Instant Attraction: Clay Authigenesis in Fossil Fungal Biofilms
Abstract
:1. Introduction
2. Methods
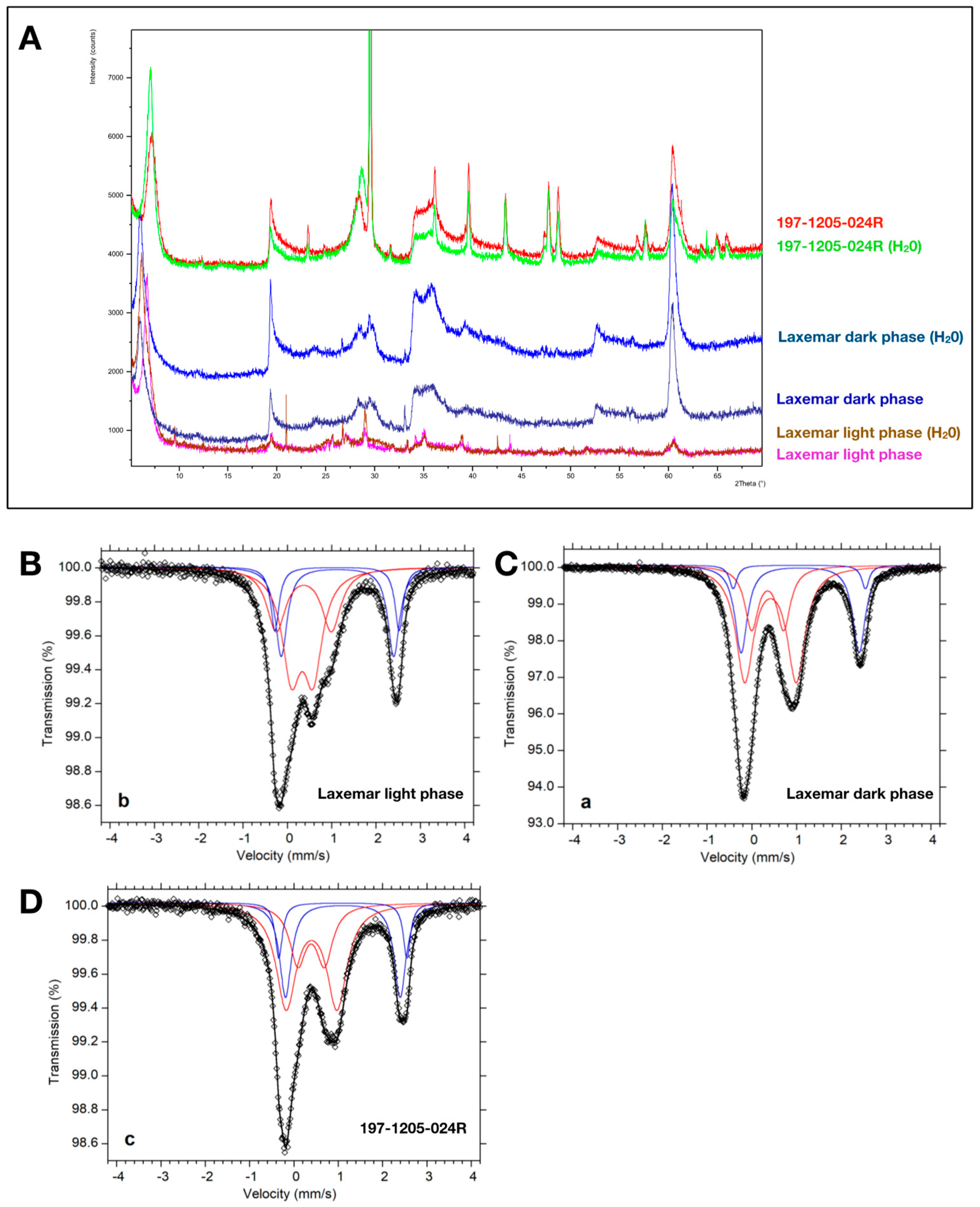
2.1. X-Ray Powder Diffraction
2.2. Mössbauer Spectroscopy
3. Microbial Clay Mineralization
3.1. Benefits of Clay Authigenesis for Microbial Communities
3.2. Clays as Biosignatures
4. Microbial Clay Mineralization in the Igneous Crust: 2.4 Ga–48 Ma
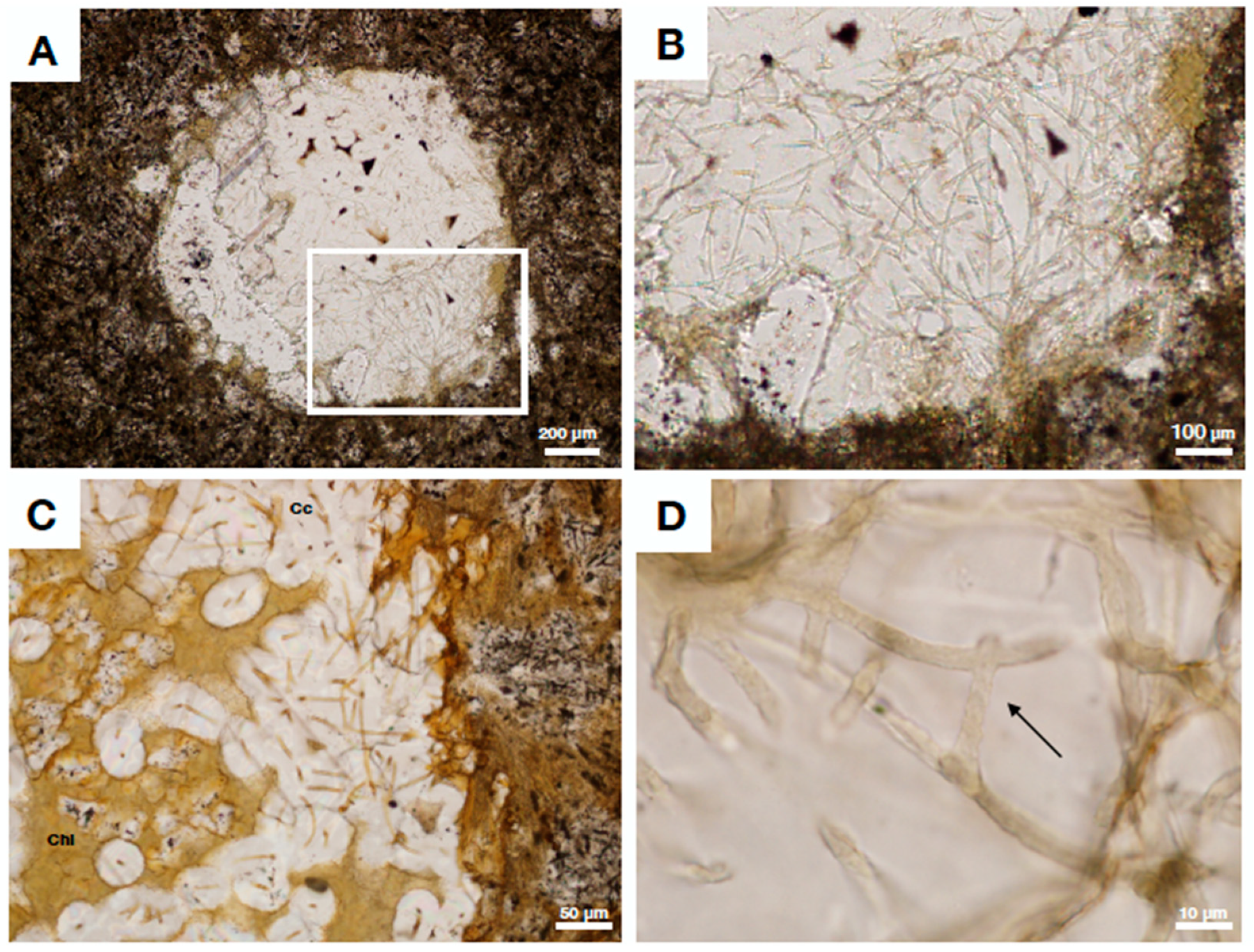
4.1. Clay Fossilized Filaments from the Ongeluk Formation, South Africa
4.2. Fungal Fossils from Fractured Granitic Rock
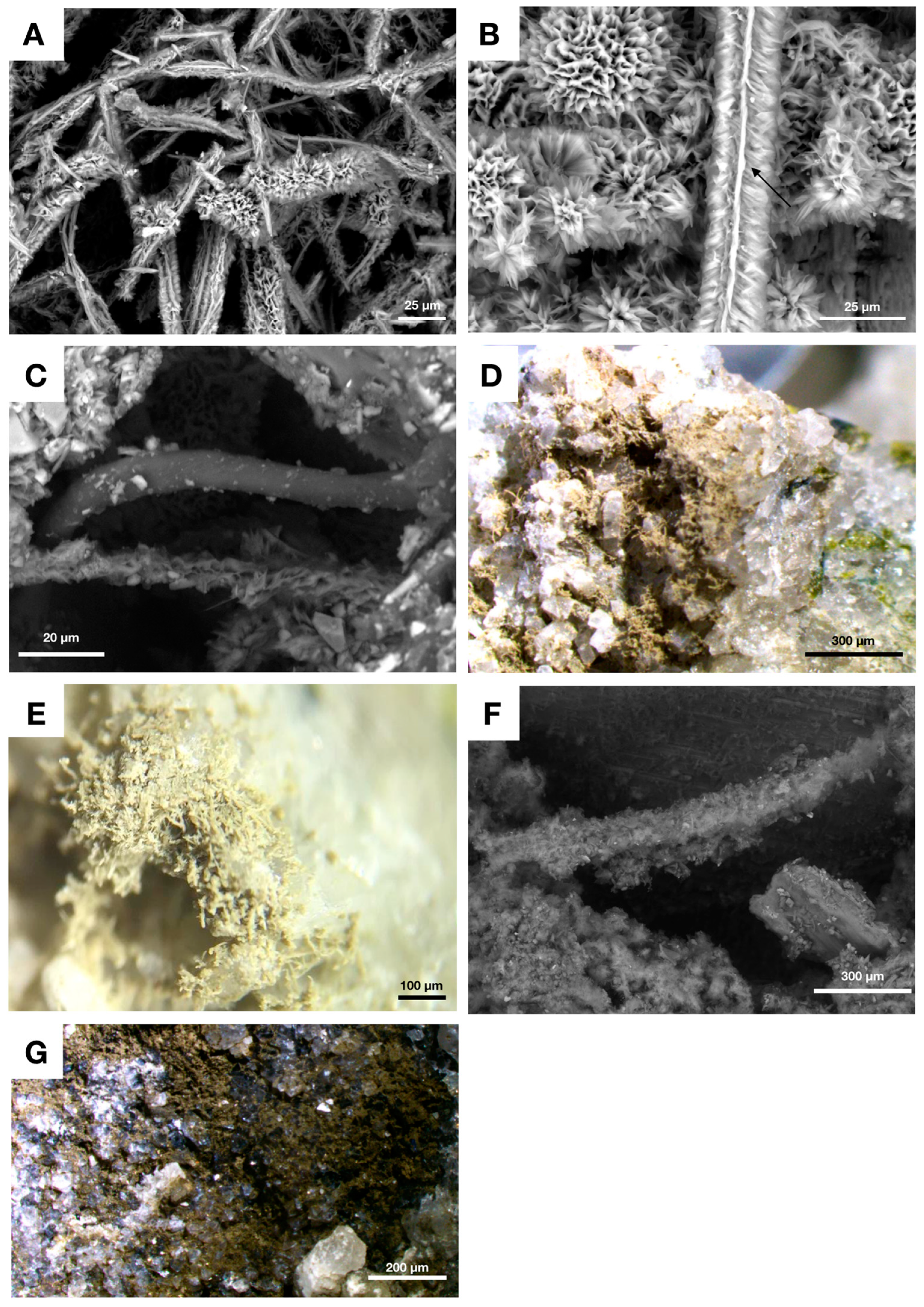
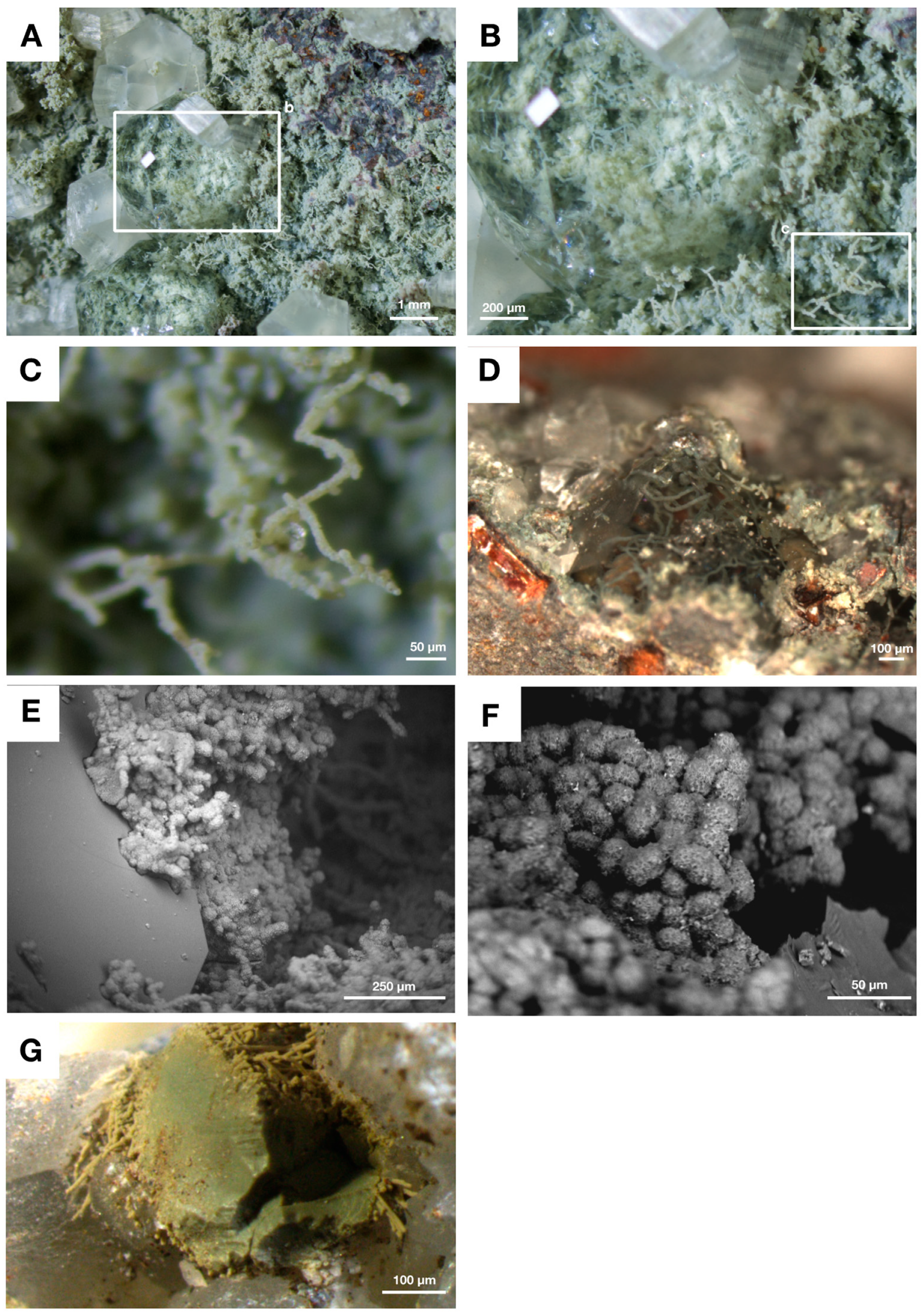
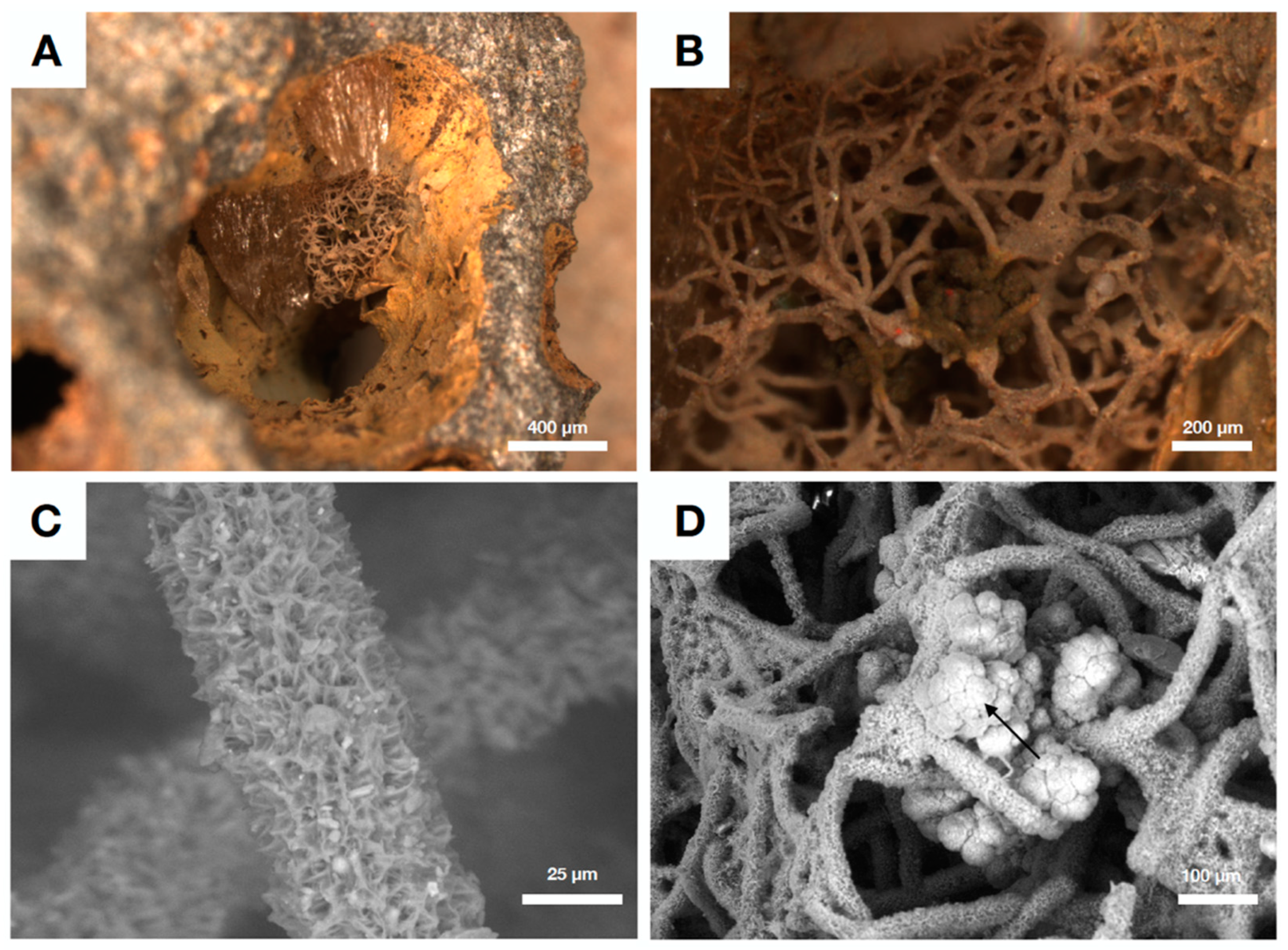
4.3. Fungal Biofilms within The Emperor Seamounts
4.4. Authigenic Clays within Late Devonian Pillow Basalts
5. Redox Variability in Crustal Biosphere Habitats
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferris, F.G.; Beveridge, T.J.; Fyfe, W.S. Iron-silica crystallite nucleation by bacteria in a geothermal sediment. Nature 1986, 320, 609–611. [Google Scholar] [CrossRef]
- Ferris, F.; Fyfe, W.; Beveridge, T. Bacteria as nucleation sites for authigenic minerals in a metal-contaminated lake sediment. Chem. Geol. 1987, 63, 225–232. [Google Scholar] [CrossRef]
- Ferris, F.G.; Fyfe, W.S.; Beveridge, T.J. Bacteria as nucleation sites for authigenic minerals. In Developments in Geochemistry; Elsevier: Amsterdam, The Netherlands, 1991; Volume 6, pp. 319–325. [Google Scholar]
- Konhauser, K.O.; Fyfe, W.S.; Ferris, F.G.; Beveridge, T.J. Metal sorption and mineral precipitation by bacteria in two Amazonian river systems: Rio Solimões and Rio Negro, Brazil. Geology 1993, 21, 1103–1106. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Schultze-Lam, S.; Ferris, F.G.; Fyfe, W.S.; Longstaffe, F.J.; Beveridge, T.J. Mineral Precipitation by Epilithic Biofilms in the Speed River, Ontario, Canada. Appl. Environ. Microbiol. 1994, 60, 549–553. [Google Scholar] [PubMed]
- Chafetz, H.S.; Buczynski, C. Bacterially induced lithification of microbial mats. Palaios 1992, 7, 277–293. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Urrutia, M.M. Bacterial clay authigenesis: a common biogeochemical process. Chem. Geol. 1999, 161, 399–413. [Google Scholar] [CrossRef]
- Knorre, H.V.; Krumbein, W.E. Bacterial calcification. In Microbial Sediments; Springer: Berlin/Heidelberg, Germany, 2000; pp. 25–31. [Google Scholar]
- Konhauser, K.O.; Hamade, T.; Raiswell, R.; Morris, R.C.; Ferris, F.G.; Southam, G.; Canfield, D.E. Could bacteria have formed the Precambrian banded iron formations? Geology 2002, 30, 1079–1082. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Warthmann, R.; McKenzie, J.A.; Visscher, P.T.; Bittermann, A.G.; van Lith, Y. Lithifying microbial mats in Lagoa Vermelha, Brazil: modern Precambrian relics? Sediment. Geol. 2006, 185, 175–183. [Google Scholar] [CrossRef]
