prostate cancer detection. Int. Braz. J. Urol. 2009, 35, 521–529; discussion 522–530. [Google Scholar] [CrossRef][Green Version]
- Wright, J.L.; Lange, P.H. Newer potential biomarkers in prostate cancer. Rev. Urol. 2007, 9, 207–213. [Google Scholar]
- Gretzer, M.B.; Partin, A.W. PSA markers in prostate cancer detection. Urol. Clin. N. Am. 2003, 30, 677–686. [Google Scholar] [CrossRef]
- Dall’Era, M.A.; Cooperberg, M.R.; Chan, J.M.; Davies, B.J.; Albertsen, P.C.; Klotz, L.H.; Warlick, C.A.; Holmberg, L.; Bailey, D.E., Jr.; Wallace, M.E.; et al. Active surveillance for early-stage prostate cancer: Review of the current literature. Cancer 2008, 112, 1650–1659. [Google Scholar] [CrossRef]
- Hughes, C.; Murphy, A.; Martin, C.; Sheils, O.; O’Leary, J. Molecular pathology of prostate cancer. J. Clin. Pathol. 2005, 58, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Kastner, C.; Armitage, J.; Kimble, A.; Rawal, J.; Carter, P.G.; Venn, S. The Charlson comorbidity score: A superior comorbidity assessment tool for the prostate cancer multidisciplinary meeting. Prostate Cancer Prostatic Dis. 2006, 9, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Cowen, M.E.; Halasyamani, L.K.; Kattan, M.W. Predicting life expectancy in men with clinically localized prostate cancer. J. Urol. 2006, 175, 99–103. [Google Scholar] [CrossRef]
- Tewari, A.; Johnson, C.C.; Divine, G.; Crawford, E.D.; Gamito, E.J.; Demers, R.; Menon, M. Long-term survival probability in men with clinically localized prostate cancer: A case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J. Urol. 2004, 171, 1513–1519. [Google Scholar] [CrossRef]
- Walz, J.; Gallina, A.; Saad, F.; Montorsi, F.; Perrotte, P.; Shariat, S.F.; Jeldres, C.; Graefen, M.; Benard, F.; McCormack, M.; et al. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J. Clin. Oncol. 2007, 25, 3576–3581. [Google Scholar] [CrossRef]
- Shariat, S.F.; Scardino, P.T.; Lilja, H. Screening for prostate cancer: An update. Can. J. Urol. 2008, 15, 4363–4374. [Google Scholar]
- Eichelberger, L.E.; Cheng, L. Does pT2b prostate carcinoma exist? Critical appraisal of the 2002 TNM classification of prostate carcinoma. Cancer 2004, 100, 2573–2576. [Google Scholar] [CrossRef]
- Rajinikanth, A.; Manoharan, M.; Soloway, C.T.; Civantos, F.J.; Soloway, M.S. Trends in Gleason score: Concordance between biopsy and prostatectomy over 15 years. Urology 2008, 72, 177–182. [Google Scholar] [CrossRef]
- Linden, R.A.; Halpern, E.J. Advances in transrectal ultrasound imaging of the prostate. Semin. Ultrasound CT MRI 2007, 28, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.B.; Ganju, A.; Sikander, M.; Kashyap, V.K.; Hafeez, Z.B.; Chauhan, N.; Malik, S.; Massey, A.E.; Tripathi, M.K.; Halaweish, F.T.; et al. Ormeloxifene Suppresses Prostate Tumor Growth and Metastatic Phenotypes via Inhibition of Oncogenic beta-catenin Signaling and EMT Progression. Mol. Cancer Ther. 2017, 16, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Masumori, N. Naftopidil for the treatment of urinary symptoms in patients with benign prostatic hyperplasia. Ther. Clin. Risk Manag. 2011, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Ishii, K.; Kanda, H.; Kato, M.; Miki, M.; Kajiwara, S.; Arima, K.; Shiraishi, T.; Sugimura, Y. Combination treatment with naftopidil increases the efficacy of radiotherapy in PC-3 human prostate cancer cells. J. Cancer Res. Clin. Oncol. 2017, 143, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-Y.; Huang, W.-Y.; Lin, C.-L.; Huang, T.-C.; Wu, Y.-Y.; Chen, J.-H.; Kao, C.-H. Propranolol Reduces Cancer Risk: A Population-Based Cohort Study. Medicine 2015, 94, e1097. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.D.; Mawji, I.A.; Anyiwe, K.; Williams, M.A.; Wang, X.; Venugopal, A.L.; Gronda, M.; Hurren, R.; Cheng, S.; Serra, S.; et al. Inhibition of the Sodium Potassium Adenosine Triphosphatase Pump Sensitizes Cancer Cells to Anoikis and Prevents Distant Tumor Formation. Cancer Res. 2009, 69, 2739. [Google Scholar] [CrossRef]
- Brohée, L.; Peulen, O.; Nusgens, B.; Castronovo, V.; Thiry, M.; Colige, A.C.; Deroanne, C.F. Propranolol sensitizes prostate cancer cells to glucose metabolism inhibition and prevents cancer progression. Sci. Rep. 2018, 8, 7050. [Google Scholar] [CrossRef]
