Copper and its Isotopes in Organic-Rich Sediments: From the Modern Peru Margin to Archean Shales
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Organic Carbon Abundance and Isotope Composition
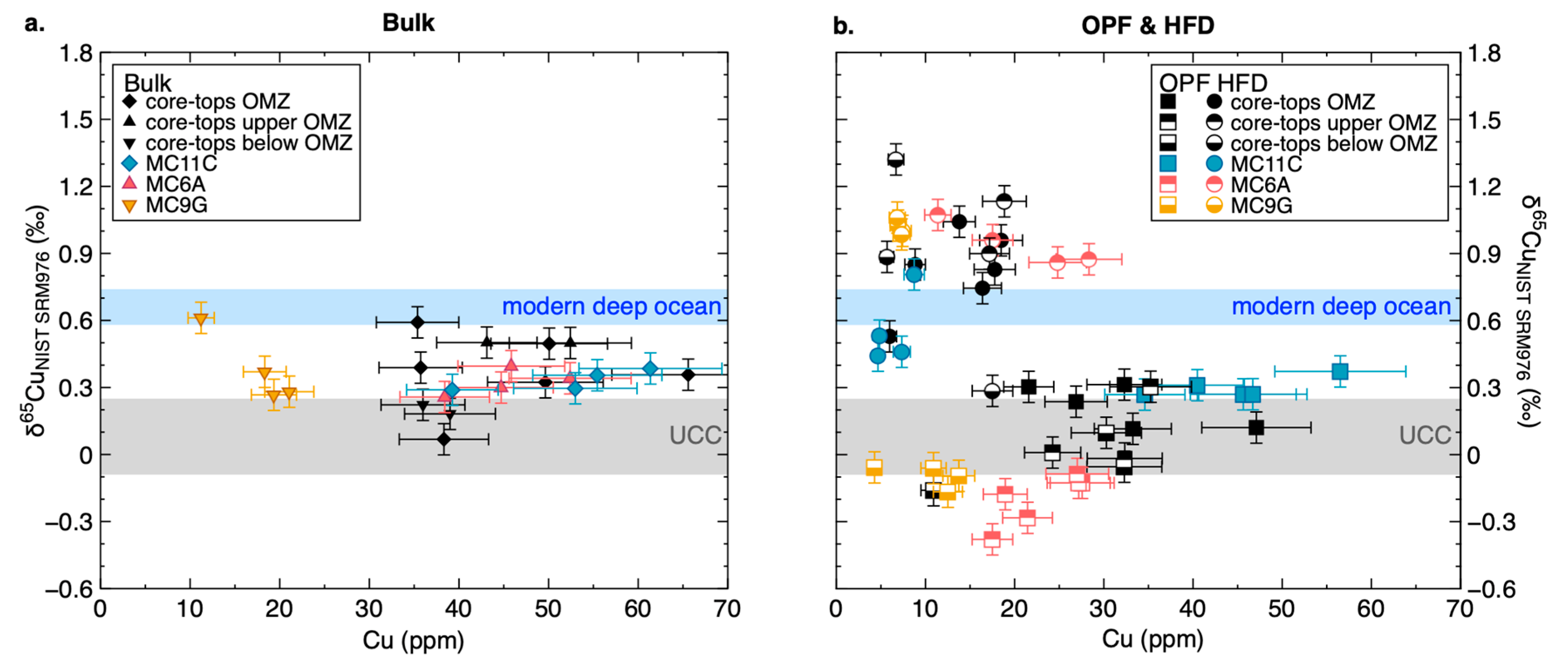
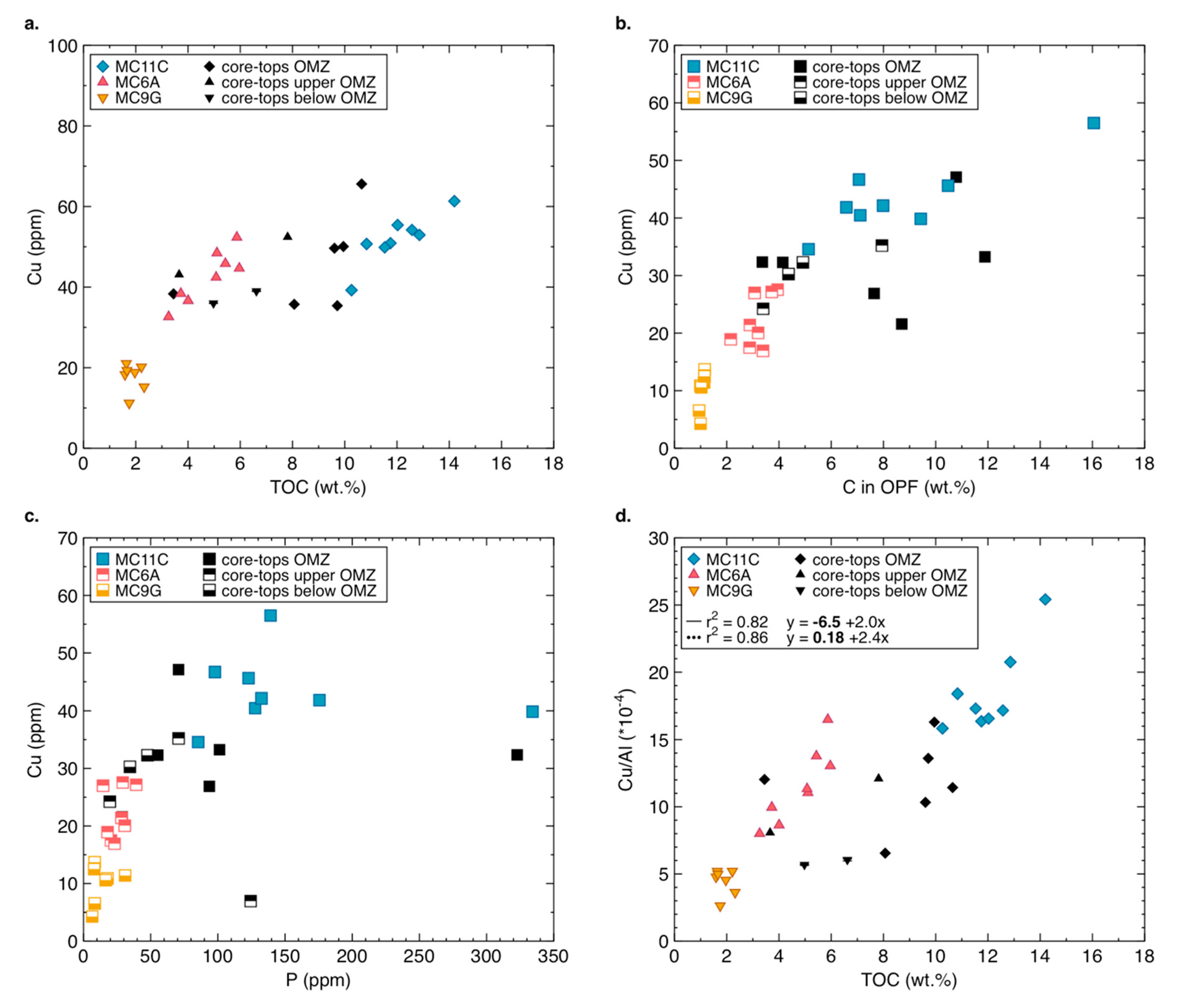
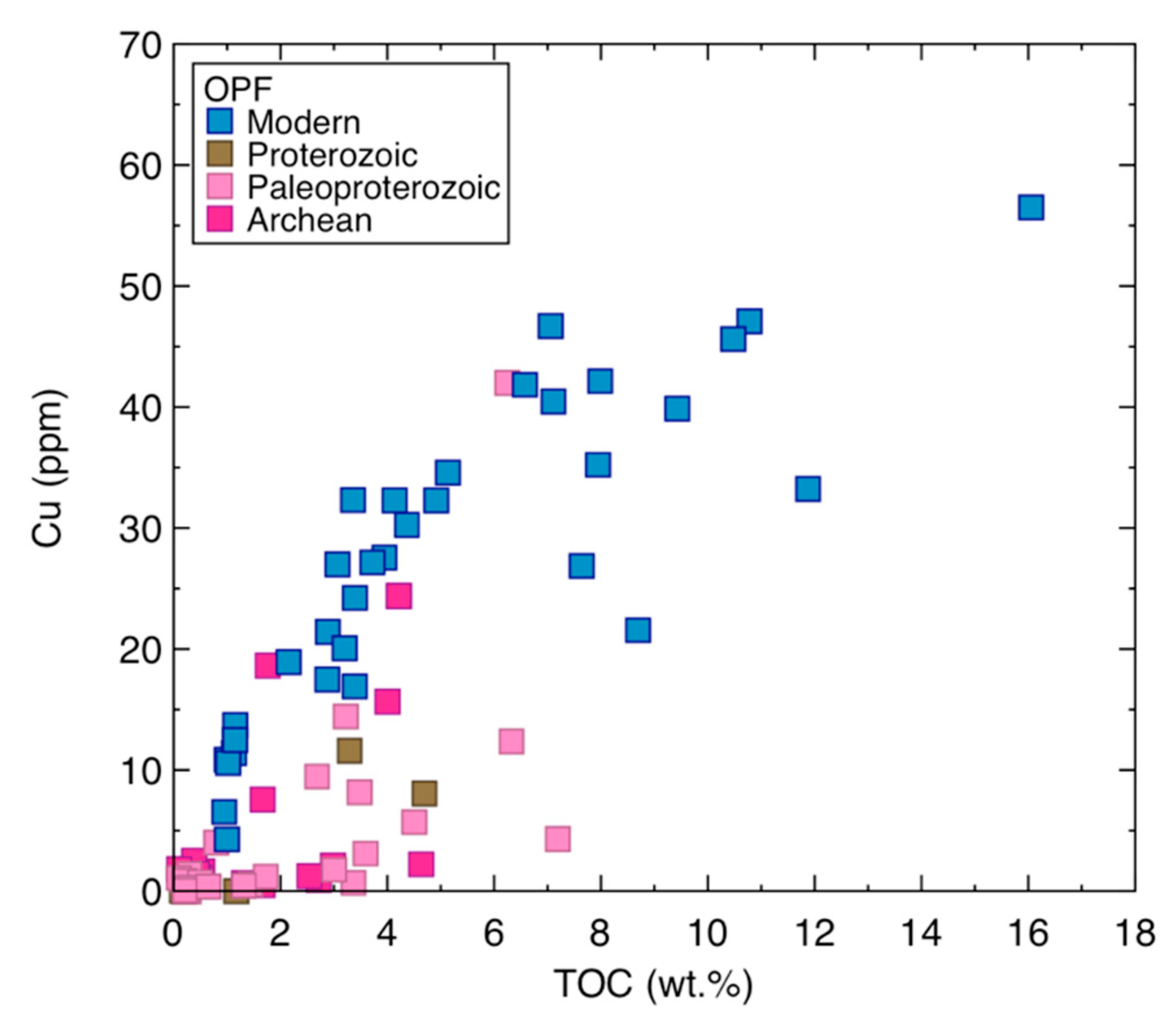
3.2. Copper Concentrations and Isotope Composition of Modern Organic-Rich Sediments