- McKinley, J.P.; Stevens, T.O.; Westall, F. Microfossils and paleoenvironments in deep subsurface basalt samples. Geomicrobiol. J. 2000, 17, 43–54. [Google Scholar]
- Schiffman, P.; Fisher, Q.J.; Konhauser, K.O. Microbial mediation of authigenic clays during hydrothermal alteration of basaltic tephra, Kilauea Volcano. Geochem. Geophys. Geosystems 2002, 3, 1–13. [Google Scholar] [Green Version]
- Peckmann, J.; Bach, W.; Behrens, K.; Reitner, J. Putative cryptoendolithic life in Devonian pillow basalt, Rheinisches Schiefergebirge, Germany. Geobiology 2008, 6, 125–135. [Google Scholar] [CrossRef]
- Eickmann, B.; Bach, W.; Kiel, S.; Reitner, J.; Peckmann, J. Evidence for cryptoendolithic life in Devonian pillow basalts of Variscan orogens, Germany. Palaeogeogr. Palaeoclim. Palaeoecol. 2009, 283, 120–125. [Google Scholar] [CrossRef]
- Ivarsson, M.; Bengtson, S.; Skogby, H.; Belivanova, V.; Marone, F. Fungal colonies in open fractures of subseafloor basalt. Geo-Marine Lett. 2013, 33, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Ivarsson, M.; Broman, C.; Gustafsson, H.; Holm, N.G. Biogenic Mn-oxides in subseafloor basalts. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Ivarsson, M.; Bengtson, S.; Skogby, H.; Lazor, P.; Broman, C.; Belivanova, V.; Marone, F. A fungal-prokaryotic consortium at the basalt-zeolite interface in subseafloor igneous crust. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Bengtson, S.; Ivarsson, M.; Astolfo, A.; Belivanova, V.; Broman, C.; Marone, F.; Stampanoni, M. Deep-biosphere consortium of fungi and prokaryotes in Eocene subseafloor basalts. Geobiology 2014, 12, 489–496. [Google Scholar] [CrossRef]
- Bengtson, S.; Rasmussen, B.; Ivarsson, M.; Muhling, J.; Broman, C.; Marone, F.; Stampanoni, M.; Bekker, A. Fungus-like mycelial fossils in 2.4-billion-year-old vesicular basalt. Nat. Ecol. Evol. 2017, 1, 141. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Influence of clay minerals on the morphology of fungal pellets. Mycol. Res. 2002, 106, 107–117. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Metal sorption by biomass of melanin-producing fungi grown in clay containing medium. J. Chem. Technol. Biotechnol. 2003, 78, 23–34. [Google Scholar] [CrossRef]
- Konhauser, K.O. Diversity of bacterial iron mineralization. Earth-Science Rev. 1998, 43, 91–121. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Ferris, F.G. Diversity of iron and silica precipitation by microbial mats in hydrothermal waters, Iceland: Implications for Precambrian iron formations. Geology 1996, 24, 323. [Google Scholar] [CrossRef]