- Olivan, M.; Rigau, M.; Colás, E.; Garcia, M.; Montes, M.; Sequeiros, T.; Regis, L.; Celma, A.; Planas, J.; Placer, J.; et al. Simultaneous treatment with statins and aspirin reduces the risk of prostate cancer detection and tumorigenic properties in prostate cancer cell lines. BioMed Res. Int. 2015, 2015, 762178. [Google Scholar] [CrossRef]
- Hsu, A.L.; Ching, T.T.; Wang, D.S.; Song, X.; Rangnekar, V.M.; Chen, C.S. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J. Biol. Chem. 2000, 275, 11397–11403. [Google Scholar] [CrossRef]
- Gayvert, K.M.; Dardenne, E.; Cheung, C.; Boland, M.R.; Lorberbaum, T.; Wanjala, J.; Chen, Y.; Rubin, M.A.; Tatonetti, N.P.; Rickman, D.S.; et al. A Computational Drug Repositioning Approach for Targeting Oncogenic Transcription Factors. Cell Rep. 2016, 15, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, F.; Roca, M.S.; Lombardi, R.; Ciardiello, C.; Grumetti, L.; De Rienzo, S.; Moccia, T.; Vitagliano, C.; Sorice, A.; Costantini, S.; et al. Synergistic antitumor interaction of valproic acid and simvastatin sensitizes prostate cancer to docetaxel by targeting CSCs compartment via YAP inhibition. J. Exp. Clin. Cancer Res. 2020, 39, 213. [Google Scholar] [CrossRef]
- Salem, O.; Hansen, C.G. The Hippo Pathway in Prostate Cancer. Cells 2019, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Cheng, L.; Mao, F.; Zhang, Z.; Zhang, Y.; Farah, E.; Bosler, J.; Bai, Y.; Ahmad, N.; Kuang, S.; et al. Inhibition of cholesterol biosynthesis overcomes enzalutamide resistance in castration-resistant prostate cancer (CRPC). J. Biol. Chem. 2018, 293, 14328–14341. [Google Scholar] [CrossRef] [PubMed]
- Zingales, V.; Distefano, A.; Raffaele, M.; Zanghi, A.; Barbagallo, I.; Vanella, L. Metformin: A Bridge between Diabetes and Prostate Cancer. Front. Oncol. 2017, 7, 243. [Google Scholar] [CrossRef]
- Zaidi, S.; Gandhi, J.; Joshi, G.; Smith, N.L.; Khan, S.A. The anticancer potential of metformin on prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 351–361. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Tong, D.; Parmar, H.; Hasenmayer, D.; Yuan, W.; Zhang, D.; Jiang, J. Metformin represses androgen-dependent and androgen-independent prostate cancers by targeting androgen receptor. Prostate 2015, 75, 1187–1196. [Google Scholar] [CrossRef]
- Qi, C.; Bin, L.; Yang, Y.; Yang, Y.; Li, J.; Zhou, Q.; Wen, Y.; Zeng, C.; Zheng, L.; Zhang, Q.; et al. Glipizide suppresses prostate cancer progression in the TRAMP model by inhibiting angiogenesis. Sci. Rep. 2016, 6, 27819. [Google Scholar] [CrossRef]
- Rushworth, L.K.; Hewit, K.; Munnings-Tomes, S.; Somani, S.; James, D.; Shanks, E.; Dufès, C.; Straube, A.; Patel, R.; Leung, H.Y. Repurposing screen identifies mebendazole as a clinical candidate to synergise with docetaxel for prostate cancer treatment. Br. J. Cancer 2020, 122, 517–527. [Google Scholar] [CrossRef]
- Liu, C.; Lou, W.; Zhu, Y.; Nadiminty, N.; Schwartz, C.T.; Evans, C.P.; Gao, A.C. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 3198–3210. [Google Scholar] [CrossRef]
- Chow, W.A.; Jiang, C.; Guan, M. Anti-HIV drugs for cancer therapeutics: Back to the future? Lancet Oncol. 2009, 10, 61–71. [Google Scholar] [CrossRef]
- Guan, M.; Su, L.; Yuan, Y.C.; Li, H.; Chow, W.A. Nelfinavir and nelfinavir analogs block site-2 protease cleavage to inhibit castration-resistant prostate cancer. Sci. Rep. 2015, 5, 9698. [Google Scholar] [CrossRef]
- Lokeshwar, B.L. Chemically modified non-antimicrobial tetracyclines are multifunctional drugs against advanced cancers. Pharmacol. Res. 2011, 63, 146–150. [Google Scholar] [CrossRef]
- Corey, E.; Brown, L.G.; Quinn, J.E.; Poot, M.; Roudier, M.P.; Higano, C.S.; Vessella, R.L. Zoledronic acid exhibits inhibitory effects on osteoblastic and osteolytic metastases of prostate cancer. Clin. Cancer Res. 2003, 9, 295–306. [Google Scholar] [PubMed]
- Xia, Q.; Sung, J.; Chowdhury, W.; Chen, C.L.; Höti, N.; Shabbeer, S.; Carducci, M.; Rodriguez, R. Chronic administration of valproic acid inhibits prostate cancer cell growth in vitro and in vivo. Cancer Res. 2006, 66, 7237–7244. [Google Scholar] [CrossRef]
- El Etreby, M.F.; Liang, Y.; Lewis, R.W. Induction of apoptosis by mifepristone and tamoxifen in human LNCaP prostate cancer cells in culture. Prostate 2000, 43, 31–42. [Google Scholar] [CrossRef]