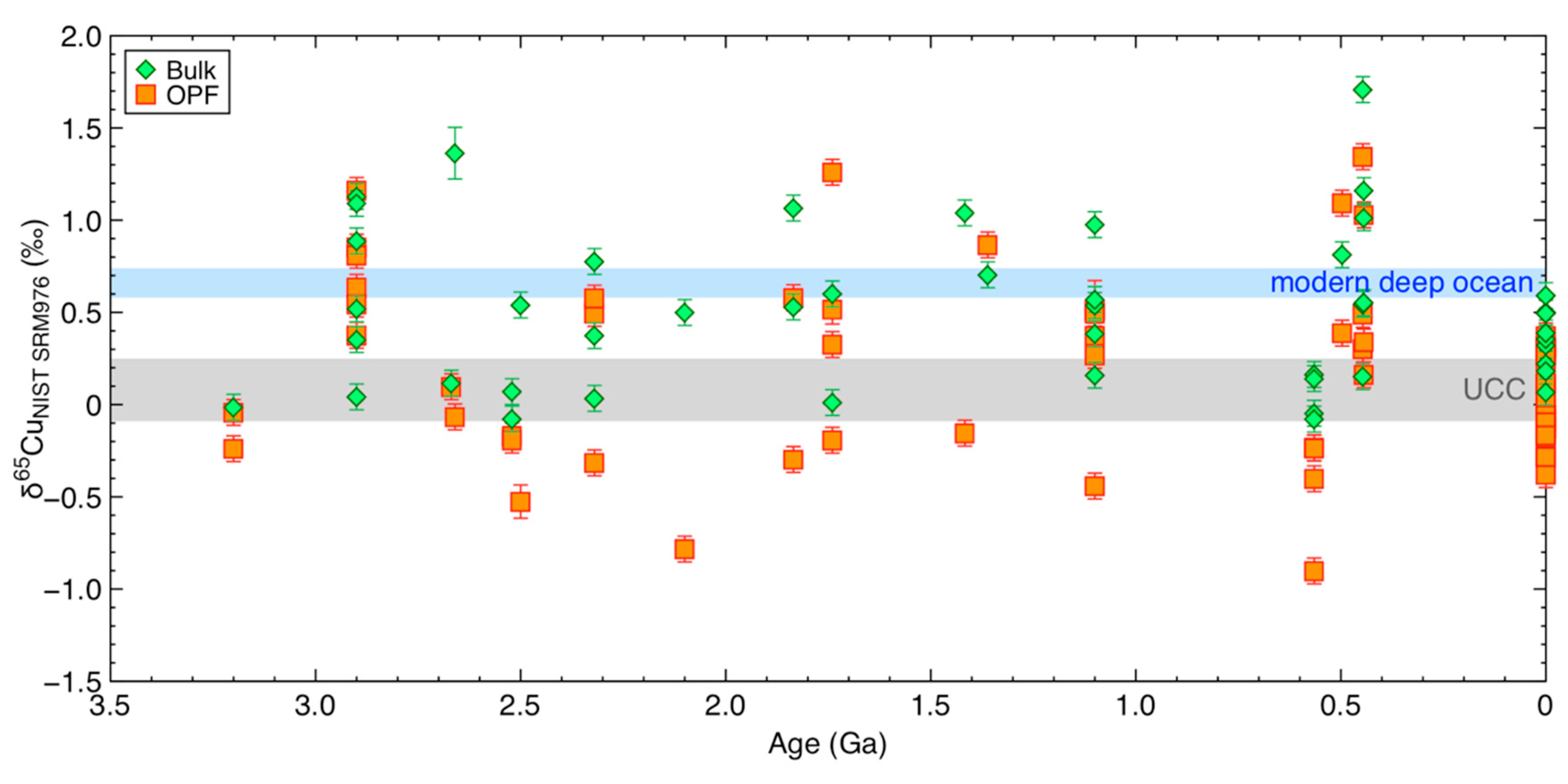
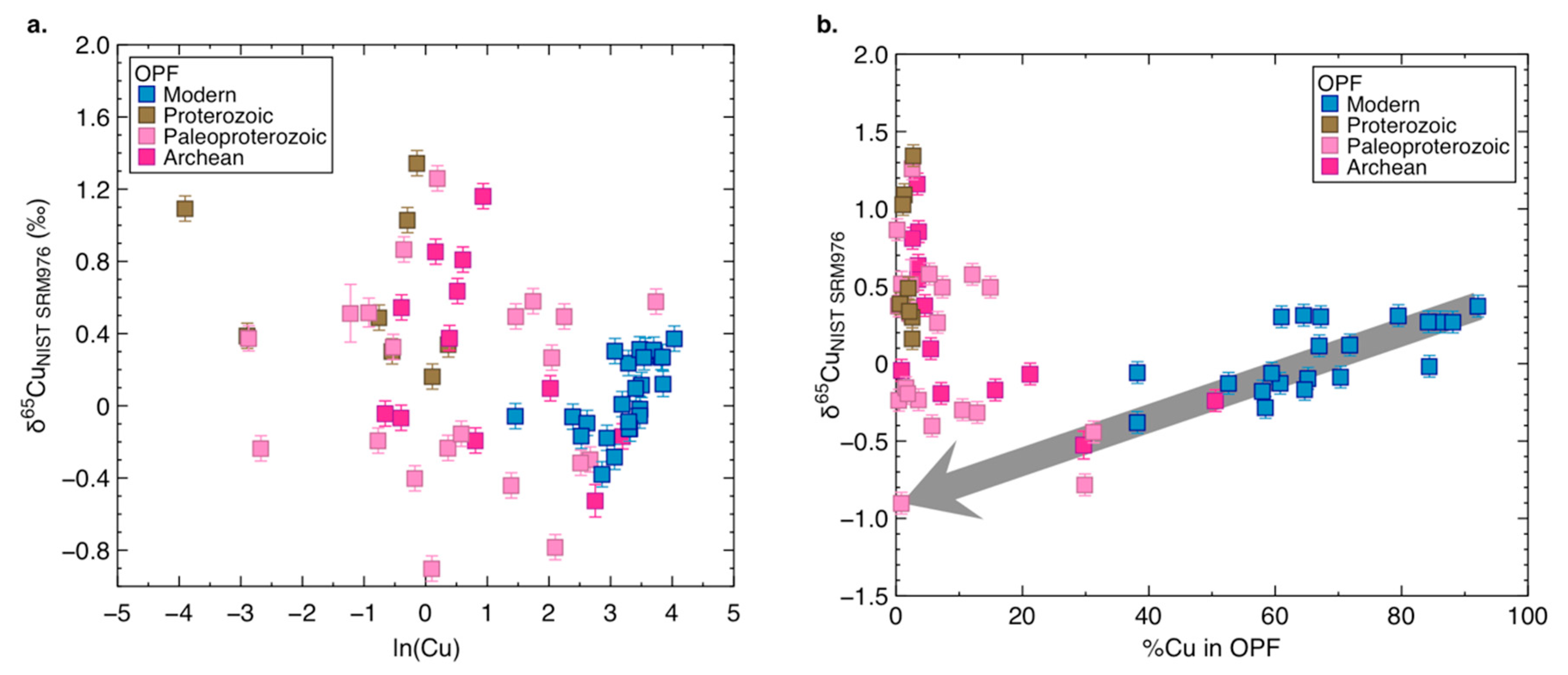
3.3. Copper Concentrations and Isotope Composition of Ancient Organic-Rich Sediments
4. Discussion
4.1. Tests of the Two-Step Digestion Methodology
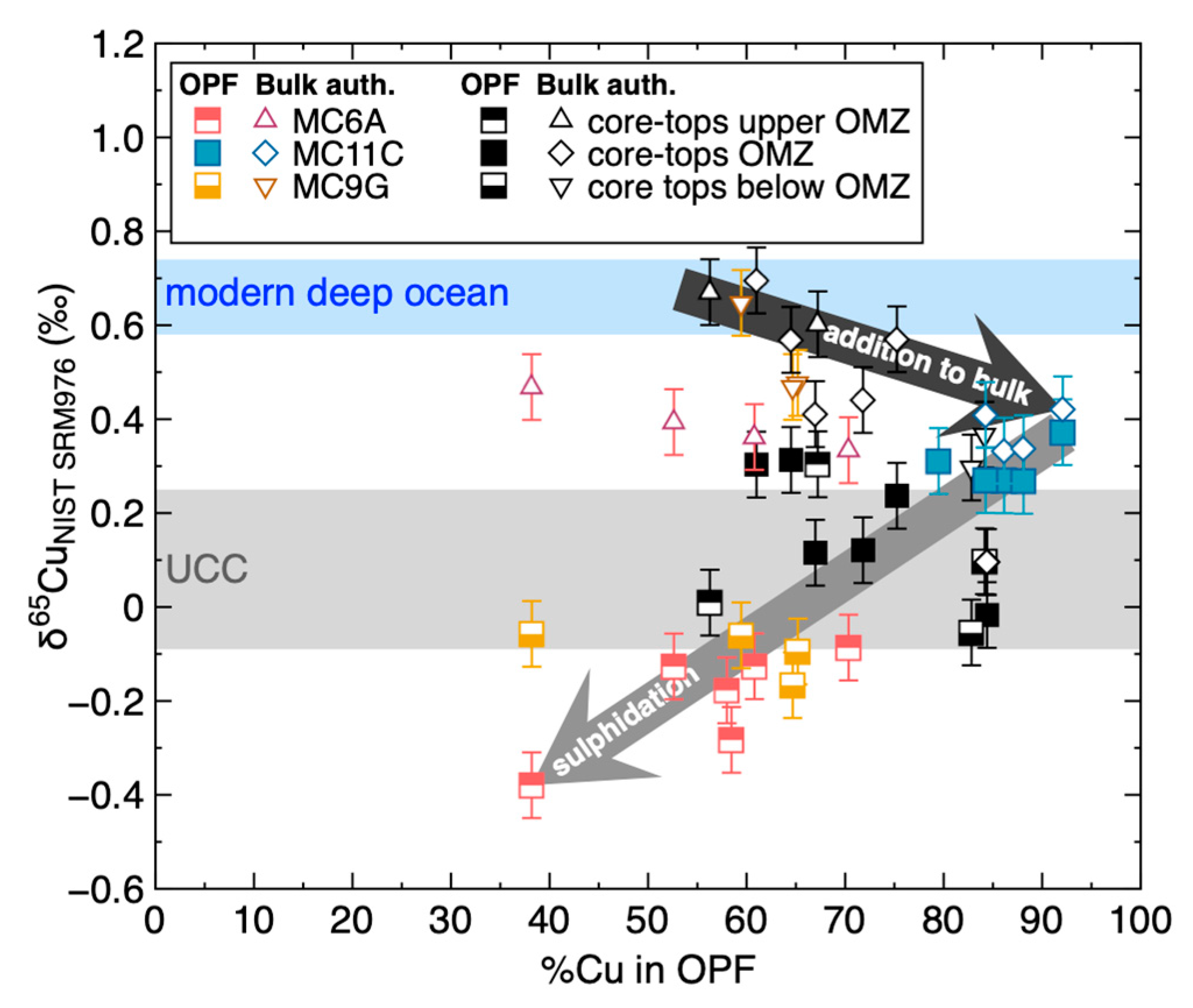
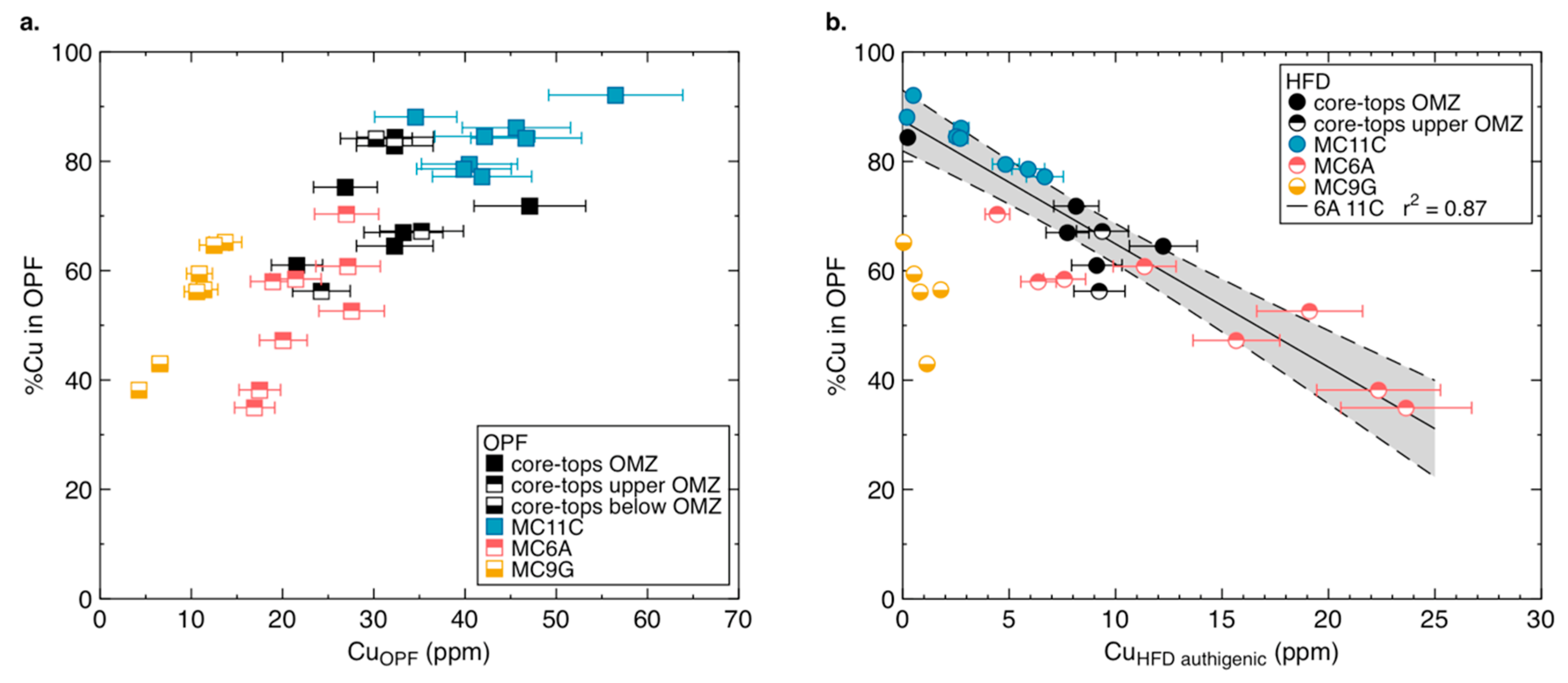
4.2. The Source of Cu in Peru Margin Organic-Rich Sediments