- Wierzchos, J.; Ascaso, C.; Sancho, L.G.; Green, A. Iron-Rich Diagenetic Minerals are Biomarkers of Microbial Activity in Antarctic Rocks. Geomicrobiol. J. 2003, 20, 15–24. [Google Scholar] [CrossRef]
- Drake, H.; Ivarsson, M.; Bengtson, S.; Heim, C.; Siljeström, S.; Whitehouse, M.J.; Broman, C.; Belivanova, V.; Åström, M.E. Anaerobic consortia of fungi and sulfate reducing bacteria in deep granite fractures. Nat. Commun. 2017, 8, 55. [Google Scholar] [CrossRef]
- Edwards, K.J.; Rogers, D.R.; Wirsen, C.O.; McCollom, T.M. Isolation and characterization of novel psychrophilic, neutrophilic, fe-oxidizing, chemolithoautotrophic α- and γ-Proteobacteria from the Deep Sea. Appl. Environ. Microbiol. 2003, 69, 2906–2913. [Google Scholar] [CrossRef]
- Edwards, K.J.; Fisher, A.T.; Wheat, C.G. The Deep Subsurface Biosphere in Igneous Ocean Crust: Frontier Habitats for Microbiological Exploration. Front. Microbiol. 2012, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Orcutt, B.N.; Sylvan, J.B.; Knab, N.J.; Edwards, K.J. Microbial ecology of the Dark Ocean above, at, and below the seafloor. Microbiol. Mol. Boil. Rev. 2011, 75, 361–422. [Google Scholar] [CrossRef]
- Thorseth, I.; Torsvik, T.; Furnes, H.; Muehlenbachs, K. Microbes play an important role in the alteration of oceanic crust. Chem. Geol. 1995, 126, 137–146. [Google Scholar] [CrossRef]
- Staudigel, H.; Furnes, H.; McLoughlin, N.; Banerjee, N.R.; Connell, L.B.; Templeton, A. 3.5 billion years of glass bioalteration: Volcanic rocks as a basis for microbial life? Earth-Sci. Rev. 2008, 89, 156–176. [Google Scholar] [CrossRef]
- Banerjee, N.R.; Izawa, M.R.M.; Sapers, H.M.; Whitehouse, M.J. Geochemical biosignatures preserved in microbially altered basaltic glass. Surf. Interface Anal. 2011, 43, 452–457. [Google Scholar] [CrossRef]
- Schumann, G.; Manz, W.; Reitner, J.; Lustrino, M. Ancient fungal life in North Pacific Eocene oceanic crust. Geomicrobiol. J. 2004, 21, 241–246. [Google Scholar] [CrossRef]
- Ivarsson, M.; Lindblom, S.; Broman, C.; Holm, N.G. Fossilized microorganisms associated with zeolite–carbonate interfaces in sub-seafloor hydrothermal environments. Geobiology 2008, 6, 155–170. [Google Scholar] [CrossRef]
- Ivarsson, M.; Lausmaa, J.; Lindblom, S.; Broman, C.; Holm, N.G. Fossilized microorganisms from the Emperor Seamounts: Implications for the search for a subsurface fossil record on Earth and Mars. Astrobiology 2008, 8, 1139–1157. [Google Scholar] [CrossRef]
- Ivarsson, M.; Bengtson, S.; Belivanova, V.; Stampanoni, M.; Marone, F.; Tehler, A. Fossilized fungi in subseafloor Eocene basalts. Geology 2012, 40, 163–166. [Google Scholar] [CrossRef]