- Sosic, I.; Mirkovic, B.; Arenz, K.; Stefane, B.; Kos, J.; Gobec, S. Development of new cathepsin B inhibitors: Combining bioisosteric replacements and structure-based design to explore the structure-activity relationships of nitroxoline derivatives. J. Med. Chem. 2013, 56, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Matsui, Y.; Bhat, S.; Nacev, B.A.; Xu, J.; Bhang, H.E.; Dhara, S.; Han, K.C.; Chong, C.R.; Pomper, M.G.; et al. Effect of nitroxoline on angiogenesis and growth of human bladder cancer. J. Natl. Cancer Inst. 2010, 102, 1855–1873. [Google Scholar] [CrossRef]
- Chang, W.L.; Hsu, L.C.; Leu, W.J.; Chen, C.S.; Guh, J.H. Repurposing of nitroxoline as a potential anticancer agent against human prostate cancer: A crucial role on AMPK/mTOR signaling pathway and the interplay with Chk2 activation. Oncotarget 2015, 6, 39806–39820. [Google Scholar] [CrossRef]
- Lv, Z.; Chu, Y.; Wang, Y. HIV protease inhibitors: A review of molecular selectivity and toxicity. HIV AIDS 2015, 7, 95–104. [Google Scholar] [CrossRef]
- Gantt, S.; Casper, C.; Ambinder, R.F. Insights into the broad cellular effects of nelfinavir and the HIV protease inhibitors supporting their role in cancer treatment and prevention. Curr. Opin. Oncol. 2013, 25, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qian, D.Z.; Tan, Y.S.; Lee, K.; Gao, P.; Ren, Y.R.; Rey, S.; Hammers, H.; Chang, D.; Pili, R.; et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc. Natl. Acad. Sci. USA 2008, 105, 19579–19586. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhan, T.; Duffy, D.; Hoffman-Censits, J.; Kilpatrick, D.; Trabulsi, E.J.; Lallas, C.D.; Chervoneva, I.; Limentani, K.; Kennedy, B.; et al. A pilot phase II Study of digoxin in patients with recurrent prostate cancer as evident by a rising PSA. Am. J. Cancer Ther. Pharmacol. 2014, 2, 21–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, M.; Shim, J.S.; Li, R.J.; Dang, Y.; He, Q.; Das, M.; Liu, J.O. Identification of an old antibiotic clofoctol as a novel activator of unfolded protein response pathways and an inhibitor of prostate cancer. Br. J. Pharmacol. 2014, 171, 4478–4489. [Google Scholar] [CrossRef] [PubMed]
- Lupu, R.; Menendez, J.A. Pharmacological inhibitors of Fatty Acid Synthase (FASN)--catalyzed endogenous fatty acid biogenesis: A new family of anti-cancer agents? Curr. Pharm. Biotechnol. 2006, 7, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, M.C.; Pouwer, R.H.; Gunter, J.H.; Lubik, A.A.; Quinn, R.J.; Nelson, C.C. The fatty acid synthase inhibitor triclosan: Repurposing an anti-microbial agent for targeting prostate cancer. Oncotarget 2014, 5, 9362–9381. [Google Scholar] [CrossRef] [PubMed]
- Rodricks, J.V.; Swenberg, J.A.; Borzelleca, J.F.; Maronpot, R.R.; Shipp, A.M. Triclosan: A critical review of the experimental data and development of margins of safety for consumer products. Crit. Rev. Toxicol. 2010, 40, 422–484. [Google Scholar] [CrossRef]
- Abou El-Magd, R.M.; Park, H.K.; Kawazoe, T.; Iwana, S.; Ono, K.; Chung, S.P.; Miyano, M.; Yorita, K.; Sakai, T.; Fukui, K. The effect of risperidone on D-amino acid oxidase activity as a hypothesis for a novel mechanism of action in the treatment of schizophrenia. J. Psychopharmacol. 2010, 24, 1055–1067. [Google Scholar] [CrossRef]
- Ayan, D.; Maltais, R.; Poirier, D. Identification of a 17beta-hydroxysteroid dehydrogenase type 10 steroidal inhibitor: A tool to investigate the role of type 10 in Alzheimer’s disease and prostate cancer. ChemMedChem 2012, 7, 1181–1184. [Google Scholar] [CrossRef]
- Tammali, R.; Srivastava, S.K.; Ramana, K.V. Targeting aldose reductase for the treatment of cancer. Curr. Cancer Drug Targets 2011, 11, 560–571. [Google Scholar] [CrossRef]
- Turanli, B.; Gulfidan, G.; Arga, K.Y. Transcriptomic-Guided Drug Repositioning Supported by a New Bioinformatics Search Tool: GeneXpharma. OMICS 2017, 21, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Frishman, W.H. Beta-Adrenergic Receptor Blockers in Hypertension: Alive and Well. Prog. Cardiovasc. Dis. 2016, 59, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.; Pergola, C.; Koeberle, A.; Dodt, G.; Steinhilber, D.; Werz, O. The role of diacylglyceride generation by phospholipase D and phosphatidic acid phosphatase in the activation of 5-lipoxygenase in polymorphonuclear leukocytes. J. Leukoc. Biol. 2008, 83, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, W.M.; Hammond, J.J.; Thomas, J.C.; Overturf, M.L.; Zama, A. Prazosin and clonidine for moderately severe hypertension. JAMA 1978, 240, 2553–2556. [Google Scholar] [CrossRef]