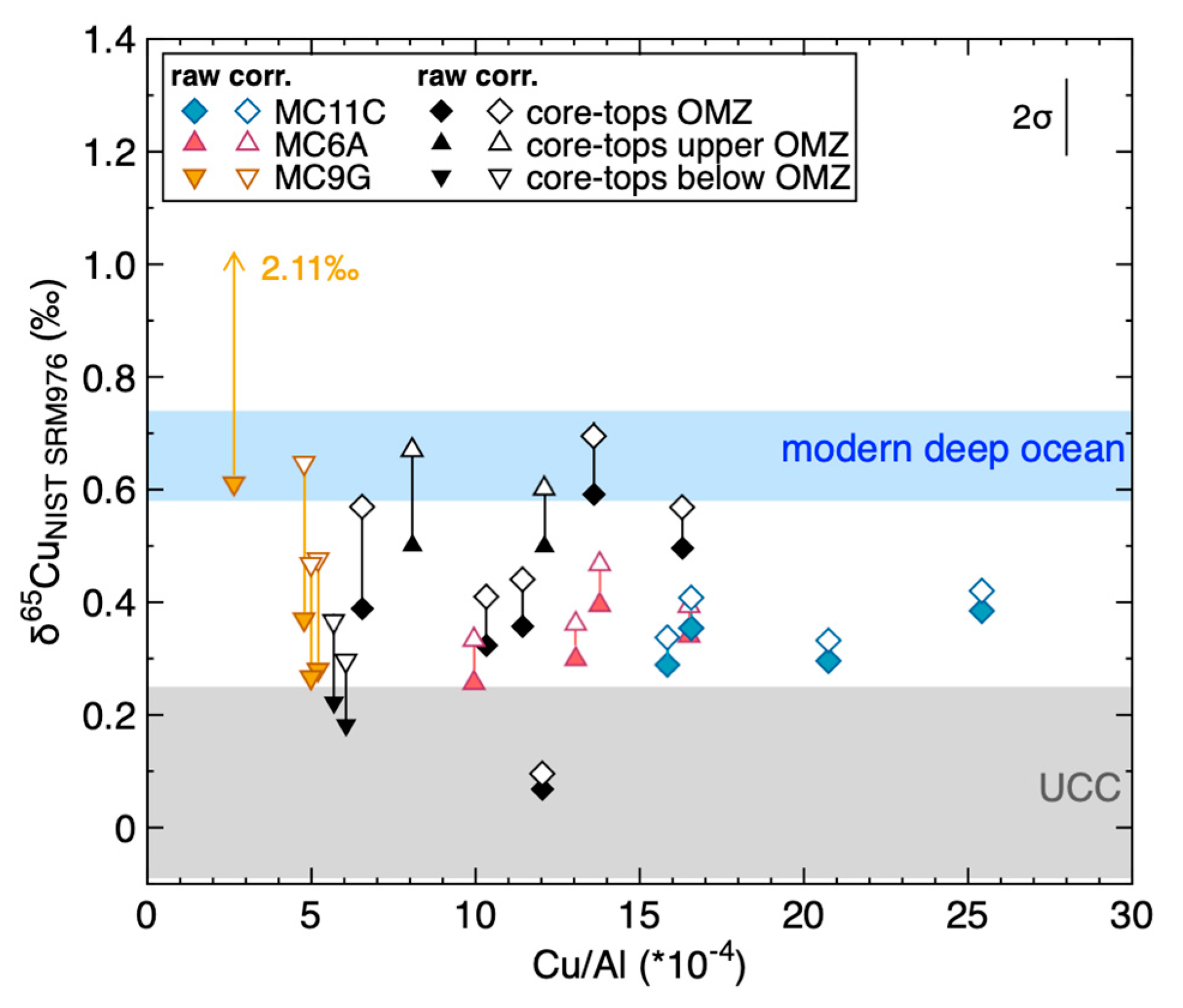
4.3. The Control of Sulphidation on Cu Isotopes in Peru Margin Sediments
4.4. Detrital Correction and Authigenic Cu in Peru Margin and Ancient Sediments
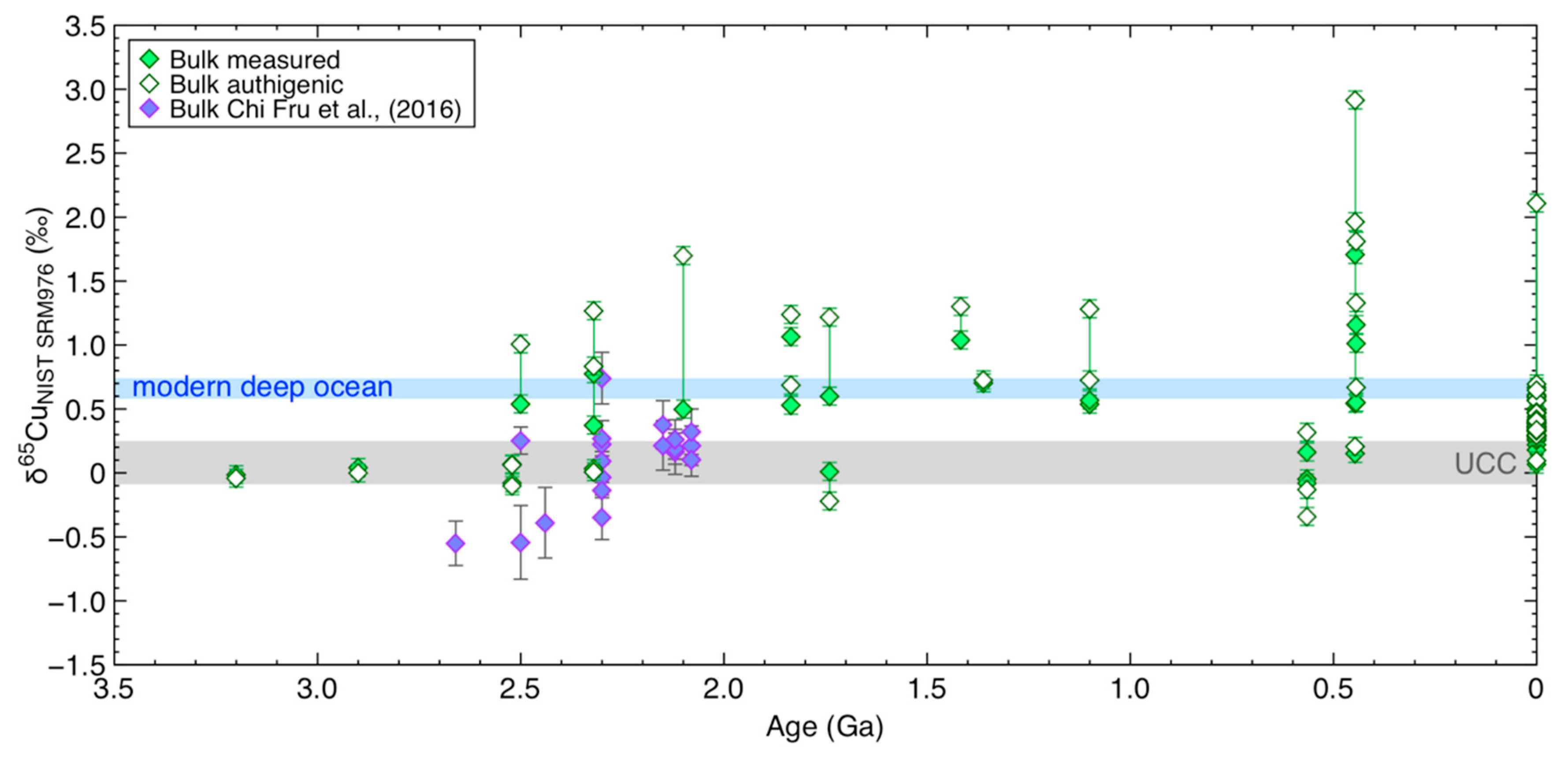
4.5. Copper and its Isotopes in Bulk Ancient Shales
4.6. Copper in the OPF of Ancient Shales: Not Only Sulphidation
5. Conclusions
- (1).