- Ivarsson, M.; Holm, N.G.; Neubeck, A. The deep biosphere of the subseafloor igneous crust. In Trace Metal Biogeochemsitry and Ecology of Deep-Sea Hydrothermal Vent Systems; Demina, L.L., Galkin, S.V., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–24. [Google Scholar]
- Ivarsson, M.; Schnürer, A.; Bengtson, S.; Neubeck, A. Anaerobic Fungi: A Potential Source of Biological H2 in the Oceanic Crust. Front. Microbiol. 2016, 7, 674. [Google Scholar] [CrossRef] [Green Version]
- Ivarsson, M.; Bengtson, S.; Neubeck, A. The igneous oceanic crust – Earth’s largest fungal habitat? Fungal Ecol. 2016, 20, 249–255. [Google Scholar] [CrossRef]
- Drake, H.; Ivarsson, M.; Tillberg, M.; Whitehouse, M.; Kooijman, E. Ancient microbial activity in deep hydraulically conductive fracture zones within the forsmark target area for geological nuclear waste disposal, Sweden. Geosciences 2018, 8, 211. [Google Scholar] [CrossRef]
- Drake, H.; Ivarsson, M. The role of anaerobic fungi in fundamental biogeochemical cycles in the deep biosphere. Fungal Boil. Rev. 2018, 32, 20–25. [Google Scholar] [CrossRef]
- Sutherland, I.W. Biofilm exopolysaccharides: a strong and sticky framework. Microbiol. 2001, 147, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: the “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Arp, G.; Reimer, A.; Reitner, J. Calcification in cyanobacterial biofilms of alkaline salt lakes. Eur. J. Phycol. 1999, 34, 393–403. [Google Scholar] [CrossRef]
- Arp, G. Photosynthesis-Induced Biofilm Calcification and Calcium Concentrations in Phanerozoic Oceans. Science 2001, 292, 1701–1704. [Google Scholar] [CrossRef] [Green Version]
- Dupraz, C.; Visscher, P.T. Microbial lithification in marine stromatolites and hypersaline mats. Trends Microbiol. 2005, 13, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Science Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Hazen, R.M.; Schiffries, C.M. Why deep carbon? Rev. Mineral. Geochem. 2013, 75, 1–6. [Google Scholar] [CrossRef]
- Prescher, C.; McCammon, C.; Dubrovinsky, L. MossA: A program for analyzing energy-domain Mössbauer spectra from conventional and synchrotron sources. J. Appl. Crystallogr. 2012, 45, 329–331. [Google Scholar] [CrossRef]
- Fortin, D.; Ferris, F.G.; Scott, S.D. Formation of Fe-silicates and Fe-oxides on bacterial surfaces in samples collected near hydrothermal vents on the Southern Explorer Ridge in the Northeast Pacific Ocean. Am. Miner. 1998, 83, 1399–1408. [Google Scholar] [CrossRef]
- Léveillé, R.J.; Datta, S. Lava tubes and basaltic caves as astrobiological targets on Earth and Mars: A review. Planet. Space Sci. 2010, 58, 592–598. [Google Scholar] [CrossRef]