- Clifford, G.M.; Farmer, R.D. Medical therapy for benign prostatic hyperplasia: A review of the literature. Eur. Urol. 2000, 38, 2–19. [Google Scholar] [CrossRef]
- Waldo, R. Prazosin relieves Raynaud’s vasospasm. JAMA 1979, 241, 1037. [Google Scholar] [CrossRef]
- Assad Kahn, S.; Costa, S.L.; Gholamin, S.; Nitta, R.T.; Dubois, L.G.; Feve, M.; Zeniou, M.; Coelho, P.L.; El-Habr, E.; Cadusseau, J.; et al. The anti-hypertensive drug prazosin inhibits glioblastoma growth via the PKCdelta-dependent inhibition of the AKT pathway. EMBO Mol. Med. 2016, 8, 511–526. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chueh, S.-C.; Hsiao, C.-J.; Li, T.-K.; Chen, T.-H.; Liao, C.-H.; Lyu, P.-C.; Guh, J.-H. Prazosin displays anticancer activity against human prostate cancers: Targeting DNA and cell cycle. Neoplasia 2007, 9, 830–839. [Google Scholar] [CrossRef]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical Pharmacology of Non-Steroidal Anti-Inflammatory Drugs: A Review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Bilani, N.; Bahmad, H.; Abou-Kheir, W. Prostate Cancer and Aspirin Use: Synopsis of the Proposed Molecular Mechanisms. Front. Pharmacol. 2017, 8, 145. [Google Scholar] [CrossRef]
- Janik, J.E.; Miller, L.L.; Longo, D.L.; Powers, G.C.; Urba, W.J.; Kopp, W.C.; Gause, B.L.; Curti, B.D.; Fenton, R.G.; Oppenheim, J.J.; et al. Phase II trial of interleukin 1 alpha and indomethacin in treatment of metastatic melanoma. J. Natl. Cancer Inst. 1996, 88, 44–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Timar, J.; Ladanyi, A.; Forster-Horvath, C.; Lukits, J.; Dome, B.; Remenar, E.; Godeny, M.; Kasler, M.; Bencsik, B.; Repassy, G.; et al. Neoadjuvant immunotherapy of oral squamous cell carcinoma modulates intratumoral CD4/CD8 ratio and tumor microenvironment: A multicenter phase II clinical trial. J. Clin. Oncol. 2005, 23, 3421–3432. [Google Scholar] [CrossRef] [PubMed]
- Guieu, R.; Blin, O.; Pouget, J.; Serratrice, G. Analgesic effect of indomethacin shown using the nociceptive flexion reflex in humans. Ann. Rheum. Dis. 1992, 51, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Percy, J.S.; Stephenson, P.; Thompson, M. Indomethacin in the Treatment of Rheumatic Diseases. Ann. Rheum. Dis. 1964, 23, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Chennamaneni, S.; Zhong, B.; Lama, R.; Su, B. COX inhibitors Indomethacin and Sulindac derivatives as antiproliferative agents: Synthesis, biological evaluation, and mechanism investigation. Eur. J. Med. Chem. 2012, 56, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Touhey, S.; O’Connor, R.; Plunkett, S.; Maguire, A.; Clynes, M. Structure-activity relationship of indomethacin analogues for MRP-1, COX-1 and COX-2 inhibition. identification of novel chemotherapeutic drug resistance modulators. Eur. J. Cancer 2002, 38, 1661–1670. [Google Scholar] [CrossRef]
- Lin, C.C.; Suen, K.M.; Stainthorp, A.; Wieteska, L.; Biggs, G.S.; Leitao, A.; Montanari, C.A.; Ladbury, J.E. Targeting the Shc-EGFR interaction with indomethacin inhibits MAP kinase pathway signalling. Cancer Lett. 2019, 457, 86–97. [Google Scholar] [CrossRef]
- Mazumder, S.; De, R.; Debsharma, S.; Bindu, S.; Maity, P.; Sarkar, S.; Saha, S.J.; Siddiqui, A.A.; Banerjee, C.; Nag, S.; et al. Indomethacin impairs mitochondrial dynamics by activating the PKCzeta-p38-DRP1 pathway and inducing apoptosis in gastric cancer and normal mucosal cells. J. Biol. Chem. 2019, 294, 8238–8258. [Google Scholar] [CrossRef]
- Federica, I.; Rita, L.; Maria, R.M.; Biagio, P.; Simona De, R.; Alfredo, B.; Francesca, B. Targeting Mevalonate Pathway in Cancer Treatment: Repurposing of Statins. Recent Pat. Anti-Cancer Drug Discov. 2018, 13, 184–200. [Google Scholar] [CrossRef]
- Hindler, K.; Cleeland, C.S.; Rivera, E.; Collard, C.D. The role of statins in cancer therapy. Oncologist 2006, 11, 306–315. [Google Scholar] [CrossRef]