- Cu abundance and total organic carbon (TOC) are positively correlated in modern samples, but not in ancient ones;
- (2).
- the organic-pyrite fraction dominates the total Cu reservoir in modern samples, in most cases containing >50% of the bulk Cu;
- (3).
- the HF-digestible fraction dominates the total Cu reservoir in ancient samples, in most cases containing >80% of the bulk Cu;
- (4).
- Cu isotopes are correlated with enrichment of Cu in the organic-pyrite fraction of modern samples, but this is not the case for ancient samples.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asael, D.; Rouxel, O.; Poulton, S.W.; Lyons, T.W.; Bekker, A. Molybdenum record from black shales indicates oscillating atmospheric oxygen levels in the early Paleoproterozoic. Am. J. Sci. 2018, 318, 275–299. [Google Scholar] [CrossRef]
- Kendall, B.; Gordon, G.W.; Poulton, S.W.; Anbar, A.D. Molybdenum isotope constraints on the extent of late Paleoproterozoic ocean euxinia. Earth Planet. Sci. Lett. 2011, 307, 450–460. [Google Scholar] [CrossRef]
- Vance, D.; Archer, C.; Bermin, J.; Perkins, J.; Statham, P.J.; Lohan, M.C.; Ellwood, M.J.; Mills, R.A. The copper isotope geochemistry of rivers and the oceans. Earth Planet. Sci. Lett. 2008, 274, 204–213. [Google Scholar] [CrossRef]
- Moynier, F.; Vance, D.; Fujii, T.; Savage, P. The isotope geochemistry of zinc and copper. Rev. Mineral. Geochem. 2017, 82, 543–600. [Google Scholar] [CrossRef]
- Moffett, J.W.; Brand, L.E. Production of strong, extracellular Cu chelators by marine cyanobacteria in response to Cu stress. Limnol. Oceanogr. 1996, 41, 388–395. [Google Scholar] [CrossRef]
- Chi, F.E.; Rodriguez, N.P.; Partin, C.A.; Lalonde, S.V.; Andersson, P.; Weiss, D.J.; El Albani, A.; Rodushkin, I.; Konhauser, K.O. Cu isotopes in marine black shales record the Great Oxidation Event. Proc. Natl. Acad. Sci. 2016, 113, 4941–4946. [Google Scholar]
- Peers, G.; Price, N.M. Copper-containing plastocyanin used for electron transport by an oceanic diatom. Nature 2006, 441, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Twining, B.S.; Baines, S.B. The trace metal composition of marine phytoplankton. Annu. Rev. Mar. Sci. 2013, 5, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Bruland, K.W. Oceanographic distributions of cadmium, zinc, nickel, and copper in the North Pacific. Earth Planet. Sci. Lett. 1980, 47, 176–198. [Google Scholar] [CrossRef]
- Coale, K.H.; Bruland, K.W. Copper complexation in the Northeast Pacific. Limnol. Oceanogr. 1988, 33, 1084–1101. [Google Scholar] [CrossRef]
- Coale, K.H.; Bruland, K.W. Spatial and temporal variability in copper complexation in the North Pacific. Deep Sea Res. Part A: Oceanogr. Res. Pap. 1990, 37, 317–336. [Google Scholar] [CrossRef]
- Sunda, W.G. Trace metal/phytoplankton interactions in the sea. In Chemistry of Aquatic Systems: Local and Global Perspectives; Bidoglio, G., Stumm, W., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 213–247. [Google Scholar]
- Moffett, J.; Zika, R.; Brand, L.E. Distribution and potential sources and sinks of copper chelators in the Sargasso Sea. Deep Sea Res. Part A: Oceanogr. Res. Pap. 1990, 37, 27–36. [Google Scholar] [CrossRef]
- Bruland, K.W.; Lohan, M.C. Controls of trace metals in seawater. Treaties Geochem. 2003, 6, 23–47. [Google Scholar]
- Moffett, J.W.; Dupont, C. Cu complexation by organic ligands in the sub-arctic NW Pacific and Bering Sea. Deep Sea Res. Part A: Oceanogr. Res. Pap. 2007, 54, 586–595. [Google Scholar] [CrossRef]
- Buck, K.N.; Moffett, J.; Barbeau, K.A.; Bundy, R.M.; Kondo, Y.; Wu, J. The organic complexation of iron and copper: An intercomparison of competitive ligand exchange-adsorptive cathodic stripping voltammetry (CLE-ACSV) techniques. Limnology Oceanogr.: Methods 2012, 10, 496–515. [Google Scholar] [CrossRef]
- Jacquot, J.E.; Moffett, J.W. Copper distribution and speciation across the International GEOTRACES Section GA03. Deep Sea Res. Part II: Top. Stud. Oceanogr. 2015, 116, 187–207. [Google Scholar] [CrossRef]
- Boyle, E.A.; Sclater, F.R.; Edmond, J.M. The distribution of dissolved copper in the Pacific. Earth Planet. Sci. Lett. 1977, 37, 38–54. [Google Scholar] [CrossRef]
- Little, S.H.; Vance, D.; Siddall, M.; Gasson, E. A modeling assessment of the role of reversible scavenging in controlling oceanic dissolved Cu and Zn distributions. Glob. Biogeochem. Cycles 2013, 27, 780–791. [Google Scholar] [CrossRef]
- Little, S.; Vance, D.; Walker-Brown, C.; Landing, W. The oceanic mass balance of copper and zinc isotopes, investigated by analysis of their inputs, and outputs to ferromanganese oxide sediments. Geochim. Cosmochim. Acta 2014, 125, 673–693. [Google Scholar] [CrossRef]
- Jacobs, L.; Emerson, S.; Huested, S.S. Trace metal geochemistry in the Cariaco Trench. Deep Sea Res. Part A: Oceanogr. Res. Pap. 1987, 34, 965–981. [Google Scholar] [CrossRef]
- Huerta-Diaz, M.A.; Morse, J.W. Pyritization of trace metals in anoxic marine sediments. Geochim. Cosmochim. Acta 1992, 56, 2681–2702. [Google Scholar] [CrossRef]
- Tankéré, S.P.C.; Muller, F.L.L.; Burton, J.D.; Statham, P.J.; Guieu, C.; Martin, J.M. Trace metal distributions in shelf waters of the northwestern Black Sea. Cont. Shelf Res. 2001, 21, 1501–1532. [Google Scholar] [CrossRef]
- Ehrlich, S.; Butler, I.; Halicz, L.; Rickard, D.; Oldroyd, A.; Matthews, A. Experimental study of the copper isotope fractionation between aqueous Cu(II) and covellite, CuS. Chem. Geol. 2004, 209, 259–269. [Google Scholar] [CrossRef]
- Navarrete, J.U.; Borrok, D.M.; Viveros, M.; Ellzey, J.T. Copper isotope fractionation during surface adsorption and intracellular incorporation by bacteria. Geochim. Cosmochim. Acta 2011, 75, 784–799. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Viers, J.; Emnova, E.E.; Kompantseva, E.I.; Freydier, R. Copper isotope fractionation during its interaction with soil and aquatic microorganisms and metal oxy(hydr)oxides: Possible structural control. Geochim. Cosmochim. Acta 2008, 72, 1742–1757. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Borrok, D.M.; Wanty, R.B.; Ridley, W.I. Fractionation of Cu and Zn isotopes during adsorption onto amorphous Fe(III) oxyhydroxide: Experimental mixing of acid rock drainage and ambient river water. Geochim. Cosmochim. Acta 2008, 72, 311–328. [Google Scholar] [CrossRef]