- Sánchez- Navas, A.S.; Algarra, A.M. Nieto Bacterially-mediated authigenesis of clays in phosphate stromatolites. Sedimentolopgy 1998, 45, 519–533. [Google Scholar] [CrossRef]
- Geptner, A.; Kristmannsdottir, H.; Kristjansson, J.; Marteinsson, V. Biogenic Saponite from an Active Submarine Hot Spring, Iceland. Clays Clay Miner. 2002, 50, 174–185. [Google Scholar] [CrossRef]
- Bristow, T.F.; Milliken, R.E. Terrestrial perspective on authigenic clay mineral production in ancient Martian lakes. Clays Clay Miner. 2011, 59, 339–358. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry An Introduction Emphasizing Chemical Equilibria in Natural Waters; John Wiley & Sons: New York, NY, USA, 1981. [Google Scholar]
- Stucki, J.W. Structural iron in smectites. In Iron in Soils and Clay Minerals; Springer: Dordrecht, The Netherlands, 1988; pp. 625–675. [Google Scholar]
- Tuck, V.A.; Edyvean, R.G.J.; West, J.M.; Bateman, K.; Coombs, P.; Milodowski, A.E.; McKervey, J.A. Biologically induced clay formation in subsurface granitic environments. J. Geochem. Explor. 2006, 90, 123–133. [Google Scholar] [CrossRef]
- Castanier, S.; Le Metayer-Levrel, G.; Perthuisot, J.P. Bacterial roles in the precipitation of carbonate minerals. In Microbial Sediments; Springer: Berlin/Heidelberg, Germany, 2000; pp. 32–39. [Google Scholar]
- Braissant, O.; Decho, A.W.; Przekop, K.M.; Gallagher, K.L.; Glunk, C.; Dupraz, C.; Visscher, P.T. Characteristics and turnover of exopolymeric substances in a hypersaline microbial mat. FEMS Microbiol. Ecol. 2009, 67, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Dupraz, C.; Fowler, A.; Tobias, C.; Visscher, P.T. Stromatolitic knobs in Storr’s Lake (San Salvador, Bahamas): A model system for formation and alteration of laminae. Geobiology 2013, 11, 527–548. [Google Scholar] [CrossRef]
- Arp, G.; Thiel, V.; Reimer, A.; Michaelis, W.; Reitner, J. Biofilm exopolymers control microbialite formation at thermal springs discharging into the alkaline Pyramid Lake, Nevada, USA. Sediment. Geol. 1999, 126, 159–176. [Google Scholar] [CrossRef]
- Sandford, P.A. Exocellular, microbial polysaccharides. In Advances in Carbohydrate Chemistry and Biochemistry; Academic Press: Cambridge, MA, USA, 1979; Volume 36, pp. 265–313. [Google Scholar]
- Ueshima, M.; Tazaki, K. Possible role of microbial polysaccharides in nontronite formation. Clays Clay Miner. 2001, 49, 292–299. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.S.; Hamed, H.A.; Mouafi, F.E.; Gad, A.S. Acidic pH-shock induces the production of an exopolysaccharide by the fungus Mucor rouxii: Utilization of beet-molasses. NY Sci. J. 2012, 5, 52–61. [Google Scholar]