- Chan, K.K.; Oza, A.M.; Siu, L.L. The statins as anticancer agents. Clin. Cancer Res. 2003, 9, 10–19. [Google Scholar] [PubMed]
- Zhuang, L.; Kim, J.; Adam, R.M.; Solomon, K.R.; Freeman, M.R. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J. Clin. Investig. 2005, 115, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Klein, E.A.; Li, J.; Moussa, A.S.; Jones, J.S. Statin use and risk of prostate cancer in a population of men who underwent biopsy. J. Urol. 2011, 186, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.S.; Carroll, P.R.; Cowan, J.E.; Chan, J.M.; D’Amico, A.V. Association of statin and nonsteroidal anti-inflammatory drug use with prostate cancer outcomes: Results from CaPSURE. BJU Int. 2010, 106, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Marcella, S.W.; David, A.; Ohman-Strickland, P.A.; Carson, J.; Rhoads, G.G. Statin use and fatal prostate cancer: A matched case-control study. Cancer 2012, 118, 4046–4052. [Google Scholar] [CrossRef]
- Hutchinson, J.; Marignol, L. Clinical Potential of Statins in Prostate Cancer Radiation Therapy. Anticancer Res. 2017, 37, 5363–5372. [Google Scholar] [CrossRef][Green Version]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Whitburn, J.; Edwards, C.M.; Sooriakumaran, P. Metformin and Prostate Cancer: A New Role for an Old Drug. Curr. Urol. Rep. 2017, 18, 46. [Google Scholar] [CrossRef]
- Mouhieddine, T.H.; Nokkari, A.; Itani, M.M.; Chamaa, F.; Bahmad, H.; Monzer, A.; El-Merahbi, R.; Daoud, G.; Eid, A.; Kobeissy, F.H.; et al. Metformin and Ara-a Effectively Suppress Brain Cancer by Targeting Cancer Stem/Progenitor Cells. Front. Neurosci. 2015, 9, 442. [Google Scholar] [CrossRef]
- Jalving, M.; Gietema, J.A.; Lefrandt, J.D.; de Jong, S.; Reyners, A.K.; Gans, R.O.; de Vries, E.G. Metformin: Taking away the candy for cancer? Eur. J. Cancer 2010, 46, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Preston, M.A.; Riis, A.H.; Ehrenstein, V.; Breau, R.H.; Batista, J.L.; Olumi, A.F.; Mucci, L.A.; Adami, H.O.; Sorensen, H.T. Metformin use and prostate cancer risk. Eur. Urol. 2014, 66, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.A.; Iliopoulos, D.; Struhl, K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc. Natl. Acad. Sci. USA 2013, 110, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.N.K.; Kichenadasse, G.; Butler, L.M.; Centenera, M.M.; Morel, K.L.; Ormsby, R.J.; Michael, M.Z.; Lower, K.M.; Sykes, P.J. The Combination of Metformin and Valproic Acid Induces Synergistic Apoptosis in the Presence of p53 and Androgen Signaling in Prostate Cancer. Mol. Cancer Ther. 2017, 16, 2689–2700. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Bronsveld, H.K.; De Bruin, M.L.; Wesseling, J.; Sanders, J.; Hofland, I.; Jensen, V.; Bazelier, M.T.; Ter Braak, B.; de Boer, A.; Vestergaard, P.; et al. The association of diabetes mellitus and insulin treatment with expression of insulin-related proteins in breast tumors. BMC Cancer 2018, 18, 224. [Google Scholar] [CrossRef]
- Pollak, M. Overcoming Drug Development Bottlenecks With Repurposing: Repurposing biguanides to target energy metabolism for cancer treatment. Nat. Med. 2014, 20, 591–593. [Google Scholar] [CrossRef]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Circu, M.L.; Dykes, S.S.; Carroll, J.; Kelly, K.; Galiano, F.; Greer, A.; Cardelli, J.; El-Osta, H. A Novel High Content Imaging-Based Screen Identifies the Anti-Helminthic Niclosamide as an Inhibitor of Lysosome Anterograde Trafficking and Prostate Cancer Cell Invasion. PLoS ONE 2016, 11, e0146931. [Google Scholar] [CrossRef]
- Chen, W.; Mook, R.A., Jr.; Premont, R.T.; Wang, J. Niclosamide: Beyond an antihelminthic drug. Cell. Signal. 2018, 41, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sack, U.; Walther, W.; Scudiero, D.; Selby, M.; Kobelt, D.; Lemm, M.; Fichtner, I.; Schlag, P.M.; Shoemaker, R.H.; Stein, U. Novel effect of antihelminthic Niclosamide on S100A4-mediated metastatic progression in colon cancer. J. Natl. Cancer Inst. 2011, 103, 1018–1036. [Google Scholar] [CrossRef] [PubMed]