- Ijichi, Y.; Ohno, T.; Sakata, S. Copper isotopic fractionation during adsorption on manganese oxide: Effects of pH and desorption. Geochem. J. 2018, 52, e1–e6. [Google Scholar] [CrossRef]
- Albarède, F. The stable isotope geochemistry of copper and zinc. Rev. Mineral. Geochem. 2004, 55, 409–427. [Google Scholar] [CrossRef]
- Little, S.H.; Sherman, D.M.; Vance, D.; Hein, J.R. Molecular controls on Cu and Zn isotopic fractionation in Fe-Mn crusts. Earth and Planet. Sci. Lett. 2014, 396, 213–222. [Google Scholar] [CrossRef]
- Ryan, B.M.; Kirby, J.K.; Degryse, F.; Scheiderich, K.; McLaughlin, M.J. Copper isotope fractionation during equilibration with natural and synthetic ligands. Environ. Sci. Technol. 2014, 48, 8620–8626. [Google Scholar] [CrossRef]
- Bigeleisen, J.; Mayer, M.G. Calculation of equilibrium constants for isotopic exchange reactions. J. Chem. Phys. 1947, 15, 261–267. [Google Scholar] [CrossRef]
- Takano, S.; Tanimizu, M.; Hirata, T.; Sohrin, Y. Isotopic constraints on biogeochemical cycling of copper in the ocean. Nat. Commun. 2014, 5, 5663. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Ellwood, M.J. Dissolved copper isotope biogeochemistry in the Tasman Sea, SW Pacific Ocean. Mar. Chem. 2014, 165, 1–9. [Google Scholar] [CrossRef]
- Little, S.H.; Archer, C.; Milne, A.; Schlosser, C.; Achterberg, E.P.; Lohan, M.C.; Vance, D. Paired dissolved and particulate phase Cu isotope distributions in the South Atlantic. Chem. Geol. 2018, 502, 29–43. [Google Scholar] [CrossRef]
- Little, S.H.; Vance, D.; McManus, J.; Severmann, S.; Lyons, T.W. Copper isotope signatures in modern marine sediments. Geochim. Cosmochim. Acta 2017, 212, 253–273. [Google Scholar] [CrossRef]
- Böning, P.; Brumsack, H.-J.; Böttcher, M.E.; Schnetger, B.; Kriete, C.; Kallmeyer, J.; Borchers, S.L. Geochemistry of Peruvian near-surface sediments. Geochim. Cosmochim. Acta 2004, 68, 4429–4451. [Google Scholar] [CrossRef]
- Maréchal, C.N.; Télouk, P.; Albarède, F. Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem. Geol. 1999, 156, 251–273. [Google Scholar] [CrossRef]
- Archer, C.; Vance, D. Mass discrimination correction in multiple-collector plasma source mass spectrometry: An example using Cu and Zn isotopes. J. Anal. At. Spectrom. 2004, 19, 656. [Google Scholar] [CrossRef]
- Ciscato, E.R.; Bontognali, T.R.R.; Vance, D. Nickel and its isotopes in organic-rich sediments: Implications for oceanic budgets and a potential record of ancient seawater. Earth Planet. Sci. Lett. 2018, 494, 239–250. [Google Scholar] [CrossRef]
- Eigenbrode, J.L.; Freeman, K.H. Late Archean rise of aerobic microbial ecosystems. Proc. Natl. Acad. Sci. 2006, 103, 15759–15764. [Google Scholar] [CrossRef]
- Arthur, M.A.; Dean, W.E.; Laarkamp, K. Organic carbon accumulation and preservation in surface sediments on the Peru Margin. Chem. Geol. 1998, 152, 273–286. [Google Scholar] [CrossRef]
- Calvert, S.E.; Price, N.B. Geochemistry of Namibian shelf sediments. In Coastal Upwelling Its Sediment Record; Springer: Dordrecht, The Netherlands, 1983; pp. 337–375. [Google Scholar]
- Croudace, I.W. A possible error source in silicate wet-chemistry caused by insoluble fluorides. Chem. Geol. 1980, 31, 153–155. [Google Scholar] [CrossRef]
- Böning, P.; Shaw, T.; Pahnke, K.; Brumsack, H.-J. Nickel as indicator of fresh organic matter in upwelling sediments. Geochim. Cosmochim. Acta 2015, 162, 99–108. [Google Scholar] [CrossRef]
- Ohnemus, D.C.; Rauschenberg, S.; Cutter, G.A.; Fitzsimmons, J.N.; Sherrell, R.M.; Twining, B.S. Elevated trace metal content of prokaryotic communities associated with marine oxygen deficient zones. Limnol. Oceanogr. 2016, 62, 3–25. [Google Scholar] [CrossRef]
- Janssen, D.J.; Conway, T.M.; John, S.G.; Christian, J.R.; Kramer, D.I.; Pedersen, T.F.; Cullen, J.T. Undocumented water column sink for cadmium in open ocean oxygen-deficient zones. Proc. Natl. Acad. Sci. 2014, 111, 6888–6893. [Google Scholar] [CrossRef] [PubMed]
- Janssen, D.J.; Cullen, J.T. Decoupling of zinc and silicic acid in the subarctic northeast Pacific interior. Mar. Chem. 2015, 117, 124–133. [Google Scholar] [CrossRef]
- Bianchi, D.; Weber, T.S.; Kiko, R.; Deutsch, C. Global niche of marine anaerobic metabolisms expanded by particle microenvironments. Nat. Geosci. 2018, 11, 263–268. [Google Scholar] [CrossRef]
- Scheidegger, K.F.; Krissek, L.A. Dispersal and deposition of eolian and fluvial sediments off Peru and northern Chile. GSA Bull. 1982, 93, 150–162. [Google Scholar] [CrossRef]
- DiTullio, G.R.; Geesey, M.E.; Maucher, J.M.; Alm, M.B.; Riseman, S.F.; Bruland, K.W. Influence of iron on algal community composition and physiological status in the Peru upwelling system. Limnol. Oceanogr. 2005, 50, 1887–1907. [Google Scholar] [CrossRef]
- Ho, T.-Y.; Quigg, A.; Finkel, Z.V.; Milligan, A.J.; Wyman, K.; Falkowski, P.G.; Morel, F.M.M. The elemental composition of some marine phytoplankton. J. Phycol. 2003, 39, 1145–1159. [Google Scholar] [CrossRef]
- Ingall, E.; Jahnke, R. Evidence for enhanced phosphorus regeneration from marine sediments overlain by oxygen depleted waters. Geochim. Cosmochim. Acta 1994, 58, 2571–2575. [Google Scholar] [CrossRef]
- Paytan, A.; McLaughlin, K. The oceanic phosphorus cycle. Chem. Rev. 2007, 107, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.W. Interactions of trace metals with authigenic sulfide minerals: implications for their bioavailability. Mar. Chem. 1994, 46, 1–6. [Google Scholar] [CrossRef]
- Fujii, T.; Moynier, F.; Abe, M.; Nemoto, K.; Albarède, F. Copper isotope fractionation between aqueous compounds relevant to low temperature geochemistry and biology. Geochim. Cosmochim. Acta 2013, 110, 29–44. [Google Scholar] [CrossRef]
- Fujii, T.; Moynier, F.; Blichert-Toft, J.; Albarède, F. Density functional theory estimation of isotope fractionation of Fe, Ni, Cu, and Zn among species relevant to geochemical and biological environments. Geochim. Cosmochim. Acta 2014, 140, 553–576. [Google Scholar] [CrossRef]
- Böning, P.; Fröllje, H.; Beck, M.; Schnetger, B.; Brumsack, H.-J. Underestimation of the authigenic fraction of Cu and Ni in organic-rich sediments. Mar. Geol. 2012, 324, 24–28. [Google Scholar] [CrossRef]