- Mahapatra, S.; Banerjee, D. Fungal Exopolysaccharide: Production, Composition and Applications. Microbiol. Insights 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.; McNeil, B. Production of the fungal exopolysaccharide scleroglucan by cultivation of Sclerotium glucanicum in an airlift reactor with an external loop. J. Chem. Technol. Biotechnol. 1995, 63, 215–222. [Google Scholar] [CrossRef]
- England, L.S.; Lee, H.; Trevors, J.T. Bacterial survival in soil: Effect of clays and protozoa. Soil Boil. Biochem. 1993, 25, 525–531. [Google Scholar] [CrossRef]
- Stotzky, G.; Rem, L.T. Influence of clay minerals on microorganisms: I. Montmorillonite and kaolinite on bacteria. Can. J. Microbiol. 1966, 12, 547–563. [Google Scholar] [CrossRef]
- Morley, G.F.; Gadd, G.M. Sorption of toxic metals by fungi and clay minerals. Mycol. Res. 1995, 99, 1429–1438. [Google Scholar] [CrossRef]
- Gadd, G.M. Interactions of fungi with toxic metals. In The Genus Aspergillus; Springer: Boston, MA, USA, 1994; pp. 361–374. [Google Scholar]
- Blaudez, D.; Jacob, C.; Turnau, K.; Colpaert, J.V.; Ahonen-Jonnarth, U.; Finlay, R.; Botton, B.; Chalot, M. Differential responses of ectomycorrhizal fungi to heavy metals in vitro. Mycol. Res. 2000, 104, 1366–1371. [Google Scholar] [CrossRef]
- Perotto, S.; Martino, E. Molecular and cellular mechanisms of heavy metal tolerance in mycorrhizal fungi: What perspectives for bioremediation? Min. Biotecnol. 2001, 13, 55–63. [Google Scholar]
- Baldrián, P. Interactions of heavy metals with white-rot fungi. Enzym. Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef]
- Stucki, J.W. Properties and behaviour of iron in clay minerals. Dev. Clay Sci. 2006, 1, 423–475. [Google Scholar]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.H.; He, P.J.; Shao, L.M. Characteristics of extracellular polymeric substances (EPS) fractions from excess sludges and their effects on bioflocculability. Bioresour. Technol. 2009, 100, 3193–3198. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Löhr, S.C.; Fraser, S.A.; Baruch, E.T. Direct evidence for organic carbon preservation as clay-organic nanocomposites in a Devonian black shale; from deposition to diagenesis. Earth Planet. Sci. Lett. 2014, 388, 59–70. [Google Scholar] [CrossRef]
- Playter, T.; Konhauser, K.; Owttrim, G.; Hodgson, C.; Warchola, T.; Mloszewska, A.M.; Sutherland, B.; Bekker, A.; Zonneveld, J.-P.; Pemberton, S.G.; et al. Microbe-clay interactions as a mechanism for the preservation of organic matter and trace metal biosignatures in black shales. Chem. Geol. 2017, 459, 75–90. [Google Scholar] [CrossRef]