- Dahlmann, M.; Kobelt, D.; Walther, W.; Mudduluru, G.; Stein, U. S100A4 in Cancer Metastasis: Wnt Signaling-Driven Interventions for Metastasis Restriction. Cancers 2016, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Xiong, Y.; Yan, Y.; Xia, Y.; Song, X.; Liu, L.; Li, D.; Wang, N.; Zhang, L.; Zhu, Y.; et al. The anthelmintic drug niclosamide induces apoptosis, impairs metastasis and reduces immunosuppressive cells in breast cancer model. PLoS ONE 2014, 9, e85887. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Ward, T.; Mao, Y.; Bockhorn, J.; Liu, X.; Wang, G.; Pegram, M.; Shen, K. Niclosamide inhibits epithelial-mesenchymal transition and tumor growth in lapatinib-resistant human epidermal growth factor receptor 2-positive breast cancer. Int. J. Biochem. Cell Biol. 2016, 71, 12–23. [Google Scholar] [CrossRef]
- Guo, C.; Yeh, S.; Niu, Y.; Li, G.; Zheng, J.; Li, L.; Chang, C. Targeting androgen receptor versus targeting androgens to suppress castration resistant prostate cancer. Cancer Lett. 2017, 397, 133–143. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Gálvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Heath, E.I.; Smith, D.C.; Rathkopf, D.; Blackford, A.L.; Danila, D.C.; King, S.; Frost, A.; Ajiboye, A.S.; Zhao, M.; et al. Repurposing itraconazole as a treatment for advanced prostate cancer: A noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist 2013, 18, 163–173. [Google Scholar] [CrossRef]
- Karhadkar, S.S.; Bova, G.S.; Abdallah, N.; Dhara, S.; Gardner, D.; Maitra, A.; Isaacs, J.T.; Berman, D.M.; Beachy, P.A. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 2004, 431, 707–712. [Google Scholar] [CrossRef]
- Nacev, B.A.; Grassi, P.; Dell, A.; Haslam, S.M.; Liu, J.O. The antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cells. J. Biol. Chem. 2011, 286, 44045–44056. [Google Scholar] [CrossRef]
- Head, S.A.; Shi, W.Q.; Yang, E.J.; Nacev, B.A.; Hong, S.Y.; Pasunooti, K.K.; Li, R.J.; Shim, J.S.; Liu, J.O. Simultaneous Targeting of NPC1 and VDAC1 by Itraconazole Leads to Synergistic Inhibition of mTOR Signaling and Angiogenesis. ACS Chem. Biol. 2017, 12, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhuang, K.; Sun, K.; Yang, Q.; Ran, X.; Xu, X.; Mu, C.; Zheng, B.; Lu, Y.; Zeng, J.; et al. Itraconazole Induces Regression of Infantile Hemangioma via Downregulation of the Platelet-Derived Growth Factor-D/PI3K/Akt/mTOR Pathway. J. Investig. Dermatol. 2019, 139, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Smithuis, F.; Kyaw, M.K.; Phe, O.; Win, T.; Aung, P.P.; Oo, A.P.; Naing, A.L.; Nyo, M.Y.; Myint, N.Z.; Imwong, M.; et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: An open-label randomised trial. Lancet Infect. Dis. 2010, 10, 673–681. [Google Scholar] [CrossRef]
- Wong, Y.K.; Xu, C.; Kalesh, K.A.; He, Y.; Lin, Q.; Wong, W.S.F.; Shen, H.M.; Wang, J. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med. Res. Rev. 2017, 37, 1492–1517. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Q.; Huo, C.; Li, Y.; He, L.; Ran, B.; Chen, J.; Li, Y.; Liu, W. Ferroptosis: A Novel Mechanism of Artemisinin and its Derivatives in Cancer Therapy. Curr. Med. Chem. 2021, 28, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef]
- Yang, N.D.; Tan, S.H.; Ng, S.; Shi, Y.; Zhou, J.; Tan, K.S.; Wong, W.S.; Shen, H.M. Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin. J. Biol. Chem. 2014, 289, 33425–33441. [Google Scholar] [CrossRef]
- Abarientos, C.; Sperber, K.; Shapiro, D.L.; Aronow, W.S.; Chao, C.P.; Ash, J.Y. Hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis and its safety in pregnancy. Expert Opin. Drug Saf. 2011, 10, 705–714. [Google Scholar] [CrossRef]
- Al-Rawi, H.; Meggitt, S.J.; Williams, F.M.; Wahie, S. Steady-state pharmacokinetics of hydroxychloroquine in patients with cutaneous lupus erythematosus. Lupus 2018, 27, 847–852. [Google Scholar] [CrossRef]
- Janku, F.; McConkey, D.J.; Hong, D.S.; Kurzrock, R. Autophagy as a target for anticancer therapy. Nat. Rev. Clin. Oncol. 2011, 8, 528–539. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Gurova, K.V.; Hill, J.E.; Guo, C.; Prokvolit, A.; Burdelya, L.G.; Samoylova, E.; Khodyakova, A.V.; Ganapathi, R.; Ganapathi, M.; Tararova, N.D.; et al. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-kappaB-dependent mechanism of p53 suppression in tumors. Proc. Natl. Acad. Sci. USA 2005, 102, 17448–17453. [Google Scholar] [CrossRef] [PubMed]