- Brumsack, H.-J. Geochemistry of recent TOC-rich sediments from the Gulf of California and the Black Sea. Geol. Rundsch. 1989, 78, 851–852. [Google Scholar] [CrossRef]
- Taylor, S.; McLennan, S.M. The Continental crust: Its composition and evolution; Blackwell Scientific Publications: Hoboken, NJ, USA, 1985; p. 312. [Google Scholar]
- Och, L.M.; Shields-Zhou, G.A. The Neoproterozoic oxygenation event: Environmental perturbations and biogeochemical cycling. Earth-Sci. Rev. 2012, 110, 26–57. [Google Scholar] [CrossRef]
- Krause, A.J.; Mills, B.J.W.; Zhang, S.; Planavsky, N.; Lenton, T.M.; Poulton, S.W. Stepwise oxygenation of the Paleozoic atmosphere. Nat. Commun. 2018, 9, 4081. [Google Scholar] [CrossRef]
- Mathur, R.; Ruiz, J.; Titley, S.; Liermann, L.; Buss, H.; Brantley, S. Cu isotopic fractionation in the supergene environment with and without bacteria. Geochim. Cosmochim. Acta 2005, 69, 5233–5246. [Google Scholar] [CrossRef]
- Canfield, D. A new model for Proterozoic ocean chemistry. Nature 1998, 396, 450–453. [Google Scholar] [CrossRef]
- Rouxel, O.J.; Bekker, A.; Edwards, K. Iron isotope constraints on the Archean and Paleoprterozoic ocean redox state. Science 2005, 307, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.A.; Sigman, D.M.; Morel, F.M.M. The bioinorganic chemistry of the ancient ocean: The co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean-Proterozoic boundary. Inorg. Chim. Acta 2003, 356, 308–318. [Google Scholar] [CrossRef]
- Neaman, A.; Chorover, J.; Brantley, S.L. Element mobility patterns record organic ligands in soils on Early Earth. Geology 2005, 33, 117–120. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH diagrams. Geol. Surv. Jpn. Open File Rep. 2005, 419, 102. [Google Scholar]

| Water Depth/ | OPF | HFD | Bulk | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Depth in Coreb | BWOb | wtc | C | δ13C | Ald | Pd | Cud | % Cu | δ65Cu | 2σe | Ald | Cud | % Cu | δ65Cu | 2σe | TOCb | δ13C | Ald | Cud | Cu/Al | δ65Cu | 2σe |
| (m) or (cm) | (μM) | (%) | (wt.%) | (%) | (wt.%) | (ppm) | (ppm) | (of bulk) | (‰) | (‰) | (wt.%) | (ppm) | (of bulk) | (‰) | (‰) | (wt.%) | (‰) | (wt.%) | (ppm) | (x104) | (‰) | (‰) | |
| BC 39 | 550 | 10 | 20 | 3.4 | −20.9 | 1.0 | 323 | 32 | 84 | −0.02 | 0.03 | 2.2 | 6.0 | 16 | 0.53 | 0.07 | 3.4 | −21.1 | 3.2 | 38 | 12 | 0.07 | 0.04 |
| BC 57 | 172 | <10 | 34 | 12 | −20.8 | 0.7 | 101 | 33 | 67 | 0.12 | 0.03 | 4.1 | 16 | 33 | 0.75 | 0.04 | 9.6 | −20.5 | 4.8 | 50 | 10 | 0.32 | 0.04 |
| BC 62 | 643 | 10 | 21 | 4.4 | −21.3 | 0.9 | 35 | 30 | 84 | 0.10 | 0.03 | 5.4 | 5.7 | 16 | 0.88 | 0.03 | 5.0 | −20.9 | 6.3 | 36 | 5.7 | 0.22 | 0.03 |
| BC 76 | 725 | 15 | 30 | 4.9 | −21.2 | 1.4 | 48 | 32 | 83 | −0.05 | 0.03 | 5.0 | 6.7 | 17 | 1.32 | 0.06 | 6.6 | −21.1 | 6.4 | 39 | 6.1 | 0.18 | 0.04 |
| BC 81 | 130 | 10 | 36 | 11 | −20.6 | 1.1 | 71 | 47 | 72 | 0.12 | 0.03 | 4.6 | 18 | 28 | 0.96 | 0.04 | 10.6 | n/a | 5.7 | 66 | 11 | 0.36 | 0.03 |
| KC 83 | 106 | 10 | 44 | 7.9 | −20.4 | 1.3 | 71 | 35 | 67 | 0.30 | 0.03 | 3.0 | 17 | 33 | 0.90 | 0.04 | 7.8 | −20.2 | 4.3 | 52 | 12 | 0.50 | 0.04 |
| BC 93 | 100 | 5 | 25 | 3.4 | −20.8 | 0.3 | 20 | 24 | 56 | 0.01 | 0.03 | 5.0 | 19 | 44 | 1.13 | 0.04 | 3.7 | −20.5 | 5.3 | 43 | 8.1 | 0.50 | 0.04 |
| BC 125 | 340 | 5 | 36 | 7.6 | −21.3 | 0.7 | 94 | 27 | 75 | 0.24 | 0.04 | 4.8 | 8.8 | 25 | 0.85 | 0.03 | 8.1 | −20.7 | 5.5 | 36 | 6.5 | 0.39 | 0.04 |
| KC 127 | 309 | 5 | 45 | 4.1 | −20.9 | 0.4 | 55 | 32 | 65 | 0.31 | 0.04 | 2.7 | 18 | 35 | 0.83 | 0.04 | 9.9 | −20.6 | 3.1 | 50 | 16 | 0.50 | 0.04 |
| BC 153 | 250 | 5 | 51 | 8.7 | −20.9 | 0.1 | 29 | 22 | 61 | 0.30 | 0.03 | 2.5 | 14 | 39 | 1.04 | 0.04 | 9.7 | −20.8 | 2.6 | 35 | 14 | 0.59 | 0.03 |
| MC6A1 | 0–1 | 2.2 | 34 | 2.9 | −21.2 | 0.7 | 21 | 18 | 38 | −0.38 | 0.03 | 2.6 | 28 | 62 | 0.87 | 0.03 | 5.4 | n/a | 3.3 | 46 | 14 | 0.40 | 0.03 |
| MC6A6 | 5–6 | 2.2 | 36 | 4.0 | −20.4 | 0.8 | 29 | 28 | 53 | −0.13 | 0.02 | 2.3 | 25 | 47 | 0.86 | 0.03 | 5.9 | −20.7 | 3.2 | 52 | 17 | 0.34 | 0.03 |
| MC6A14 | 13–14 | 2.2 | 32 | 3.7 | −20.3 | 0.9 | 39 | 27 | 61 | −0.13 | 0.02 | 2.6 | 18 | 39 | 0.96 | 0.03 | 6.0 | −20.7 | 3.4 | 45 | 13 | 0.30 | 0.03 |
| MC6A16 | 15–16 | 2.2 | 30 | 3.4 | −21.2 | 1.1 | 23 | 17 | 35 | n/a | n/a | 3.3 | 32 | 65 | n/a | n/a | 5.1 | −21.2 | 4.4 | 48 | 11 | n/a | n/a |
| MC6A22 | 21–22 | 2.2 | 22 | 2.2 | −22.2 | 0.9 | 18 | 19 | 58 | −0.18 | 0.02 | 3.2 | 14 | 42 | n/a | n/a | 3.3 | −22.2 | 4.1 | 33 | 8.0 | n/a | n/a |
| MC6A26 | 25–26 | 2.2 | 24 | 2.9 | −21.8 | 1.2 | 28 | 21 | 58 | −0.28 | 0.03 | 3.1 | 15 | 42 | n/a | n/a | 4.0 | −21.8 | 4.2 | 37 | 8.7 | n/a | n/a |
| MC6A37 | 36–37 | 2.2 | 19 | 3.2 | −20.8 | 0.5 | 31 | 20 | 47 | n/a | n/a | 3.2 | 22 | 53 | n/a | n/a | 5.1 | −20.9 | 3.7 | 42 | 11 | n/a | n/a |
| MC6A4446 | 44–46 | 2.2 | 20 | 3.1 | −21.0 | 0.8 | 15 | 27 | 70 | −0.09 | 0.03 | 3.0 | 11 | 30 | 1.07 | 0.04 | 3.7 | n/a | 3.9 | 38 | 10 | 0.26 | 0.03 |
| MC11C4 | 3–4 | 2.1 | 43 | 16 | −21.2 | 0.7 | 139 | 57 | 92 | 0.37 | 0.03 | 1.7 | 4.8 | 7.9 | 0.53 | 0.05 | 14.2 | −21.3 | 2.4 | 61 | 25 | 0.38 | 0.03 |
| MC11C8 | 7–8 | 2.1 | 35 | 10 | −21.8 | 0.7 | 123 | 46 | 86 | 0.27 | 0.02 | 1.8 | 7.3 | 14 | 0.46 | 0.02 | 12.9 | −21.7 | 2.6 | 53 | 21 | 0.30 | 0.02 |
| MC11C17 | 16–17 | 2.1 | 31 | 7.1 | −22.0 | 0.9 | 128 | 40 | 79 | 0.31 | 0.02 | 2.2 | 10 | 21 | n/a | n/a | 11.8 | −21.7 | 3.1 | 51 | 16 | n/a | n/a |
| MC11C23 | 22–23 | 2.1 | 30 | 8.0 | −21.9 | 0.8 | 133 | 42 | 85 | n/a | n/a | 2.1 | 7.7 | 15 | n/a | n/a | 11.5 | −21.7 | 2.9 | 50 | 17 | n/a | n/a |
| MC11C28 | 27–28 | 2.1 | 30 | 7.1 | −21.8 | 0.7 | 98 | 47 | 84 | 0.27 | 0.03 | 2.6 | 8.7 | 16 | 0.81 | 0.03 | 12.0 | −21.8 | 3.3 | 55 | 17 | 0.35 | 0.03 |
| MC11C35 | 34–35 | 2.1 | 15 | 5.1 | −22.0 | 0.3 | 86 | 35 | 88 | 0.27 | 0.03 | 2.2 | 4.7 | 12 | 0.44 | 0.04 | 10.3 | n/a | 2.5 | 39 | 16 | 0.29 | 0.03 |
| MC11C37 | 36–37 | 2.1 | 29 | 6.6 | −21.7 | 0.9 | 176 | 42 | 77 | n/a | n/a | 2.3 | 12 | 23 | n/a | n/a | 12.6 | −21.4 | 3.2 | 54 | 17 | n/a | n/a |
| MC11C4648 | 46–48 | 2.1 | 31 | 9.4 | −21.6 | 1.0 | 334 | 40 | 79 | n/a | n/a | 1.7 | 11 | 21 | n/a | n/a | 10.8 | −21.4 | 2.8 | 51 | 18 | n/a | n/a |
| MC9G1 | 0–1 | 80 | 19 | 1.0 | −21.5 | 0.4 | 18 | 11 | 59 | −0.06 | 0.03 | 3.4 | 7.4 | 41 | 1.00 | 0.03 | 1.6 | n/a | 3.8 | 18 | 4.8 | 0.37 | 0.03 |
| MC9G3 | 2–3 | 80 | 19 | 1.1 | −21.4 | 0.4 | 31 | 11 | 57 | n/a | n/a | 3.5 | 8.8 | 43 | n/a | n/a | 2.2 | −21.6 | 3.9 | 20 | 5.2 | n/a | n/a |