- LaLonde, K.; Mucci, A.; Ouellet, A.; Gelinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [Green Version]
- Parenteau, M.N.; Jahnke, L.L.; Farmer, J.D.; Cady, S.L. Production and Early Preservation of Lipid Biomarkers in Iron Hot Springs. Astrobiology 2014, 14, 502–521. [Google Scholar] [CrossRef] [Green Version]
- Summons, R.E.; Amend, J.P.; Bish, D.; Buick, R.; Cody, G.D.; Marais, D.J.D.; Dromart, G.; Eigenbrode, J.L.; Knoll, A.H.; Sumner, D.Y. Preservation of Martian Organic and Environmental Records: Final Report of the Mars Biosignature Working Group. Astrobiology 2011, 11, 157–181. [Google Scholar] [CrossRef]
- Magnabosco, C.; Lin, L.-H.; Dong, H.; Bomberg, M.; Ghiorse, W.; Stan-Lotter, H.; Pedersen, K.; Kieft, T.L.; Van Heerden, E.; Onstott, T.C. The biomass and biodiversity of the continental subsurface. Nat. Geosci. 2018, 11, 707. [Google Scholar] [CrossRef]
- Beaufort, D.; Rigault, C.; Billon, S.; Billault, V.; Inoue, A.; Inoue, S.; Patrier, P. Chlorite and chloritization processes through mixed-layer mineral series in low-temperature geological systems—a review. Clay Miner. 2015, 50, 497–523. [Google Scholar] [CrossRef]
- Canfield, D.E. A new model for Proterozoic ocean chemistry. Nature 1998, 396, 450–453. [Google Scholar] [CrossRef]
- Tarduno, J.A.; Duncan, R.A.; Scholl, D.W. Leg 197 Summary: Proceedings of the Ocean Drilling Program, Initial Reports; Ocean Drilling Program Texas A&M University: College Station TX, USA, 2002; Volume 197, pp. 1–92. [Google Scholar]
- Orcutt, B.N.; Wheat, C.G.; Rouxel, O.; Hulme, S.; Edwards, K.J.; Bach, W. Oxygen consumption rates in subseafloor basaltic crust derived from a reaction transport model. Nat. Commun. 2013, 4, 2539. [Google Scholar] [CrossRef] [Green Version]
- Bach, W.; Edwards, K.J. Iron and sulfide oxidation within the basaltic ocean crust: implications for chemolithoautotrophic microbial biomass production. Geochim. Cosmochim. Acta 2003, 67, 3871–3887. [Google Scholar] [CrossRef]
- Tosca, N.J.; Knoll, A.H. Juvenile chemical sediments and the long term persistence of water at the surface of Mars. Earth Planet. Sci. Lett. 2009, 286, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Jaisi, D.P.; Kim, J.; Zhang, G. Microbe-clay mineral interactions. Am. Mineral. 2009, 94, 1505–1519. [Google Scholar] [CrossRef]
- Sakakibara, M.; Sugawara, H.; Tsuji, T.; Ikehara, M. Filamentous microbial fossil from low-grade metamorphosed basalt in northern Chichibu belt, central Shikoku, Japan. Planet. Space Sci. 2014, 95, 84–93. [Google Scholar] [CrossRef]

| Reference | Age | Setting | Preservation | Clay Phase | Color of Clay | Assumed Source Fluid (This Review) |
|---|---|---|---|---|---|---|