- Gasparian, A.V.; Burkhart, C.A.; Purmal, A.A.; Brodsky, L.; Pal, M.; Saranadasa, M.; Bosykh, D.A.; Commane, M.; Guryanova, O.A.; Pal, S.; et al. Curaxins: Anticancer compounds that simultaneously suppress NF-kappaB and activate p53 by targeting FACT. Sci. Transl. Med. 2011, 3, 95ra74. [Google Scholar] [CrossRef] [PubMed]
- Nesher, E.; Safina, A.; Aljahdali, I.; Portwood, S.; Wang, E.S.; Koman, I.; Wang, J.; Gurova, K.V. Role of Chromatin Damage and Chromatin Trapping of FACT in Mediating the Anticancer Cytotoxicity of DNA-Binding Small-Molecule Drugs. Cancer Res. 2018, 78, 1431–1443. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Intravenous zoledronic acid for the treatment of osteoporosis: The evidence of its therapeutic effect. Core Evid. 2010, 4, 13–23. [Google Scholar] [CrossRef][Green Version]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Chen, B.; et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002, 94, 1458–1468. [Google Scholar] [CrossRef]
- Mason, M.D.; Clarke, N.W.; James, N.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Attard, G.; Cross, W.; Jones, R.J.; Parker, C.C.; et al. Adding Celecoxib With or Without Zoledronic Acid for Hormone-Naive Prostate Cancer: Long-Term Survival Results From an Adaptive, Multiarm, Multistage, Platform, Randomized Controlled Trial. J. Clin. Oncol. 2017, 35, 1530–1541. [Google Scholar] [CrossRef]
- Niraula, S.; Templeton, A.J.; Vera-Badillo, F.; Dodd, A.; Nugent, Z.; Joshua, A.M.; Tannock, I.F. Duration of suppression of bone turnover following treatment with zoledronic acid in men with metastatic castration-resistant prostate cancer. Future Sci. OA 2018, 4, FSO253. [Google Scholar] [CrossRef]
- Ghodke-Puranik, Y.; Thorn, C.F.; Lamba, J.K.; Leeder, J.S.; Song, W.; Birnbaum, A.K.; Altman, R.B.; Klein, T.E. Valproic acid pathway: Pharmacokinetics and pharmacodynamics. Pharm. Genom. 2013, 23, 236–241. [Google Scholar] [CrossRef]
- Yu, S.; Yang, X.; Zhu, Y.; Xie, F.; Lu, Y.; Yu, T.; Yan, C.; Shao, J.; Gao, Y.; Mo, F.; et al. Systems pharmacology of mifepristone (RU486) reveals its 47 hub targets and network: Comprehensive analysis and pharmacological focus on FAK-Src-Paxillin complex. Sci. Rep. 2015, 5, 7830. [Google Scholar] [CrossRef]
- Mobbs, B.G.; Johnson, I.E. Suppression of the growth of the androgen-insensitive R3327 HI rat prostatic carcinoma by combined estrogen and antiprogestin treatment. J. Steroid Biochem. Mol. Biol. 1991, 39, 713–722. [Google Scholar] [CrossRef]
- Tieszen, C.R.; Goyeneche, A.A.; Brandhagen, B.N.; Ortbahn, C.T.; Telleria, C.M. Antiprogestin mifepristone inhibits the growth of cancer cells of reproductive and non-reproductive origin regardless of progesterone receptor expression. BMC Cancer 2011, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Taplin, M.E.; Manola, J.; Oh, W.K.; Kantoff, P.W.; Bubley, G.J.; Smith, M.; Barb, D.; Mantzoros, C.; Gelmann, E.P.; Balk, S.P. A phase II study of mifepristone (RU-486) in castration-resistant prostate cancer, with a correlative assessment of androgen-related hormones. BJU Int. 2008, 101, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.J.; Pettinati, H.M.; Kampman, K.M.; O’Brien, C.P. The status of disulfiram: A half of a century later. J. Clin. Psychopharmacol. 2006, 26, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Hald, J.; Jacobsen, E. A drug sensitizing the organism to ethyl alcohol. Lancet 1948, 2, 1001–1004. [Google Scholar] [CrossRef]
- Tacconi, E.M.; Lai, X.; Folio, C.; Porru, M.; Zonderland, G.; Badie, S.; Michl, J.; Sechi, I.; Rogier, M.; Matia Garcia, V.; et al. BRCA1 and BRCA2 tumor suppressors protect against endogenous acetaldehyde toxicity. EMBO Mol. Med. 2017, 9, 1398–1414. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Sheshukova, E.V.; Bialik, T.E.; Komarova, T.V. Human Endogenous Formaldehyde as an Anticancer Metabolite: Its Oxidation Downregulation May Be a Means of Improving Therapy. Bioessays 2018, 40, e1800136. [Google Scholar] [CrossRef] [PubMed]
- Pontel, L.B.; Rosado, I.V.; Burgos-Barragan, G.; Garaycoechea, J.I.; Yu, R.; Arends, M.J.; Chandrasekaran, G.; Broecker, V.; Wei, W.; Liu, L.; et al. Endogenous Formaldehyde Is a Hematopoietic Stem Cell Genotoxin and Metabolic Carcinogen. Mol. Cell 2015, 60, 177–188. [Google Scholar] [CrossRef]