| MC9G5 | 4–5 | 80 | 16 | 1.0 | −21.1 | 0.4 | 17 | 11 | 56 | n/a | n/a | 3.7 | 8.3 | 44 | n/a | n/a | 2.0 | −21.6 | 4.1 | 19 | 4.6 | n/a | n/a |
| MC9G7 | 6–7 | 80 | 14 | 1.0 | −20.9 | 0.2 | 6.6 | 4.3 | 38 | −0.06 | 0.03 | 4.0 | 6.9 | 62 | 1.03 | 0.03 | 1.8 | −21.7 | 4.2 | 11 | 2.6 | 0.61 | 0.03 |
| MC9G9 | 8–9 | 80 | 15 | 1.0 | −21.2 | 0.4 | 8.5 | 6.6 | 43 | n/a | n/a | 3.8 | 8.7 | 57 | n/a | n/a | 2.3 | −21.6 | 4.2 | 15 | 3.6 | n/a | n/a |
| MC9G11 | 10–11 | 80 | 17 | 1.2 | −20.5 | 0.3 | 8.4 | 14 | 65 | −0.09 | 0.03 | 3.7 | 7.3 | 35 | 0.99 | 0.03 | 1.6 | n/a | 4.0 | 21 | 5.2 | 0.28 | 0.03 |
| MC9G13 | 12–13 | 80 | 14 | 1.2 | −20.6 | 0.4 | 8.1 | 13 | 65 | −0.17 | 0.02 | 3.5 | 6.8 | 35 | 1.06 | 0.05 | 1.7 | −21.8 | 3.9 | 19 | 5.0 | 0.27 | 0.04 |
| Sample ID | Core ID | Group, Formation | Lithology | Redox | Age | Depth in Core | OPF | HFD | Bulk | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wta | δ13C | Alb | Pb | Cub | % Cu | δ65Cu | 2σc | Alb | Cub | % Cu | δ65Cu | 2σc | TOCd | Alb | Cub | Cu/Al | δ65Cu | 2σc | |||||||
| (Ma) | (m) | (%) | (‰) | (wt.%) | (ppm) | (ppm) | (of bulk) | (‰) | (‰) | (wt.%) | (ppm) | (of bulk) | (‰) | (‰) | (wt.%) | (wt.%) | (ppm) | (x104) | (‰) | (‰) | |||||
| GYX GC3 | n/a | Wufeng Fm. Guizhou Province | black shale | 446.34 | n/a | 13 | n/a | 1.7 | 4.4 | 0.58 | 2.6 | 0.30 | 0.03 | 3.7 | 22 | 97 | 0.55 | 0.03 | n/a | 5.4 | 22 | 4.1 | 0.54 | 0.03 | |
| GYX GC7 | n/a | Wufeng Fm. Guizhou Province | black shale | 446.34 | n/a | 4.6 | n/a | 0.0094 | 2.6 | 0.47 | 2.0 | 0.49 | 0.02 | 3.3 | 23 | 98 | 0.15 | 0.04 | n/a | 3.3 | 24 | 7.1 | 0.15 | 0.04 | |
| GYX GC11a | n/a | Wufeng Fm. Guizhou Province | black shale | 446.34 | n/a | 5.5 | n/a | 0.052 | 4.0 | 0.87 | 2.7 | 1.34 | 0.03 | 4.3 | 31 | 97 | 1.72 | 0.03 | n/a | 4.4 | 32 | 7.3 | 1.71 | 0.03 | |
| GRSW15 | n/a | Longmaxi Fm. Guizhou Province | black shale | 443.83 | n/a | 8.3 | n/a | 0.55 | 6.5 | 1.4 | 2.1 | 0.34 | 0.04 | 5.0 | 66 | 98 | 1.03 | 0.03 | n/a | 5.5 | 68 | 12 | 1.01 | 0.03 | |
| GRSW21 | n/a | Longmaxi Fm. Guizhou Province | black shale | 443.83 | n/a | 4.4 | n/a | 0.21 | 3.7 | 0.74 | 1.1 | 1.03 | 0.04 | 4.1 | 67 | 99 | 0.55 | 0.02 | n/a | 4.4 | 68 | 16 | 0.55 | 0.02 | |
| GRSW27 | n/a | Longmaxi Fm. Guizhou Province | black shale | 443.83 | n/a | 6.0 | n/a | 1.5 | 9.9 | 1.1 | 2.6 | 0.16 | 0.03 | 3.7 | 42 | 97 | 1.19 | 0.03 | n/a | 5.2 | 43 | 8.3 | 1.16 | 0.03 | |
| Baldwin1 B1 | Baldwin-1 | Red Heart Dolomite | black shale | 521 | 968.5 | 6.7 | n/a | 1.0 | 8.0 | 0.012 | 0.86 | n/a | n/a | 6.5 | 1.4 | 99 | 1.86 | 0.03 | 0.20 | 7.5 | 1.4 | 0.19 | n/a | n/a | |
| Baldwin1 B2 | Baldwin-1 | Red Heart Dolomite | black shale | 521 | 968.2 | 7.8 | n/a | 1.5 | 9.2 | 0.055 | 0.65 | 0.39 | 0.05 | 7.9 | 8.5 | 99 | 0.82 | 0.03 | 0.16 | 9.3 | 8.5 | 0.91 | 0.81 | 0.03 | |
| Baldwin1 B13 | Baldwin-1 | Thorntonia Limestone | black shale | 515 | 899.5 | 16 | n/a | 0.13 | 6.7 | 0.020 | 1.3 | 1.09 | 0.06 | 0.3 | 1.5 | 99 | n/a | n/a | 1.2 | 0.4 | 1.5 | 3.4 | n/a | n/a | |
| Baldwin1 B22 | Baldwin-1 | Arthur Creek | black shale | 505 | 888.0 | 13 | −32.0 | 0.21 | 101 | 8.1 | 15 | n/a | n/a | 3.6 | 46 | 85 | n/a | n/a | 4.7 | 3.8 | 54 | 14 | n/a | n/a | |
| Baldwin1 B25 | Baldwin-1 | Arthur Creek | black shale | 505 | 878.0 | 23 | −32.1 | 0.88 | 1139 | 12 | 13 | n/a | n/a | 2.0 | 77 | 87 | n/a | n/a | 3.3 | 2.9 | 89 | 30 | n/a | n/a | |
| M1 866.8 | Munta-1 | Officer Basin, Observatory Hill | shale | 520 | 866.8 | 20 | −30.9 | 2.8 | 3.9 | 1.4 | 3.6 | −0.23 | 0.04 | 6.2 | 39 | 96 | −0.04 | 0.04 | n/a | 9.1 | 40 | 4.5 | −0.05 | 0.04 | |
| M1 953.2 | Munta-1 | Officer Basin, Narana | shale | 565 | 953.2 | 19 | −32.8 | 1.7 | 0.7 | 0.069 | 0.44 | −0.24 | 0.04 | 1.6 | 16 | 100 | 0.17 | 0.04 | 0.23 | 3.3 | 16 | 4.8 | 0.16 | 0.04 | |
| M1 1172 | Munta-1 | Officer Basin, Munyara | shale | 565 | 1172.0 | 16 | −22.3 | 3.6 | 13 | 1.1 | 0.84 | −0.90 | 0.03 | 6.7 | 131 | 99 | −0.07 | 0.04 | 0.10 | 10 | 132 | 13 | −0.08 | 0.04 | |
| M1 1215 | Munta-1 | Officer Basin, Tanana | shale | 570 | 1215.9 | 10 | −29.1 | 1.9 | 7.4 | 0.84 | 5.7 | −0.40 | 0.03 | 7.4 | 14 | 94 | 0.17 | 0.03 | 0.20 | 9.3 | 15 | 1.6 | 0.14 | 0.03 | |
| Wallara W40 | Wallara-1 | Amadeus Basin, Finke beds Fm. | black shale | ferruginous | 750 | 1486.3 | 32 | −27.3 | 0.39 | 3.9 | 0.74 | 2.8 | n/a | n/a | 1.7 | 26 | 97 | n/a | n/a | 0.50 | 2.1 | 27 | 12 | n/a | n/a |
| AEM 59.18 | S3 | Mauritania, Aguelt El Mabha | lam black shale | ferruginous | 1100 | 59.2 | n/a | n/a | 1.7 | 5.8 | 0.30 | 2.4 | 0.51 | 0.16 | 10 | 12 | 98 | 0.38 | 0.05 | 0.28 | 12 | 12 | 1.1 | 0.38 | 0.06 |
| AEM 59.33 | S3 | Mauritania, Aguelt El Mabha | lam black shale | ferruginous | 1100 | 59.33 | n/a | n/a | 0.024 | 0.6 | 0.057 | 0.42 | 0.37 | 0.06 | 10 | 13 | 100 | 0.16 | 0.05 | 0.22 | 10 | 14 | 1.3 | 0.16 | 0.05 |
| Tourist S2 141 | S2 | Mauritania, Tourist | dark grey/black shale | ferruginous | 1100 | 141.8 | n/a | n/a | 0.81 | 25 | 7.7 | 6.6 | 0.27 | 0.04 | 10 | 110 | 93 | 0.56 | 0.04 | 23 | 11 | 118 | 11 | 0.54 | 0.04 |
| Tourist S2 171 | S2 | Mauritania, Tourist | dark grey/black shale | ferruginous | 1100 | 151.7 | n/a | n/a | 0.82 | 29 | 4.3 | 7.3 | 0.50 | 0.04 | 10 | 55 | 93 | 0.58 | 0.04 | 7.2 | 11 | 59 | 5.2 | 0.57 | 0.04 |
| Tourist S3 181 | S3 | Mauritania, Tourist | dark grey/black shale | ferruginous | 1100 | 181.5 | n/a | n/a | 0.69 | 2.8 | 4.0 | 31 | −0.44 | 0.04 | 5.4 | 8.8 | 69 | 1.62 | 0.06 | 0.80 | 6.1 | 13 | 2.1 | 0.98 | 0.05 |
| RP 4.12 | BMR Urapunga4 | Roper Gr., McArthur Basin, Velkerri | shale | euxinic | 1361 | 216.7 | 4.3 | n/a | 0.21 | 3.5 | 0.70 | 0.20 | 0.87 | 0.03 | 4.0 | 353 | 100 | 0.70 | 0.03 | 3.4 | 4.2 | 353 | 84 | 0.70 | 0.03 |
| RP 4.22 | BMR Urapunga4 | Roper Gr., McArthur Basin, Velkerri | shale | euxinic | 1417 | 363.4 | 8.5 | n/a | 0.91 | 8.6 | 1.8 | 1.6 | −0.15 | 0.03 | 6.9 | 112 | 98 | 1.06 | 0.04 | 3.0 | 7.8 | 114 | 15 | 1.04 | 0.04 |
| MB14 | GRNT 79-7 | Barney Creek, McArthur Basin | shale | euxinic | 1639 | 491 | 5.8 | n/a | 0.56 | 15 | 0.59 | 2.2 | 0.33 | 0.05 | 6.1 | 26 | 98 | 0.00 | 0.03 | 1.5 | 6.7 | 27 | 4.0 | 0.01 | 0.03 |
| MB19 | GRNT 79-7 | Barney Creek, McArthur Basin | shale | euxinic | 1639 | 496 | 5.9 | n/a | 0.68 | 33 | 1.2 | 2.6 | 1.26 | 0.04 | 7.5 | 46 | 97 | 0.58 | 0.04 | 1.7 | 8.2 | 47 | 5.7 | 0.60 | 0.04 |