| [19] | Bedrock 2.4 Ga (filament ages ~2.06 Ga) | Ongeluk ophiolite, South Africa | Clay mineralization, slightly metamorphic and chloritized | Chlorite (transformed from precursor clay) | Pale yellow-brown | Anoxic |
| [25] | Bedrock 1.8 Ga (filament ages not constrained, Phanerozoic) | Continental fractured granite Laxemar, Sweden (drill cores) | Clay mineralization/carbonaceous filaments | Fe/Mg/Ca-rich clay, minor Fe-oxides | Cream white (sample XLX09) | Anoxic |
| [13] | Presumed Devonian | Arnstein ophiolite, Germany (pillow basalt) | Clay mineralization | TiO2 filament-strands surrounded by illite and chamosite | Pale yellow-brown | Presumed oxic-sub oxic |
| [14] | Presumed Devonian | Frankenwald and Thüringer Wald, Germany (pillow basalt) | Clay mineralization | Illite center/chamosite rim or TiO2 filament-strands surrounded by illite and chamosite | Green | Presumed oxic-sub oxic |
| [16] | Cretaceous, 81 Ma | Oceanic crust, Detroit seamount (basalt drill cores) | Clay mineralization | Smectite (montmorillonite) | Cream yellow (sample 197-1204B-16R-01) | Oxic -slightly sub oxic |
| [17] | Paleocene-Eocene boundary, 56 Ma | Oceanic crust, Nintoku seamount (basalt drill cores) | Clay mineralization | Smectite (montmorillonite) | Green (sample 197-1205-34R-5) | Oxic -slightly sub oxic |
| [15] | Paleocene-Eocene boundary, 56 Ma (Nintoku seamount)/ Eocene, 48 Ma (Koko seamount) | Oceanic crust, Nintoku and Koko seamounts (basalt drill cores) | Clay mineralization | Smectite (montmorillonite) | Green (sample 197-1205-34R-5), Yellow-cream/red (sample 197-1206-4R-2) | Oxic -slightly sub oxic |
| [18] | Eocene, 48 Ma | Oceanic crust, Koko seamount (basalt drill cores) | Clay mineralization | Smectite (montmorillonite) | Yellow-cream/red (sample 197-1206-4R-2) | Oxic -slightly sub oxic |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallstedt, T.; Ivarsson, M.; Drake, H.; Skogby, H. Instant Attraction: Clay Authigenesis in Fossil Fungal Biofilms. Geosciences 2019, 9, 369. https://doi.org/10.3390/geosciences9090369
Sallstedt T, Ivarsson M, Drake H, Skogby H. Instant Attraction: Clay Authigenesis in Fossil Fungal Biofilms. Geosciences. 2019; 9(9):369. https://doi.org/10.3390/geosciences9090369
Chicago/Turabian StyleSallstedt, Therese, Magnus Ivarsson, Henrik Drake, and Henrik Skogby. 2019. "Instant Attraction: Clay Authigenesis in Fossil Fungal Biofilms" Geosciences 9, no. 9: 369. https://doi.org/10.3390/geosciences9090369
APA StyleSallstedt, T., Ivarsson, M., Drake, H., & Skogby, H. (2019). Instant Attraction: Clay Authigenesis in Fossil Fungal Biofilms. Geosciences, 9(9), 369. https://doi.org/10.3390/geosciences9090369