- Liu, P.; Brown, S.; Goktug, T.; Channathodiyil, P.; Kannappan, V.; Hugnot, J.P.; Guichet, P.O.; Bian, X.; Armesilla, A.L.; Darling, J.L.; et al. Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br. J. Cancer 2012, 107, 1488–1497. [Google Scholar] [CrossRef]
- Guo, F.; Yang, Z.; Kulbe, H.; Albers, A.E.; Sehouli, J.; Kaufmann, A.M. Inhibitory effect on ovarian cancer ALDH+ stem-like cells by Disulfiram and Copper treatment through ALDH and ROS modulation. Biomed. Pharmacother. 2019, 118, 109371. [Google Scholar] [CrossRef]
- Lin, J.; Haffner, M.C.; Zhang, Y.; Lee, B.H.; Brennen, W.N.; Britton, J.; Kachhap, S.K.; Shim, J.S.; Liu, J.O.; Nelson, W.G.; et al. Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth. Prostate 2011, 71, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Lin, J.; Blackford, A.; Bardia, A.; King, S.; Armstrong, A.J.; Rudek, M.A.; Yegnasubramanian, S.; Carducci, M.A. Pharmacodynamic study of disulfiram in men with non-metastatic recurrent prostate cancer. Prostate Cancer Prostatic Dis. 2013, 16, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Safi, R.; Nelson, E.R.; Chitneni, S.K.; Franz, K.J.; George, D.J.; Zalutsky, M.R.; McDonnell, D.P. Copper signaling axis as a target for prostate cancer therapeutics. Cancer Res. 2014, 74, 5819–5831. [Google Scholar] [CrossRef] [PubMed]
- Skrott, Z.; Mistrik, M.; Andersen, K.K.; Friis, S.; Majera, D.; Gursky, J.; Ozdian, T.; Bartkova, J.; Turi, Z.; Moudry, P.; et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 2017, 552, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A. Sirolimus after kidney transplantation. BMJ 2014, 349, g6808. [Google Scholar] [CrossRef]
- Farb, A.; John, M.; Acampado, E.; Kolodgie, F.D.; Prescott, M.F.; Virmani, R. Oral everolimus inhibits in-stent neointimal growth. Circulation 2002, 106, 2379–2384. [Google Scholar] [CrossRef][Green Version]
- Fattori, R.; Piva, T. Drug-eluting stents in vascular intervention. Lancet 2003, 361, 247–249. [Google Scholar] [CrossRef]
- Benjamin, D.; Colombi, M.; Moroni, C.; Hall, M.N. Rapamycin passes the torch: A new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011, 10, 868–880. [Google Scholar] [CrossRef]
- Brown, V.I.; Fang, J.; Alcorn, K.; Barr, R.; Kim, J.M.; Wasserman, R.; Grupp, S.A. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 15113–15118. [Google Scholar] [CrossRef]
- Altman, J.K.; Sassano, A.; Kaur, S.; Glaser, H.; Kroczynska, B.; Redig, A.J.; Russo, S.; Barr, S.; Platanias, L.C. Dual mTORC2/mTORC1 targeting results in potent suppressive effects on acute myeloid leukemia (AML) progenitors. Clin. Cancer Res. 2011, 17, 4378–4388. [Google Scholar] [CrossRef]
- Mancini, M.; Petta, S.; Martinelli, G.; Barbieri, E.; Santucci, M.A. RAD 001 (everolimus) prevents mTOR and Akt late re-activation in response to imatinib in chronic myeloid leukemia. J. Cell. Biochem. 2010, 109, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Sillaber, C.; Mayerhofer, M.; Bohm, A.; Vales, A.; Gruze, A.; Aichberger, K.J.; Esterbauer, H.; Pfeilstocker, M.; Sperr, W.R.; Pickl, W.F.; et al. Evaluation of antileukaemic effects of rapamycin in patients with imatinib-resistant chronic myeloid leukaemia. Eur. J. Clin. Investig. 2008, 38, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Bahmad, H.F.; Mouhieddine, T.H.; Chalhoub, R.M.; Assi, S.; Araji, T.; Chamaa, F.; Itani, M.M.; Nokkari, A.; Kobeissy, F.; Daoud, G.; et al. The Akt/mTOR pathway in cancer stem/progenitor cells is a potential therapeutic target for glioblastoma and neuroblastoma. Oncotarget 2018, 9, 33549–33561. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.; Anderson, K. Thalidomide and dexamethasone: A new standard of care for initial therapy in multiple myeloma. J. Clin. Oncol. 2006, 24, 334–336. [Google Scholar] [CrossRef][Green Version]
- Eleutherakis-Papaiakovou, V.; Bamias, A.; Dimopoulos, M.A. Thalidomide in cancer medicine. Ann. Oncol. 2004, 15, 1151–1160. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Samman, H.; Monzer, A.; Hadadeh, O.; Cheaito, K.; Abdel-Samad, R.; Hayar, B.; Pisano, C.; Msheik, H.; Liu, Y.N.; et al. The synthetic retinoid ST1926 attenuates prostate cancer growth and potentially targets prostate cancer stem-like cells. Mol. Carcinog. 2019, 58, 1208–1220. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).