| MB27 | GRNT 79-7 | Barney Creek, McArthur Basin | shale | euxinic | 1639 | 508 | 1.9 | n/a | 0.24 | 6.1 | 0.40 | 0.90 | 0.52 | 0.08 | 8.8 | 44 | 99 | n/a | n/a | 0.66 | 9.0 | 44 | 4.9 | n/a | n/a |
| MB36 | GRNT 79-7 | Barney Creek, McArthur Basin | shale | euxinic | 1639 | 851 | 12 | n/a | 0.65 | 11 | 0.46 | 1.9 | −0.19 | 0.05 | 4.6 | 24 | 98 | n/a | n/a | 1.3 | 5.3 | 25 | 4.6 | n/a | n/a |
| 981 05_08 | PR98-1 | Animikie Basin, Rove | shale | ferruginous | 1835 | 55.5 | 14 | n/a | 1.6 | 14 | 5.7 | 5.3 | 0.58 | 0.04 | 3.6 | 103 | 95 | 1.09 | 0.04 | 4.5 | 5.2 | 109 | 21 | 1.07 | 0.04 |
| MGS2 22 | MGS2 | Animikie Basin, Virginia | shale | euxinic | 1835 | 99.1 | 7.0 | −32.3 | 1.6 | 4.9 | 14 | 11 | −0.30 | 0.03 | 9.8 | 123 | 89 | 0.63 | 0.04 | 3.2 | 11 | 138 | 12 | 0.53 | 0.04 |
| PC104 | 77BLD3 | Mt. Partridge, Pine Creek, Wildman Sltst | black shale | ferruginous | 2019 | 85.0 | 24 | n/a | 1.4 | 4.3 | 8.2 | 30 | −0.78 | 0.07 | 5.1 | 19 | 70 | 1.05 | 0.04 | 3.5 | 6.5 | 27 | 4.2 | 0.50 | 0.05 |
| PC110 | 77BLD3 | Mt. Partridge, Pine Creek, Wildman Sltst | black shale | ferruginous | 2019 | 96.2 | 14 | −30.6 | 1.8 | 4.4 | 3.1 | 21 | n/a | n/a | 11 | 12 | 79 | 0.31 | 0.03 | 3.6 | 13 | 15 | 1.1 | n/a | n/a |
| EBA1 826.2 | EBA-1 | Pretoria, Timeball Hill | black shale | ferruginous | 2316 | 826.2 | 14 | −30.9 | 2.2 | 5.4 | 9.5 | 15 | 0.49 | 0.04 | 13 | 54 | 85 | 0.35 | 0.03 | 2.7 | 15 | 64 | 4.1 | 0.37 | 0.03 |
| EBA1 1158.5 | EBA-1 | Pretoria, Timeball Hill | black shale | ferruginous | 2316 | 1158.5 | 8.8 | −35.0 | 0.52 | 177 | 12 | 13 | −0.32 | 0.04 | 10 | 84 | 87 | 0.09 | 0.04 | 6.3 | 11 | 96 | 9.0 | 0.03 | 0.04 |
| EBA1 1160 | EBA-1 | Pretoria, Timeball Hill | black shale | ferruginous | 2316 | 1160 | 10 | n/a | 0.70 | 12 | 42 | 12 | 0.58 | 0.02 | 8.2 | 306 | 88 | 0.80 | 0.04 | 6.3 | 8.9 | 348 | 39 | 0.78 | 0.04 |
| McRS10 | n/a | Hamersley Basin, Pilbara Craton, Mt. McRae | black shale | 2479 | n/a | 7.4 | n/a | 0.0076 | 0.42 | 16 | 30 | −0.53 | 0.09 | 2.7 | 37 | 70 | 0.99 | 0.03 | 4.0 | 2.7 | 53 | 20 | 0.54 | 0.06 | |
| WS 2 | n/a | Hamersley Basin, Pilbara Craton, Whaleback Shale | black shale | 2463 | n/a | 59 | −31.4 | 1.3 | 14 | 2.1 | 86 | n/a | n/a | 3.0 | 0.36 | 14 | 0.06 | 0.11 | 3.0 | 4.3 | 2.5 | 0.58 | n/a | n/a | |
| WS 4 | n/a | Hamersley Basin, Pilbara Craton, Whaleback Shale | black shale | 2463 | n/a | 46 | −31.8 | 0.48 | 3.8 | 0.94 | 71 | n/a | n/a | 3.5 | 0.38 | 29 | −0.10 | 0.13 | 2.7 | 4.0 | 1.3 | 0.33 | n/a | n/a | |
| GKP01 310.2 | GKP-01 | Transvaal, Klein Naute | black shale | interm. eux | 2521 | 310.2 | 8.0 | −39.2 | 0.32 | 38 | 24 | 16 | −0.17 | 0.04 | 5.8 | 131 | 84 | 0.12 | 0.03 | 4.2 | 6.2 | 155 | 25 | 0.07 | 0.04 |
| GKP01 337 | GKP-01 | Transvaal, Upper Nauga | BIF/chert | interm. eux | 2521 | 337 | 1.0 | −34.7 | 0.17 | 0.42 | 2.2 | 7 | −0.19 | 0.04 | 0.2 | 29 | 93 | −0.07 | 0.04 | 4.6 | 0.4 | 31 | 82 | −0.08 | 0.04 |
| GKP01 1209.6 | GKP-01 | Transvaal, Vryburg | org-rich silicic mdst | 2669 | 1209.6 | 26 | −34.1 | 4.9 | 27 | 7.6 | 5.5 | 0.10 | 0.04 | 9.0 | 130 | 94 | 0.12 | 0.04 | 1.7 | 14 | 137 | 9.9 | 0.12 | 0.04 | |
| WRL1 HM21 | WRL-1 | Fortescue Gr., Maramamba | shale | 2597 | 590.5 | 28 | n/a | 2.5 | 2.8 | 0.67 | 21 | −0.07 | 0.03 | 0.7 | 2.5 | 79 | 1.75 | 0.16 | 1.3 | 3.2 | 3.2 | 1.0 | 1.36 | 0.14 | |
| PGA 46.27 | TSB07-26 | Mozaan Gr., Ntombe | shale | 2954 | 46.27 | 21 | n/a | 0.91 | 0.71 | 1.7 | 3.5 | 0.64 | 0.04 | 4.2 | 46 | 96 | 1.15 | 0.04 | 0.54 | 5.1 | 48 | 9.3 | 1.13 | 0.04 | |
| PGA 46.53 | TSB07-27 | Mozaan Gr., Ntombe | shale | 2950 | 46.53 | 19 | n/a | 0.50 | 0.10 | 1.2 | 3.6 | 0.85 | 0.04 | 3.2 | 32 | 96 | 0.51 | 0.03 | 0.49 | 3.7 | 33 | 8.8 | 0.52 | 0.03 | |
| PGA 46.67 | TSB07-28 | Mozaan Gr., Ntombe | shale | 2950 | 46.67 | 18 | n/a | 0.67 | 1.1 | 2.5 | 3.4 | 1.16 | 0.04 | 3.3 | 73 | 97 | 0.00 | 0.02 | 0.39 | 4.0 | 75 | 19 | 0.04 | 0.02 | |
| PGA 70.18 | TSB07-29 | Mozaan Gr., Ntombe | shale | 2950 | 70.18 | 8.3 | n/a | 0.67 | 0.35 | 0.68 | 3.5 | 0.54 | 0.04 | 3.5 | 19 | 97 | 0.35 | 0.04 | n/a | 4.2 | 20 | 4.7 | 0.35 | 0.04 | |
| PGA 93.36 | TSB07-30 | Mozaan Gr., Ntombe | shale | 2950 | 93.36 | 17 | n/a | 1.1 | 0.70 | 1.5 | 4.6 | 0.38 | 0.03 | 3.9 | 31 | 95 | 0.91 | 0.04 | 0.44 | 5.0 | 32 | 6.5 | 0.89 | 0.04 | |
| PGA 162.4 | TSB07-31 | Mozaan Gr., Sinqeni | shale | 2950 | 162.4 | 41 | n/a | 3.9 | 2.6 | 1.8 | 2.6 | 0.81 | 0.03 | 4.0 | 68 | 97 | 1.10 | 0.03 | 0.11 | 8.0 | 70 | 8.8 | 1.09 | 0.03 | |
| BARB3 ETH1 | BARB 3 | Buck Reef Chert | black shale | 3400 | 208.7 | 1.8 | n/a | 0.06 | 3.6 | 0.25 | 51 | −0.24 | 0.04 | 3.6 | 0.25 | 49 | 1.66 | 0.03 | 3.2 | 3.7 | 0.50 | 0.14 | 0.70 | 0.04 | |
| BARB3 ETH2 | BARB 3 | Buck Reef Chert | black shale | 3400 | 213.1 | 3.5 | −31.2 | 0.0 | 0.00 | 1.3 | 35 | n/a | n/a | 6.3 | 2.3 | 65 | 1.38 | 0.06 | 2.6 | 6.3 | 3.6 | 0.58 | n/a | n/a | |
| BARB3 ETH9 | BARB 3 | Buck Reef Chert | black laminated chert | 3400 | 871.5 | 0.6 | n/a | 0.19 | 1.6 | 0.52 | 0.88 | −0.04 | 0.04 | 1.1 | 58 | 99 | −0.01 | 0.03 | 1.7 | 1.3 | 59 | 44 | −0.01 | 0.03 | |
| BARB4 ETH24 | BARB 4 | Upper Mendon Formation | black chert | 3280 | 434.7 | 15 | −26.8 | 1.2 | 2.0 | 19 | 19 | n/a | n/a | 12 | 78 | 81 | n/a | n/a | 1.8 | 13 | 96 | 7.4 | n/a | n/a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciscato, E.R.; Bontognali, T.R.R.; Poulton, S.W.; Vance, D. Copper and its Isotopes in Organic-Rich Sediments: From the Modern Peru Margin to Archean Shales. Geosciences 2019, 9, 325. https://doi.org/10.3390/geosciences9080325
Ciscato ER, Bontognali TRR, Poulton SW, Vance D. Copper and its Isotopes in Organic-Rich Sediments: From the Modern Peru Margin to Archean Shales. Geosciences. 2019; 9(8):325. https://doi.org/10.3390/geosciences9080325
Chicago/Turabian StyleCiscato, Emily R., Tomaso R. R. Bontognali, Simon W. Poulton, and Derek Vance. 2019. "Copper and its Isotopes in Organic-Rich Sediments: From the Modern Peru Margin to Archean Shales" Geosciences 9, no. 8: 325. https://doi.org/10.3390/geosciences9080325
APA StyleCiscato, E. R., Bontognali, T. R. R., Poulton, S. W., & Vance, D. (2019). Copper and its Isotopes in Organic-Rich Sediments: From the Modern Peru Margin to Archean Shales. Geosciences, 9(8), 325. https://doi.org/10.3390/geosciences9080325





